Abstract
This study sought to further understand how environmental conditions influence the outcomes of early developmental insults. It compared changes in monoamine levels in frontal cortex, nucleus accumbens and striatum of male and female Long-Evans rat offspring subjected to maternal Pb exposure (0, 50 or 150 ppm in drinking water from 2 mos pre-breeding until pup weaning) +/− prenatal (PS) (restraint on GD16-17) or PS + offspring stress (OS; 3 variable stress challenges to young adults) determined at 2 mos of age and at 6 mos of age in littermates subsequently exposed either to experimental manipulations (EM: daily handling and performance on an operant fixed interval (FI) schedule of food reward), or to no experience (NEM; time alone). Time alone (NEM conditions), even in normal (control) animals, modified the trajectory of neurochemical changes between 2 and 6 mos across brain regions and monoamines. EM significantly modified the NEM trajectories, and except NE and striatal DA, which increased, blunted the changes in monoamine levels that occurred over time alone. Pb+/stress modified the trajectory of monoamine changes in both EM and NEM conditions, but these predominated under NEM conditions. Stress-associated modifications, occurring mainly with NEM OS groups, were fully reversed by EM procedures, while reversals of Pb+/-stress-associated modifications occurred primarily in nucleus accumbens, a region critical to mediation of FI response rates. These results extend the known environmental conditions that modify developmental Pb+/-stress insults, which is critical to ultimately understanding whether early insults lead to adaptive or maladaptive behavior and to devising behavioral therapeutic strategies. That time alone and a set of EM conditions typically used as outcome measures in intervention studies can themselves invoke neurochemical changes, moreover, has significant implications for experimental design of such studies.
Keywords: behavior, lead, prenatal stress, offspring stress, dopamine, serotonin, norepinephrine, enrichment
INTRODUCTION
Early life insults, such as prenatal stress and lead (Pb) exposure, have been shown to incur virtually lifelong effects in experimental studies. Prenatal restraint stress in rodents, for example, is associated with enhanced anxiety and depression-like behaviors and, under some conditions, with cognitive impairments, increased propensity to drug abuse, and alterations in function of the HPA axis and response to stress challenges in adult offspring [1–6]. Developmental Pb exposure also exerts long-term effects on cognitive functions as well as on HPA axis reactivity [1, 4, 6–11]. In addition to their common effects on brain mesocorticolimbic dopamine and glutamate systems [12–18], elevated Pb burden and stress are also co-occurring risk factors, particularly for low socioeconomic status communities [19, 20]. Correspondingly, our studies in rats have demonstrated that Pb exposure and stress can produce protracted synergistic behavioral and neurochemical effects at blood Pb levels (11 μg/dl) just above those deemed to be of concern for children by the Centers for Disease Control. For example, marked elevations of response rates on Fixed Interval schedules of reinforcement were observed at low levels of Pb exposure that were markedly further enhanced under conditions of Pb exposure combined with prenatal (PS) and offspring stress (OS), raising FI response rates to levels associated with higher Pb exposure levels. Similar Pb+ stress additive effects were noted for a broad range of neurochemical changes [8, 21, 22].
Whether such developmental insults ultimately resolve (adaptation), exhibit recovery, or lead to further dysfunction or even psychopathology is likely to reflect the course of subsequent environmental influences and events. To determine the subsequent evolution of adaptive vs. maladaptive behavior will require evaluation of the influence of various environmental conditions on the altered CNS milieu arising from such early developmental insults. Only with such an understanding can more precise and refined behavioral therapeutic strategies for these developmental insults be devised.
In that context, procedures such as handling and various other forms of what is referred to as ‘environmental enrichment’ have been reported in experimental animal studies to have an ameliorating influence on the adverse consequences of prenatal and early postnatal stressors, as well as more broadly in models of drug addiction and psychiatric and neurodegenerative diseases [23–26]. Environmental enrichment comprised of complex housing has also been reported to reverse effects of early Pb exposures in rodents [27–29].
