Abstract
Alpha, beta-unsaturated carbonyls are highly reactive mutagens and carcinogens to which humans are exposed on a daily basis. This study demonstrates that aldo-keto reductase family 1 member B10 (AKR1B10) is a critical protein in detoxifying dietary and lipid-derived unsaturated carbonyls. Purified AKR1B10 recombinant protein efficiently catalyzed the reduction to less toxic alcohol forms of crotonaldehyde at 0.90 µM, 4-hydroxynonenal (HNE) at 0.10 µM, trans-2-hexanal at 0.10 µM, and trans-2, 4-hexadienal at 0.05 µM, the concentrations at or lower than physiological exposures. Ectopically expressed AKR1B10 in 293T cells eliminated immediately HNE at 1 (subtoxic) or 5 µM (toxic) by converting to 1, 4-dihydroxynonene, protecting the cells from HNE toxicity. AKR1B10 protein also showed strong enzymatic activity toward glutathione-conjugated carbonyls. Taken together, our study results suggest that AKR1B10 specifically expressed in the intestine is physiologically important in protecting the host cell against dietary and lipid-derived cytotoxic carbonyls.
Keywords: aldo-keto reductase family 1 member B10, alpha, beta-unsaturated carbonyls, glutathione-conjugated carbonyls, steady-state kinetics, cytotoxicity
Introduction
Aldo-keto reductase family 1 member B10 (AKR1B10, also designated aldose reductase-like-1, ARL-1) is primarily expressed in the human colon and small intestine, but overexpressed in hepatocellular carcinoma, lung squamous cell carcinoma and lung adenocarcinoma in smokers [1–3]. AKR1B10 belongs to aldo-keto reductase (AKR) superfamily, a protein cluster that is widely implicated in carbonyl detoxification, osmolytic regulation, hormonal metabolism, fatty acid synthesis, diabetic complications, tumorigenesis, and cancer therapeutics [4–10].
AKR1B10 is a monomeric enzyme, efficiently converting carbonyls to corresponding alcohols with NADPH as a co-enzyme [1, 11–14]. AKR1B10 also shows strong enzymatic activity toward all-trans-retinal, 9-cis-retinal, and 13-cis-retinal, which may regulate the intracellular retinoic acid, a signaling molecule modulating cell proliferation and differentiation [15, 16]; and in lung cancer AKR1B10 activates pro-carcinogen polycyclic aromatic hydrocarbons (PHA) in cigarette smoke, promoting tumorigenesis [17]. These data suggest the potential role for AKR1B10 in cell differentiation and carcinogenesis in abnormal situations, but do not enlighten its physiological function in the intestine, where retinals and PHA are not routinely present.
To explore the biological function of AKR1B10 in the intestine, we deepened the observation on its activity to electrophilic carbonyls. Alpha, beta-unsaturated carbonyls are highly reactive and cytotoxic. Through interaction with proteins, peptides and DNA, the carbonyls cause protein dysfunction and DNA damage (breaks and mutations), resulting in mutagenesis, carcinogenesis, or cell death [18–21]. Gastrointestinal (GI) cells are exposed to various carbonyls on a daily basis via food consumption and lumen-microbial production in addition to cellular lipid peroxidation [22–24]. For instance, humans are exposed to crotonaldehyde through the consumption of vegetables (1.4–100 µg/kg), fruits (5.4–78 µg/kg), fish (71.4–1000 µg/kg), meat (10–270 µg/kg), and alcoholic beverages such as wine (300–700 µg/l) and whisky (30–210 µg/l) [24]. Human exposure to trans-2-hexanal is up to 350 µg/day [25]. The dietary exposures are persistent, and although subtoxic, may have far-reaching effects. Therefore, the host needs an effective defense system to protect the intestinal cells. This study demonstrated that AKR1B10 may be a critical protein in this carbonyl defense system.
Intracellular carbonyls are eliminated by three metabolic pathways, through which carbonyls are reduced to less toxic alcohols, oxidized to carboxylic acids, or conjugated with glutathione. Aldehyde reductase and aldose reductase are involved in the reduction of carbonyls [26]; aldehyde dehydrogenases mediate the oxidative pathway [27]; and glutathione-Stransferases catalyze the carbonyl conjugation with glutathione [26, 28]. Glutathione conjugates are further reduced to alcohol forms and then pumped out of the cell by glutathione-S-conjugate transporters; and aldose reductase acts on this reduction step [26]. This study recognized for the first time the enzymatic activity of AKR1B10 to the glutathione conjugates of carbonyls (GS-carbonyls). The data suggest that AKR1B10 detoxifies α, β-unsaturated carbonyls at both free and glutathione-conjugated forms.
Materials and Methods
Cell culture, EGFP-AKR1B10 expression vector and transient transfection
Transformed human embryonic kidney cell (293T) maintenance, enhanced green fluorescent protein (EGFP)-AKR1B10 expression vector construction, and transient delivery into the 293T cells were conducted as previously described [14].