Often in such studies, the enrichment intervention is imposed, or not, over some period of time, and is then followed by measurement of specific outcomes, with differences in that outcome generally presumed to reflect the environmental enrichment intervention. In the case of neurochemical and behavioral outcomes, however, it is also possible that: 1) time itself can result in changes in neurotransmitter levels/function, and 2) the outcome being measured after the intervention (e.g., handling, weighing, behavioral paradigm and reinforcer in the case of behavioral outcomes) can alter neurotransmitter levels/function independently of the intervention.
With respect to the first possibility, age–related changes in all aspects of dopamine systems have been reported that differ by brain region and gender [30–36]. While most such studies have compared early to late stages of the life span, more recent reports demonstrate changes in neurochemical function even between 2 and 6 mos of age in mice [34].
In relation to the second possibility, the effects of behavioral history/training on subsequent behavioral performance, pharmacological drug response and neurochemical and biochemical functions have long been recognized. For example, cocaine self-administration is influenced by behavioral history in both rhesus monkeys [37–39] and rats [40]. Prior undrugged test experience has been shown to significantly reduce or even eliminate the efficacy of benzodiazepines in the elevated plus maze anxiety paradigm [41, 42]. Thus, behavioral performance itself can directly influence neurochemical function, and differences in behavioral history are likely to be associated with different underlying neurochemical profiles.
Evaluation of the possibilities posed above also has direct relevance to the design of experiments aimed at resolving questions related to efficacious intervention strategies. The current study sought to determine whether such possibilities influenced the trajectory of neurochemical changes occurring following maternal Pb exposure alone or combined with prenatal stress (PS) or PS followed by offspring stress (OS) challenges. For this purpose, changes in monoamine levels in frontal cortex, nucleus accumbens and striatum of male and female Long-Evans rat offspring that had been subjected to maternal Pb alone +/− PS and/or OS were determined in offspring taken at 2 mos of age and in littermates at 6 mos of age that had subsequently been subjected to experimental manipulations (EM) consisting of daily handling and weighing and performance on an operant fixed interval (FI) schedule of food reward, or that had no such experimental experiences (NEM; time alone manipulation) and remained in their home cages. This permitted a determination of the trajectory of neurochemical changes between 2 and 6 mos of age in relation to time alone (NEM conditions) and alterations to time associated changes produced by assessment of the behavioral outcome of interest (EM conditions). It also examined the extent to which the trajectories of neurochemical changes under EM vs. NEM conditions were modified by stress and Pb+/-stress, and whether the specific EM conditions used here could reverse any such modifications.
MATERIALS AND METHODS
Animals and Pb Exposure
Three-week-old female Long Evans rats (Charles River, Germantown, NY) were randomly assigned to one of the following drinking solutions: 0, 50 or 150 ppm Pb acetate dissolved in distilled deionized water. More recent studies of young adult males in our laboratory show that 50 ppm exposure initiated postweaning results in blood Pb values averaging 7–12 μg/dl [13, 43], just at and above the CDC’s currently designated level of concern for children. These PbBs are also associated with similar behavioral deficits in rodents and children [44–46]. The 150 ppm exposure was associated with mean PbBs in dams ranging from 32.6 ± 4.4 to 42.7 ± 4.0 μg/dl and was used because our previous studies had demonstrated synergistic effects of this Pb exposure level with stress [1, 8].