MTT assay
HNE toxicity was detected using a MTT ((3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) cell proliferation kit (Roche, IN) as described previously [29]. Briefly, cells (5,000/well) were seeded in 96-well plates overnight and then exposed to 50 µl of serum-free medium containing HNE at different concentrations for 2 hours, followed by addition of 50 µl of fresh medium containing 20% FBS. After incubation for 48 hours, viable cells were detected.
Intracellular metabolism of HNE
The 293T cells transfected with the EGFP-AKR1B10 or control vector EGFP-C3 were collected by trypsinization after incubation for 36 hours. Trypsin was neutralized by a complete medium containing 10% FBS. After being washed by PBS, cells were re-suspended at 1.0 × 107 cells/ml in a serum-free DMEM medium containing 1.0 or 5.0 µM HNE. At indicated time points, 40 µl of the cell suspension (4.0 × 105 cells) was transferred into a tube containing 20 µl of perchloric acid (45%), vortexed immediately, and then kept on ice. The aqueous phase was collected by centrifugation at 14,000 rpm, 4°C for 10 min, and 30 µl was used for high-performance liquid chromatography-ultraviolet (HPLC-UV) analysis.
HPLC-UV procedures
Enzymatic products were analyzed by a HPLC system equipped with a dual UV detector (Shimadzu, Japan). A premier C18 column (4.0 × 250mm) with 5 µm particles, preceded by a pre-column with the same materials, was used. Mobile phase was a mixture of Buffer A (0.2%, v/v, formic acid in deionized water) and Buffer B (acetonitrile; Sigma, MO). Separation and detecting conditions are summarized in Table 1S in Supplementary Data. Standards purchased from Sigma, MO were used to calibrate the column and retention time.
HPLC-MS procedures
GS-carbonyl conjugates and enzymatic products were analyzed by an injection of one microliter of each sample into a ThermoFinnigan Surveyor HPLC equipped with a Gemini® narrow bore column (150 × 20 mm) packed with 3 µM C18 (110Å) at a flow rate of 0.150 ml/min of a binary mobile phase gradient at 50:50 in 20 min (mobile phase A = 0.01% formic acid; mobile phase B = acetonitrile) until the proper peak shape, and separation and reduction of interferences were obtained. A ThermoFinnigan (TSQ7000) triple stage quadrupole (TSQ) mass spectrometer equipped with an electrospray ionization source (ESI) was calibrated with MRFA for both the single and double charge state (m/z 524.2 and 262.6, respectively) to provide a 0.1 amu mass accuracy for each [M+H]+ parent ion (mp ·+). A full scan collection in a mass range from 150–600 m/z through a quadrupole filter (Q1) for each substrate/product of interest, e.g. m/z 406.1 for GS-trans-2, 4-hexadienol, was obtained with a capillary temperature of 250°C maintained at 4.5 kV. The mp ·+ ions from Q1 were passed through a collision chamber (Q2), operating in a radio-frequency-only mode, and subsequently scanned through a third mass filter (Q3).
Preparation of AKR1B10 recombinant protein and enzyme activity assay
AKR1B10 protein preparation and activity assays were performed as previously described [14]. Two µg AKR1B10 proteins were used in each reaction and the mixture without protein was used as a control. Oxidized NADPH was monitored by a spectrophotometer at 340 nm for activity assessment, presented as oxidized NADPH (nmol)/mg protein/min, or 100 µl of reaction mixture was loaded onto HPLC for enzymatic product analysis. Michaelis-Menten constants (Km and Vmax) were calculated with GraphPad Prism 4 (Graph Pad Software, CA). kcat = Vmax/[E]. [E] denotes enzyme concentrations in molar.
Synthesis of glutathione conjugates
Glutathione conjugates of carbonyls (GS-carbonyls) were synthesized by incubating 3 mM glutathione with 600 µM of acrolein, crotonaldehyde, HNE, trans-2-hexanal, or trans-2, 4-hexadienal in 100 mM sodium phosphate (pH 7.0) at room temperature for 90 min. Progress of the reactions was monitored by the decrease in absorbance (A210) for acrolein, A220 for crotonaldehyde, A223 for HNE, A225 for trans-2-hexanal, and A278 for trans-2, 4-hexadienal. Resulting products were purified by HPLC-UV (see above) and confirmed by HPLC-mass spectroscopy (HPLC-MS). Amounts of formed conjugates were calculated by quantitating residual carbonyls left over considering a 1:1 molar ratio conjugation between glutathione and carbonyls. Reduced GS-carbonyl standards were prepared by complete reduction with excessive AKR1B1 proteins (50 µg) prepared previously [13].
Statistical analysis
Student’s t-tests or Chi-square tests of independence, as appropriate, were used for statistically significant tests of the data with p < 0.05.