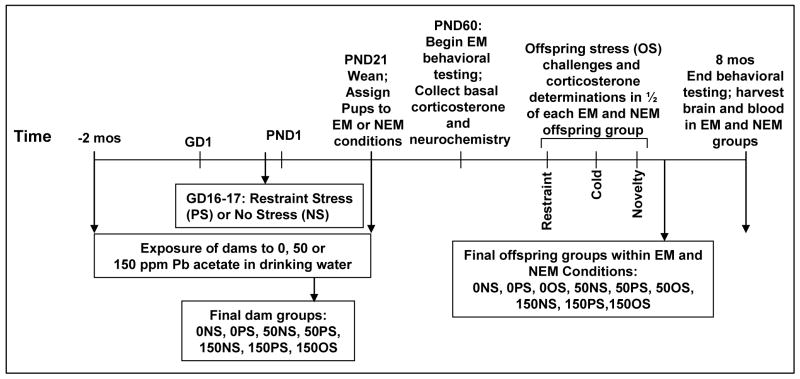
As shown in Figure 1, Pb exposure of dams was initiated two months prior to breeding and was continued throughout lactation to ensure elevated bone Pb levels and Pb body burden [47], consistent with human exposure. When females reached 3 mos of age, they were paired with 3 mos old male Long-Evans rats for breeding. Animals were housed in a vivarium room maintained at 22 ± 5 °C with a 12h light-dark cycle (lights on at 0700h). Standard rat chow diet was provided ad libitum. All experiments were carried out according to NIH Guidelines and were approved by the University of Rochester’s University Committee on Animal Resources.
Figure 1.
Schematic of experimental design.
Breeding and Prenatal Stress
At pro-estrus, as determined by vaginal smears, female rats were mated with males (2:1) across two estrous cycles. The presence of vaginal plugs or sperm in vaginal smears collected in the early morning indicative of pregnancy was considered gestational day 1 (GD1). Pregnant females in each Pb-treated group were weighed and further randomly subdivided to a non-stress (NS) or prenatal stress (PS) sub-groups and individually housed for the remainder of pregnancy and lactation.
On gestational days 16 and 17, dams assigned to PS groups were weighed and subjected to three 45 min restraint sessions (0900, 1200 and 1500h) in plastic cylindrical devices using procedures modified from Ward and Weisz [48] (Figure 1). This procedure, or an even more protracted restraint stress paradigm, has been widely employed [49–53]. NS dams were weighed and subsequently left undisturbed in their home cages. This protocol, as used in our previous study of Pb and stress, elevated corticosterone levels and altered catecholamine levels in frontal cortex and nucleus accumbens of dams [1]. At the end of the last restraint session on GD16, blood was collected for corticosterone determinations. GD16 rather than GD17 was chosen for corticosterone determination to prevent potential habituation effects from obscuring any treatment-related differences. This resulted in six Pb-stress conditions with the following numbers of dams: 0NS (n=20), 0PS (n=23), 50NS (n=21), 50PS (n=31), 150NS (n=19), and 150PS (n=33) as shown in Figure 1. Differences in sample sizes reflect both differences in pregnancy rates and initial assignment of greater number of dams to Pb+PS groups.
Offspring Procedures
At delivery (postnatal day 1: PND1), litter size was recorded and number of pups culled to 8 per litter, maintaining equal numbers of males and females wherever possible. Cross-fostering was not performed, as the intent of the study was to model the human environment and culture. Pups were weaned at PND21, when Pb exposure effectively ended, separated by gender, and housed in same litter/gender pairs for the duration of the experiment.
From weaning, pups were provided with unrestricted access to tap water (0 ppm) and food (Laboratory Rodent Diet 5001, POMI Foods Inc.) until approximately 2 mos of age. At this point, brains from a subset of male and female offspring from each of the 6 Pb-stress treatment groups defined above were sacrificed to provide basal determinations of corticosterone and levels of monoamines in frontal cortex, nucleus accumbens and striatum.
Remaining pups from each of the 6 Pb-stress groups per gender were then allocated to either an experimental manipulation (EM) condition or a non experimental manipulation (NEM) condition. In addition, a subset of offspring from each PS group (0PS, 50PS and 150PS) was allocated to receive offspring stress (OS) treatment as adults that consisted of a series of three additional stressor exposures. Thus final Pb-stress conditions included 9 groups for each gender in each of the EM and NEM conditions: 0NS, 0PS, 0OS, 50NS, 50PS, 50OS, 150NS, 150PS and 150OS (Figure 1).
EM Conditions
Rats in EM groups were provided with sufficient food to allow a 2–3 gm weight gain per day until male pups reached approximately 300 g and females 220 g. At this point, caloric intake was restricted to maintain the above-stated body weights as required for behavioral evaluation for the duration of the experiment. Because animals were pair-housed, a cage divider was placed in the cage at the time of feeding each day to separate residents and remained there for approximately 90 min.
At approximately 2–3 mos of age, EM groups began behavioral testing which involved daily weighing and handling, transport to and from the room housing the behavioral test chambers, and testing on a fixed interval (FI) 1 min schedule of food reinforcement. The FI schedule of reinforcement was used because of its documented sensitivity to Pb exposure across species and developmental periods of exposure [54], and which would therefore facilitate interpretation of Pb-stress interactions. This schedule provided a 45 mg food pellet (Bioserv, Frenchtown, NJ) for the first occurrence of a designated response after a 1 min interval had elapsed over the course of 20 min behavioral test sessions that were conducted 5 days a week (M-F) between 1000 and 1500h as described in detail previously [1, 8].
Offspring stress challenges for EM OS groups were imposed immediately prior to an FI session to measure impact on subsequent FI performance. A single 45 min restraint stress was imposed prior to session 13 in both males and females, using the same procedure described above for the dams. Prior to session 21 for females, and session 20 for males, cold stress, considered a mild physical stress, was imposed. Animals were placed in cages similar to home cages (without the bedding) at 4° C in a temperature-controlled room for 30 min prior to being placed in the operant chambers. Prior to session 30 for females and session 31 for males, animals underwent a 15 min test of motor activity in locomotor activity cages as a novel environment stressor. The period of stress challenge in FI animals covered the 14–18 weeks of age range. Blood from tail nicks was collected at the end of the FI session following the stress challenge for the determination of corticosterone levels in FI tested offspring.
NEM Conditions
NEM groups remained in home cages throughout the period of time occupied by EM behavioral testing, were weight restricted (although not as precisely as EM groups because of the number of animals involved in the experiment) at the same time as EM groups, and minimally handled (early weighing and culling of litters). NEM offspring allocated to the OS groups, however, were subjected to the same schedule of stressors and post-session determination of corticosterone levels as EM littermates. Given the numbers of animals involved in the study, stress challenges were carried out according to the same schedule in EM and NEM rats, but began approximately 4 weeks later in NEM offspring and covered the 18–22 weeks of age range. Corticosterone determinations were carried out at the same time points post stress challenge in NEM rats as in EM rats. NEM rats remained in transport cages between the stress challenge and corticosterone determination and were then returned to home cages.
Multiple variable stressors rather than a single homotypical stress challenge were used for OS conditions to more closely mimic the human experience across the life span and to preclude any habituation effects. Stress challenges were carried out during the acquisition phase of FI training so that the ability to elicit group differences was maximized. Stress challenges were imposed in the order of restraint, followed by cold, followed by novelty. The use of the most intense stressor, restraint, before other stress challenges was based on its potential to increase sensitivity of the animals to subsequent stressors [55], thereby potentially maximizing the ability to detect treatment-related differences.
In all cases, only a single male and female from each litter were included in each Pb-stress EM and NEM group to preclude litter-specific effects.
Within 24–48 hr after FI behavioral testing was completed, brain regions were rapidly harvested following decapitation from all EM and NEM groups for the determination of brain monoamine levels, and blood collected for corticosterone determinations. Results of the FI performance, neurochemical changes following behavioral testing, as well as the corticosterone and behavioral responses to stress challenges are described elsewhere [4, 8, 56].
Blood Pb Analysis
PbB levels were measured by anodic stripping voltammetry according to methods described previously [21, 47] in dams (n=7–8 for each group) and pups (n=3–9) at the time of offspring weaning (PND21) from each treatment group.
Neurochemical Determinations of Monoamines
Levels of (DA (dopamine), DOPAC (dihydroxyphenylacetic acid), HVA (homovanillic acid), NE (norepinephrine), 5-HT (serotonin) and 5-HIAA (5 hydroxyindoleacetic acid) from frontal cortex, nucleus accumbens and striatum were analyzed using HPLC as described in detail previously [8]. Concentrations of neurotransmitters were expressed in terms of ng/mg protein. DA turnover was calculated as the DOPAC/DA ratio.