Results
AKR1B10 catalyzes reduction of free carbonyls at subtoxic levels
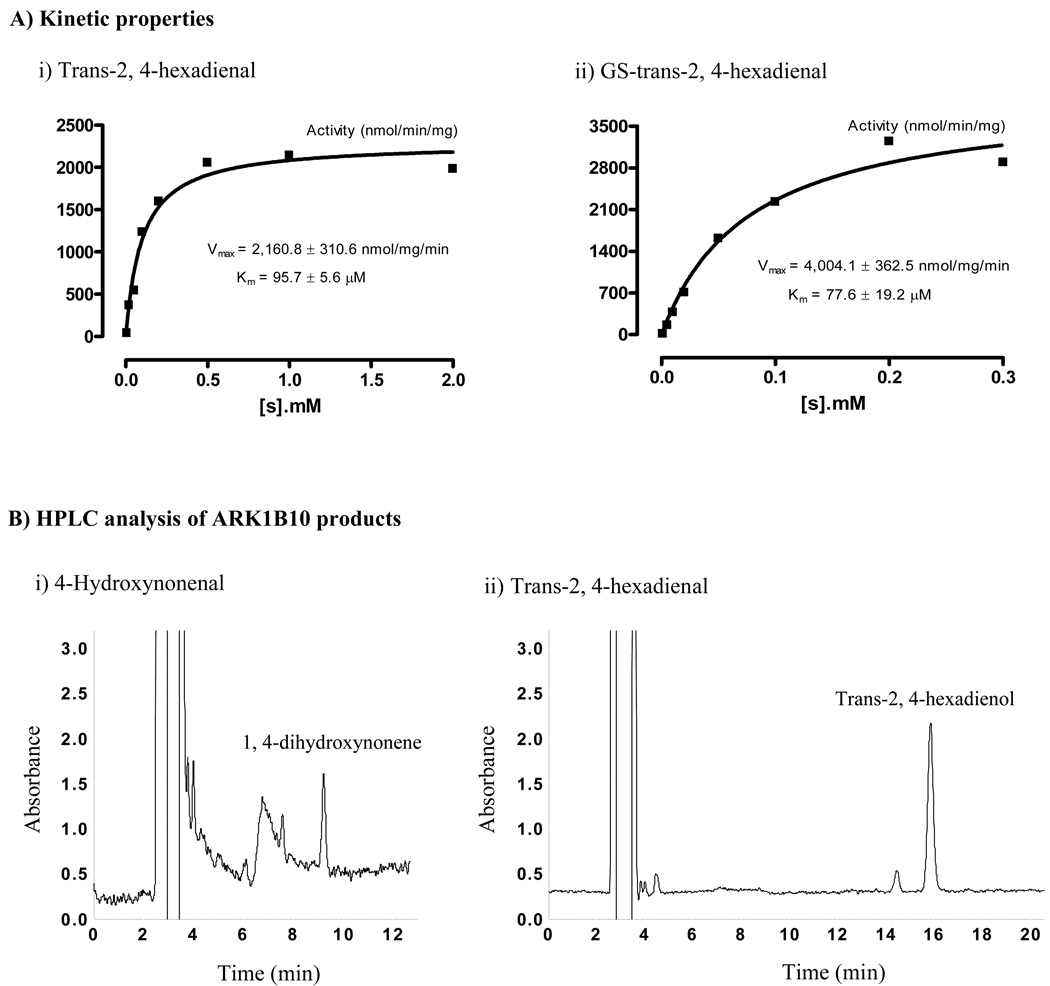
Previous studies from us and other investigators have shown the enzymatic activity of AKR1B10 toward acrolein, crotonaldehyde, and HNE [12, 14]. Using the purified AKR1B10 protein (Figure 1S), this study further assessed AKR1B10 activity toward trans-2-hexanal and trans-2, 4-hexadienal, two highly electrophilic unsaturated carbonyls with lipid and dietary origins [25]. AKR1B10 activity to HNE was also evaluated in our experimental conditions. The results showed that trans-2-hexanal and trans-2, 4-hexadienal both were appreciable substrates of AKR1B10, displaying steady-state kinetics (Figure 1A-i). Interestingly, HNE had a Km value at 30.9 ± 7.1 µM in our experimental conditions, approximately 10 fold lower than that previously reported [12] (see comments in discussion). The kinetic parameters are summarized and compared in Table 1.
Fig. 1. Substrate-velocity curves and HPLC chromatographs of AKR1B10 products.
A) Substrate-velocity curves. AKR1B10 enzymatic activity to trans-2, 4-hexadienal and glutathione (GS)-trans-2, 4-hexadienal was measured as described in Materials and Methods. Substrate-velocity curves were produced with GraphPad Prism 4 (Graph Pad Software, CA). B) HPLC analysis of the AKR1B10 products. Enzymatic reactions and HPLC separations of mixtures (100 µl) were performed as described in the Materials and Methods. (i) HNE products. Image shows the product DHN when HNE was used at 0.2 µM. The small peak at 7½ min is β-mercaptoethanol present in the AKR1B10 protein preparation. Noise at 7 min constantly occurred at the detection wavelength of 190 nm. (ii) Trans-2, 4-hexadienal products. Image shows the product trans-2, 4-hexadienol when trans-2, 4-hexadienal was used at 0.2 µM. The small peak at 14½ min is noise at 228 nm. DHN and trans-2, 4-hexadienol standards were prepared by complete reduction with excessive aldose reductase proteins. HNE, 4-hydroxynonenal; DHN, 1, 4-dihydroxynonene; and HPLC, high-performance liquid chromatography.
Table 1. Kinetic parameters of AKR1B10 to carbonyls and glutathione conjugates.
AKR1B10 activity was measured as described in Materials and Methods. Constants were calculated by a Lineweaver-Burk plot of the reduction rate at various substrate concentrations. Vmax is expressed at nmol/mg protein/min. GS, glutathione; ND, not detectable; Cn, carbon chain length.
| AKR1B10 | |||||
|---|---|---|---|---|---|
| Substrates | Cn |
Vmax (nmol/mg/min) |
Km (µM) |
kcat (min−1) |
kcat/Km (min−1 µM−1) |
| Acrolein a | 3 | 3,122.0 ± 64.7 | 110.1 ± 12.2 | 115.9 ± 7.8 | 1.07 |
| Crotonaldehyde a | 4 | 2,647.5 ± 132.2 | 86.7 ± 14.3 | 103.4 ± 3.5 | 1.20 |
| Trans-2-hexanal | 6 | 2,658.1 ± 222.8 | 60.6 ± 18.5 | 97.4 ± 8.0 | 1.62 |
| Trans-2, 4-hexadienal | 6 | 2,160.8 ± 310.6 | 95.7 ± 5.6 | 82.8 ± 7.4 | 0.87 |
| 4-Hydroxynonenal | 9 | 3,298.7 ± 245.9 | 30.9 ± 7.1 | 120.7 ± 6.5 | 3.92 |
| GS-acrolein | 64.36 ± 5.6 | 532.8 ± 70.6 | 2.8 ± 0.1 | 0.005 | |
| GS-crotonaldehyde | 1,900.7 ± 90.9 | 245.7 ± 21.2 | 70.2 ± 3.5 | 0.29 | |
| GS-trans-2-hexanal | 2,049.0 ± 116.3 | 145.7 ± 20.5 | 71.3 ± 2.6 | 0.51 | |
| GS-trans-2, 4-hexadienal | 4,004.1 ± 362.5 | 77.6 ± 19.2 | 147.1 ± 10.1 | 1.91 | |
| GS-4-hydroxynonenal | ND | ND | ND | ND | |
Reference [13]
Under physiological conditions, human exposure to carbonyls is subtoxic [24]. To understand the protective role for AKR1B10 in the physiological conditions we examined its activity to carbonyls at subtoxic levels through HPLC analysis of the enzymatic products. Figure 1B shows the HPLC chromatographs for the reduction products of the HNE and trans-2, 4-hexadienal at 0.2 µM. Our results showed that AKR1B10 efficiently catalyzed the reduction of acrolein at 3.0 µM, crotonaldehyde at 0.9 µM, HNE at 0.1 µM trans-2-hexanal at 0.1 µM, and trans-2, 4-hexadienal at 0.05 µM. These concentrations are at or lower than the doses to which humans are exposed at physiological conditions.
AKR1B10 enzymatic activity toward GS-carbonyl conjugates
Conjugation with glutathione is one of the major pathways for a live cell to eliminate the carbonyls and aldose reductase catalyzes the reduction of the glutathione conjugates (GS-carbonyls) for further detoxification [26, 28]. This study evaluated the enzymatic activity of AKR1B10 toward the GS-carbonyls. Chemically synthesized GS-carbonyls were purified by HPLC-UV and verified by HPLC-MS. Figure 2S shows the production and characterization of GS-HNE conjugates. Similarly, GS-acrolein, GS-crotonaldehyde, GS-trans-2-hexanal, and GStrans-2, 4-hexadienal were chemically synthesized, purified by HPLC-UV and characterized with HPLC-MS (data not shown).