Statistical Analysis
Blood Pb Analyses
Blood Pb levels were analyzed using ANOVAs that included group (dams, male offspring, female offspring) and stress as between group factors. In the event of main effects or interactions, lower order ANOVAs and post-hoc tests as appropriate were carried out.
Neurotransmitter Level Analysis
At the 6 mos time point, values for frontal cortex DOPAC and HVA of NEM females were generally below detection limits, and brains from 50 ppm NEM females were lost due to a refrigeration problem. Thus data shown for NEM female frontal cortex from 6 mos time points include only DA and NE, and for all brain regions, NEM female data include only 0 and 150 ppm data. To depict the trajectory of change in neurotransmitter levels from the 2 mos time point, all data from the 6 mos time point were calculated as a percent change from corresponding gender group mean 2 mos 0NS values.
The specific purpose of the statistical analyses carried out and the factors analyzed are summarized in Table 1. For the evaluation of basal neurotransmitter levels at 2 mos of age, and the determination of the effects of time alone and of stress on the trajectory of neurotransmitter changes between 2 and 6 mos, overall ANOVAs including gender were first carried out. These were followed by lower order ANOVAs and post-hoc tests as appropriate based on main effects and interactions. For the determination of the modification by Pb or Pb+stress on the trajectory of neurochemical changes between 2 and 6 mos, overall ANOVAs that included gender were not carried out for three reasons: 1) missing data from the female 50 ppm NEM groups would have meant elimination of all 50 ppm data from any analysis representing a significant omission; 2) pronounced gender differences in neurotransmitter levels were already found at 2 mos of age, and 3) in analysis of the effects of time and stress (based on 0 ppm data from 6 mos of age), gender differences were confirmed for 2 of the 4 neurotransmitters in frontal cortex, all 6 comparisons in nucleus accumbens and all 5 comparisons in striatum; the latter two are already indicative of broad gender differences. Consequently, these analyses were carried out separately for males and females. In all analyses, a p value of ≤0.05 was considered statistically significant. Because of the number of analyses, only p values for specific effects from the ANOVAS are reported for comparisons described.
Table 1.
Summary of Methods of Statistical Analyses by ANOVA1
| Purpose of Assessment and Comparisons | Factors and Analyses |
|---|---|
| Comparison of basal neurotransmitter levels in relation to Pb exposure (0, 50 or 150 pm) and prenatal stress condition (NS or PS) |
|
| Trajectory of Neurochemical Changes Between 2 and 6 months with Time Alone (NS) or following prenatal stress (PS) in normal non-Pb treated (0 ppm) conditions in relation to experimental manipulation condition (EM or NEM). |
|
| Modification by Pb or Pb+Stress of the Trajectory of Neurochemical Changes between 2 and 6 mos in relation to experimental manipulation condition (EM vs NEM) |
|
Abbreviations: Pb = lead; NS=no stress; PS= prenatal stress; OS = maternal and offspring stress; EM= experimental manipulation; NEM=no experimental manipulation
Effects of Pb and Stress on Basal Neurotransmitter Levels
Analyses were carried out to determine the influence of Pb and of stress on neurotransmitter levels prior to the implementation of any EM conditions or OS challenges from brains collected at the 2 mos of age time point. For these comparisons, neurotransmitter levels from 2 mos old NS and PS groups were analyzed using ANOVAs that included Pb, gender and stress for each neurotransmitter in each brain region. Given main effects or interactions involving gender, subsequent analyses were carried out with Pb and stress as factors separately for males and females. At the 2 mos time point, levels of HVA in frontal cortex of females were generally below detection limits.
Changes in the Trajectory of Neurochemical Changes Between Two and Six Months in Relation to Time Alone vs. Prenatal Stress
To attempt to both simplify and clarify results, data from all normal non-Pb treated groups (i.e., all 0 ppm groups; Figures 3–5) at the 6 mos time point were analyzed by overall ANOVAs including gender (except frontal cortex DOPAC and DATO), condition (EM vs NEM) and stress (NS, PS, OS) for each neurotransmitter in each region. This allowed a determination as to how neurotransmitter levels had changed under EM vs. NEM conditions in relation to time alone as well as any changes produced specifically by PS. Analyses that revealed main effects or interactions involving gender were followed by subsequent ANOVAs and post-hoc tests to further elaborate the nature of the effects.