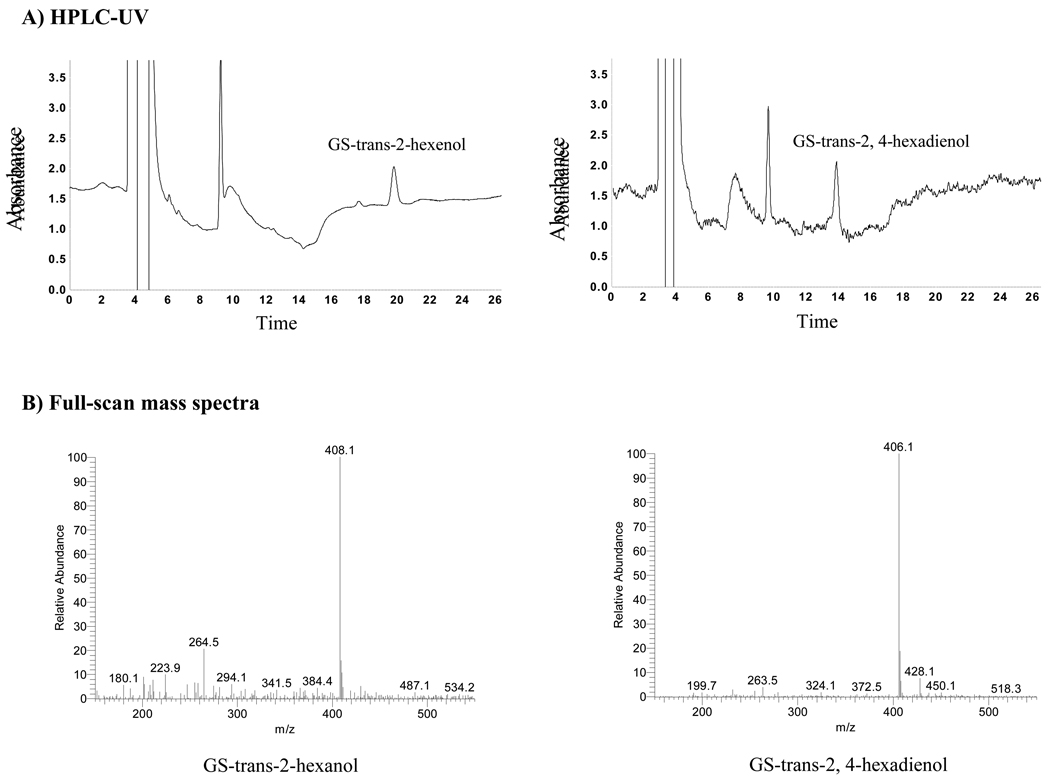
Enzymatic products (reduced forms) of GS-carbonyls were analyzed by HPLC-UV and verified by HPLC-MS. Figure 2A demonstrates the AKR1B10 enzymatic products of GS-trans 2-hexanal and GS-trans-2, 4-hexadienal at 0.5 µM, detected by HPLC-UV. Mass spectrum analysis confirmed that these two products have the expected m/z 408.1 and m/z 406.1, respectively (Figure 2B). The detected [M+H]+ parent ions (mp ·+) of GS-carbonyls/reduced forms are at m/z 363.89/365.90 for GS-acrolein/GS-allyl alcohol, at m/z 378.1/379.9 for GS-crotonaldehyde/GS-crotyl alcohol, at m/z 406.1/408.1 for GS-trans-2-hexanal/GS-trans-2-hexanol, and at m/z 404.1/406.1 for GS-trans-2, 4-hexadienal/GS-trans-2, 4-hexadienol.
Fig. 2. AKR1B10 activity to GS-trans-2-hexanal and GS-trans-2, 4-hexadienal.
Enzymatic reactions were conducted as described in the Materials and methods with GS-trans-2-hexanal and GS-trans-2, 4-hexadienal at 0.2 µM. A) HPLC-UV analysis. Reaction mixtures (100 µl) were loaded onto HPLC and separated as described in the Materials and methods. Images show the products GS-trans-2-hexanol and GS-trans-2, 4-hexadienol (labeled). The peak at about 10 min is β-mercaptoethanol present in the AKR1B10 protein preparation. (B) Full scan mass spectra (m/z 150–600), performed as described in the Materials and Methods. GS-trans-2-hexanol and GS-trans-2, 4-hexadienol have [M+H]+ 408.1 and 406.1, respectively.
The tested GS-carbonyls, except GS-HNE, are appreciable substrates of AKR1B10, displaying steady-state kinetics (Table 1). Figure 1A-ii shows the substrate-velocity curve of GS-trans-2, 4-hexadienal as an example. Similarly, we also assessed AKR1B10 activity toward these GS-carbonyls at low concentrations, and the results showed that AKR1B10 efficiently catalyzed the reduction of GS-acrolein at 5.0 µM, GS-crotonaldehyde at 0.5 µM, GS-trans-2-hexanal at 0.05 µM, and GS-trans-2, 4-hexadienal at 0.1 µM.