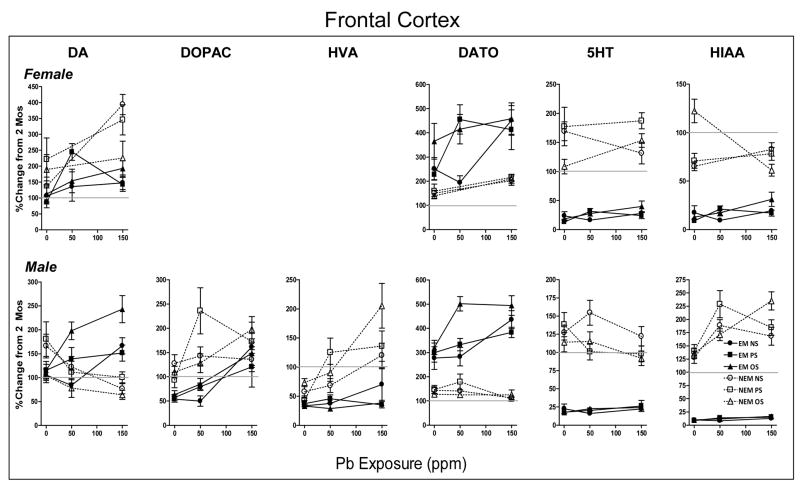
Figure 3.
Group mean ± S.E. levels of DA, DOPAC, DA TO, NE, 5HT and 5HIAA in frontal cortex of females (top row) and males (bottom row) at 6 mos of age in relation to Pb exposure concentration (ppm) for NS, PS and OS groups in EM and NEM conditions, as indicated. Data are plotted as percent change from corresponding gender group mean 0NS values of littermates determined at 2 mos of age (Figure 1). Sample sizes are as described in Figure 2 legend. Outcomes from overall ANOVAs: EM= experimental manipulation, Pb=Pb exposure, S=stress. Horizontal line at 100% shown to facilitate visualization of direction and magnitude of change.
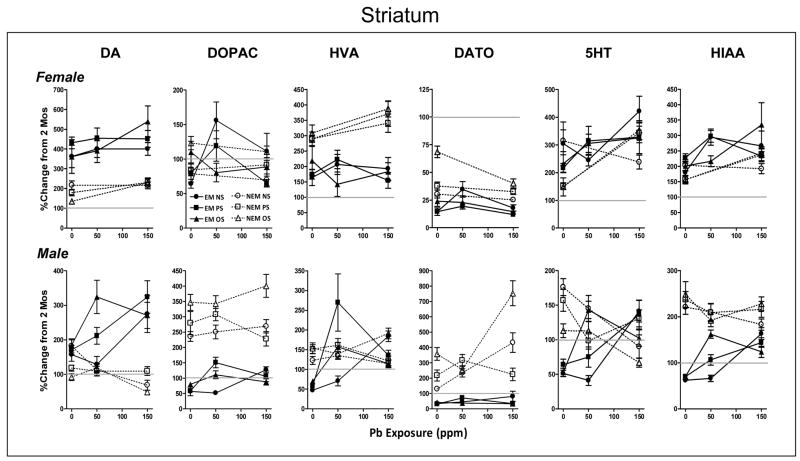
Figure 5.
Group mean ± S.E. levels of DA, DOPAC, DA TO, NE, 5HT and 5HIAA in striatum of females (top row) and males (bottom row) at 6 mos of age in relation to Pb exposure concentration (ppm) for NS, PS and OS groups in EM and NEM conditions, as indicated. Data are plotted as percent change from corresponding gender group mean 0NS values of littermates determined at 2 mos of age (Figure 1). Sample sizes are as described in Figure 4 legend. Outcomes from ANOVAs: EM= experimental manipulation, Pb=Pb exposure, S=stress. Horizontal line at 100% shown to facilitate visualization of direction and magnitude of change.