AKR1B10 efficiently detoxifies HNE in 293T cells
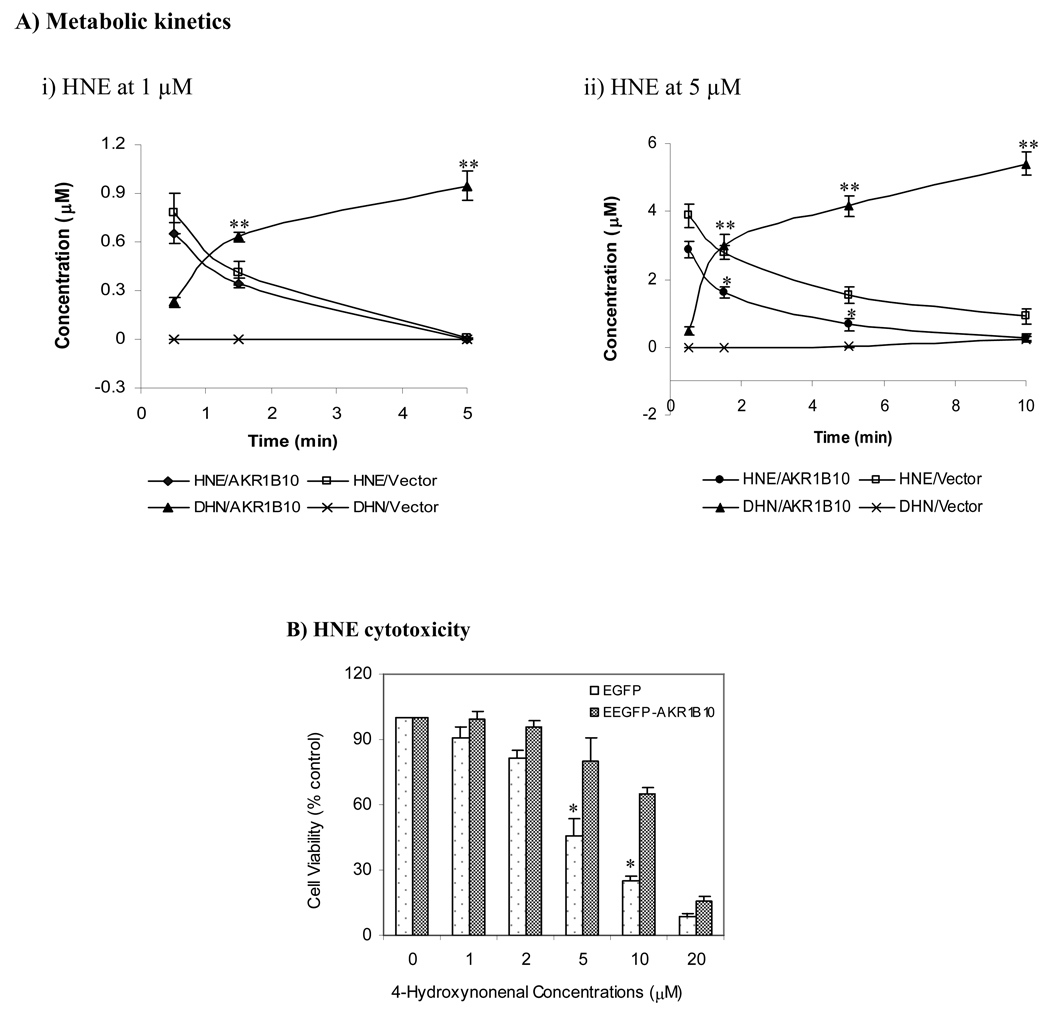
In vitro studies above indicate that AKR1B10 has strong enzymatic activity to carbonyls and their GS-conjugates. To confirm its intracellular role, we evaluated the effects of ectopically expressed EGFP-AKR1B10 (Figure 3S) [14] on the metabolic kinetics and cytotoxicity of HNE, a most toxic lipid hydroxyperoxide. Our results showed that the HNE treated at 1 (subtoxic) or 5 µM (toxic), respectively, was rapidly cleared up by reduction to DHN in the 293T cells expressing EGFP-AKR1B10 (Figure 3A). DHN was detected within 30 seconds and then rapidly accumulated. However, DHN was not detected in the vector control cells until 5 min. Half-life of HNE in the cells with EGFP-AKR1B10 was less than 1 min, compared to approximately 2½ min in the vector control. As a result, the 293T cells with EGFP-AKR1B10 were significantly (p < 0.05) resistant to HNE exposures (Figure 3B), proving the detoxicating role for AKR1B10.
Fig. 3. HNE metabolism and cytotoxicity in 293T cells.
AKR1B10 delivery into 293T cells and HNE treatment were performed as described in the Materials and Methods. A) Metabolic kinetics. Cells applied with 1 or 5 µM of HNE were collected at indicated time points. HNE and DHN were extracted and quantitated by HPLC as described in the Materials and Methods. Data represent mean ± SD from three independent experiments. *, p < 0.05 and **, p < 0.01, compared to the vector control. B) HNE cytotoxicity, detected by MTT cell proliferation kit as described in the Materials and Methods. Values indicate mean ± SD from three independent experiments. * p < 0.05, compared to EGFP-AKR1B10 cells. HNE, 4-hydroxynonenal; DHN, 1, 4-dihydroxynonene; and EGFP-AKR1B10, enhanced green fluorescent protein/aldo-keto reductase family member B10 fusion protein.
Discussion
Alpha, beta-unsaturated carbonyls are highly electrophilic, with strong cytotoxicity, mutagenicity, and carcinogenicity. This study demonstrates that AKR1B10 is a highly efficient detoxicant of the tested unsaturated carbonyls (acrolein, crotonaldehyde, HNE, trans-2-hexanal, and trans-2, 4-hexadienal) and their GS-conjugates, except for GS-HNE. In view of the wide presence in diets and constant production by lipid peroxidation [19, 24, 25], these unsaturated carbonyls represent important carcinogenic threats to humans, particularly for their gastrointestinal cells. Therefore, the study results are of important significance in several aspects, particularly to the intestinal cells where AKR1B10 is specifically expressed.
AKR1B10 shows reduction activity to the tested carbonyls at subtoxic levels, which denotes its role in host defense against carbonyls in physiological conditions. For instance, human exposure to cellular HNE is at 0.1 to 3.0 µM in normal conditions, but up to ~10 µM at oxidative stress [22]; human dietary exposure to crotonaldehyde is at 71.4–1000 µg/kg (1.02–14.27 µM) in fish and at 300–700 µg/l (4.28–9.99 µM) in wine [24]. Our data exhibited AKR1B10’s strong activity toward HNE at 0.1 µM and crotonaldehyde at 0.9 µM, which are lower than the physiological exposures above, indicating its protection role. It is noteworthy that in this study HNE had a Km at 30.9 ± 7.1 µM, approximately 10 fold lower than that previously reported [12]. This may be due to the less AKR1B10 protein used in our assays (2 µg vs. 20 – 25 µg in previous report), which will allow for a maximal use of its activity. This result is supported by an intracellular study with HNE.
In vitro enzymatic activity study is cell-free and thus somewhat artificial. To confirm the in vitro findings, we performed an intracellular study by transiently introducing AKR1B10 into the 293T cells in which it is not endogenously expressed for a clear datum interpretation. The results showed that AKR1B10 effectively eliminated the HNE treated at either subtoxic (1 µM) or toxic (5 µM) levels and thus protected the cells from HNE cytotoxicity. This result proves the high affinity (Km) and turnover rate (kcat) of AKR1B10 to HNE as observed in the in vitro enzymatic activity assays. In the vector cells, the reduction product DHN was not detected, and HNE may be eliminated by aldehyde dehydrogenase and/or glutathione conjugation (see below). In this study, serum-free medium was used to exclude the potential effect of serum on HNE metabolism.
Intracellular carbonyls are eliminated through reduction to less toxic alcohols, oxidation to carboxylic acids, or conjugation with glutathione [26–28]. The glutathione-conjugates are further reduced to alcohol forms and then pumped out of the cell. This study recognized for the first time the enzymatic activity of AKR1B10 to GS-acrolein, GS-crotonaldehyde, GS-trans-2-hexanal and GS-trans-2, 4-hexadienal, and determined their kinetic constants. This finding denotes that AKR1B10 detoxifies carbonyls at two chemical forms, i.e., the free and glutathione-conjugated carbonyls, indicating its importance in carbonyl detoxification and host protection. It is currently unknown why the GS-HNE is not an appreciable substrate of the AKR1B10. A possible explanation is that the long carbon chain of HNE prevents its GS-conjugate from efficiently fitting into the active pocket.
In summary, this study demonstrated that AKR1B10 is an important protein protecting the host cell against α, β-unsaturated carbonyls and their GS-conjugates. The specific expression of AKR1B10 in the intestine may imply its protective role in detoxifying the dietary, lumen-microbial, and lipid-derived carbonyls.
Supplementary Material
Acknowledgement
This work was supported by American Cancer Society [RSG-04-031-01-CCE] and National Cancer Institute [CA122327, 122622]. We greatly thank Ms. Barbara Nowack and Ms. Rhona Kelley for the proofreading of the manuscript.
Abbreviations used
- AKR1B10
aldo-keto reductase family 1 B10
- DHN
1, 4-dihydroxynonene
- EGFP
enhanced green fluorescent protein
- ESI
electrospray ionization; 4-hydroxynonenal
- HPLC-MS
high performance liquid chromatography-mass spectroscopy
- HPLC-UV
high-performance liquid chromatography-ultraviolet detection
- MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TIC
total ion chromatogram.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273:11429–11435. doi: 10.1074/jbc.273.19.11429. [DOI] [PubMed] [Google Scholar]
- 2.Fukumoto S, Yamauchi N, Moriguchi H, Hippo Y, Watanabe A, Shibahara J, Taniguchi H, Ishikawa S, Ito H, Yamamoto S, Iwanari H, Hironaka M, Ishikawa Y, Niki T, Sohara Y, Kodama T, Nishimura M, Fukayama M, Dosaka-Akita H, Aburatani H. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers' non-small cell lung carcinomas. Clin Cancer Res. 2005;11:1776–1785. doi: 10.1158/1078-0432.CCR-04-1238. [DOI] [PubMed] [Google Scholar]
- 3.Scuric Z, Stain SC, Anderson WF, Hwang JJ. New member of aldose reductase family proteins overexpressed in human hepatocellular carcinoma. Hepatology. 1998;27:943–950. doi: 10.1002/hep.510270408. [DOI] [PubMed] [Google Scholar]
- 4.Lee KW, Ko BC, Jiang Z, Cao D, Chung SS. Overexpression of aldose reductase in liver cancers may contribute to drug resistance. Anticancer Drugs. 2001;12:129–132. doi: 10.1097/00001813-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci U S A. 1995;92:2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko BC, Ruepp B, Bohren KM, Gabbay KH, Chung SS. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J Biol Chem. 1997;272:16431–16437. doi: 10.1074/jbc.272.26.16431. [DOI] [PubMed] [Google Scholar]
- 7.Jin J, Krishack PA, Cao D. Role of aldo-keto reductases in development of prostate and breast cancer. Front Biosci. 2006;11:2767–2773. doi: 10.2741/2006. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Yan R, Zu X, Cheng JM, Rao K, Liao DF, Cao D. Aldo-keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. J Biol Chem. 2008;283:3418–3423. doi: 10.1074/jbc.M707650200. [DOI] [PubMed] [Google Scholar]
- 9.Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev. 2008;40:553–624. doi: 10.1080/03602530802431439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y, Penning TM. Aldo-keto reductases and bioactivation/detoxication. Annu Rev Pharmacol Toxicol. 2007;47:263–292. doi: 10.1146/annurev.pharmtox.47.120505.105337. [DOI] [PubMed] [Google Scholar]
- 11.Spite M, Baba SP, Ahmed Y, Barski OA, Nijhawan K, Petrash JM, Bhatnagar A, Srivastava S. Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes. Biochem J. 2007;405:95–105. doi: 10.1042/BJ20061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin HJ, Maser E. Role of human aldo-keto-reductase AKR1B10 in the protection against toxic aldehydes. Chem Biol Interact. 2009;178:145–150. doi: 10.1016/j.cbi.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Yan R, Zu X, Ma J, Liu Z, Adeyanju M, Cao D. Aldo-keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: Implication for cancer intervention. Int J Cancer. 2007;121:2301–2306. doi: 10.1002/ijc.22933. [DOI] [PubMed] [Google Scholar]
- 14.Zu X, Yan R, Robbins S, Krishack PA, Liao DF, Cao D. Reduced 293T cell susceptibility to acrolein due to aldose reductase-like-1 protein expression. Toxicol Sci. 2007;97:562–568. doi: 10.1093/toxsci/kfm033. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz FX, Gallego O, Ardevol A, Moro A, Dominguez M, Alvarez S, Alvarez R, de Lera AR, Rovira C, Fita I, Pares X, Farres J. Aldo-keto reductases from the AKR1B subfamily: retinoid specificity and control of cellular retinoic acid levels. Chem Biol Interact. 2009;178:171–177. doi: 10.1016/j.cbi.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Gallego O, Ruiz FX, Ardevol A, Dominguez M, Alvarez R, de Lera AR, Rovira C, Farres J, Fita I, Pares X. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proc Natl Acad Sci U S A. 2007;104:20764–20769. doi: 10.1073/pnas.0705659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn AM, Harvey RG, Penning TM. Oxidation of PAH trans-Dihydrodiols by Human Aldo-Keto Reductase AKR1B10. Chem Res Toxicol. 2008 doi: 10.1021/tx8002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto M, Sibata T, Wasada H, Toyokuni S, Uchida K. Structural basis of protein-bound endogenous aldehydes. Chemical and immunochemical characterizations of configurational isomers of a 4-hydroxy-2-nonenal-histidine adduct. J Biol Chem. 2003;278:5044–5051. doi: 10.1074/jbc.M210129200. [DOI] [PubMed] [Google Scholar]
- 19.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 20.Eder E, Scheckenbach S, Deininger C, Hoffman C. The possible role of alpha, beta-unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicol Lett. 1993;67:87–103. doi: 10.1016/0378-4274(93)90048-3. [DOI] [PubMed] [Google Scholar]
- 21.Choudhary S, Xiao T, Srivastava S, Zhang W, Chan LL, Vergara LA, Van Kuijk FJ, Ansari NH. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol. 2005;204:122–134. doi: 10.1016/j.taap.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Esterbauer H, Eckl P, Ortner A. Possible mutagens derived from lipids and lipid precursors. Mutat Res. 1990;238:223–233. doi: 10.1016/0165-1110(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 23.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 24.Schuler BD, Eder E. Development of a 32P-postlabelling method for the detection of 1,N2-propanodeoxyguanosine adducts of crotonaldehyde in vivo. Arch Toxicol. 2000;74:404–414. doi: 10.1007/s002040000142. [DOI] [PubMed] [Google Scholar]
- 25.Stout MD, Bodes E, Schoonhoven R, Upton PB, Travlos GS, Swenberg JA. Toxicity, DNA binding, and cell proliferation in male F344 rats following short-term gavage exposures to trans-2-hexenal. Toxicol Pathol. 2008;36:232–246. doi: 10.1177/0192623307311758. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, Ansari NH. Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem Biophys Res Commun. 1995;217:741–746. doi: 10.1006/bbrc.1995.2835. [DOI] [PubMed] [Google Scholar]
- 27.Vasiliou V, Pappa A, Petersen DR. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact. 2000;129:1–19. doi: 10.1016/s0009-2797(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 28.Coles BF, Kadlubar FF. Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors. 2003;17:115–130. doi: 10.1002/biof.5520170112. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Xu C, Sun M, Luo D, Liao DF, Cao D. Acetyl-CoA carboxylase-alpha inhibitor TOFA induces human cancer cell apoptosis. Biochem Biophys Res Commun. 2009;385:302–306. doi: 10.1016/j.bbrc.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.