Modification by Pb+/-Stress of the Trajectory of Neurochemical Changes Between Two and Six Months
To examine whether Pb exposure or Pb+stress altered the trajectory of neurochemical changes between the two and 6 mos time points in EM vs. NEM conditions, ANOVAs were carried out that included Pb exposure (0, 50 and 150 ppm), stress (NS, PS and OS) and condition (EM and NEM) for all 6 mos time point data for each neurotransmitter in each region. For females, this was restricted to 0 and 150 ppm data based on missing 50 ppm NEM values. In the event of main effects or interactions, lower order ANOVAs and post-hoc tests were carried out as appropriate to address specific questions.
RESULTS
Blood Pb Levels
Group mean PbBs (μg/dl) of dams (n=7–9/group) and pups (n=3–9/gender/group) determined at pup weaning (21 days of age) increased with increasing Pb exposure concentration (Table 2) as previously reported [4,8]. Overall ANOVA revealed significant main effects of Pb and group (both p values <0.0001) as well as a group × Pb interaction (p<0.0001), but no main effects or interactions that included stress. The group × Pb interaction reflected the approximately 7 μg/dl higher PbBs of male and female offspring relative to dams at 50 ppm. At 150 ppm, PbBs of female offspring were higher than those of male offspring, an effect largely due to the slightly lower PbBs of the 150PS males relative to other groups. Our prior studies have demonstrated that following maternal Pb exposure, pup PbBs are below detection limits by 40 days of age [57].
Table 2.
Blood Pb Values at Weaning in Dams and Male and Female Offspring
| Group | ||||||
|---|---|---|---|---|---|---|
| Pb Exposure (ppm) | Dam NS | Dam PS | Male NS | Male PS | Female NS | Female PS |
| 0 | .89 ± 0.081 | 0.79 ± 0.10 | 1.78 ± 0.6 | 1.58 ± 0.35 | 1.2 ± 0.45 | 1.43 ± 0.3 |
| 50 | 11.6 ± 0.51 | 11.8 ± 0.5 | 19.6 ± 6.0 | 18.8 ± 0.85 | 19.3 ± 4.8 | 19 ± 2.0 |
| 150 | 30.8 ± 1.34 | 31.2 ± 0.78 | 32.00 ± 3.0 | 25.4 ± 2.5 | 35.5 ± 2.7 | 32.6 ± 1.2 |
Mean ± SE values in μg/dl; NS=no stress; PS=prenatal stress
Neurochemical Profiles at Two Months of Age
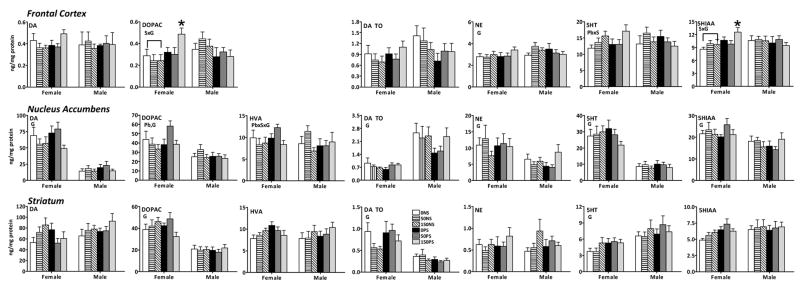
Monoamine levels in frontal cortex, nucleus accumbens and striatum at 2 mos of age exhibited multiple gender-based differences, but only a few occurrences of Pb+/-stress effects (Figure 2).
Figure 2.
Group mean ± S.E. levels of neurotransmitters (ug/mg protein) in frontal cortex, nucleus accumbens and striatum of 0NS, 50NS, 150NS, 0PS, 50PS and 150PS female and male offspring determined at 2 mos of age. Sample sizes were 4–10 for frontal cortex, 7–12 for nucleus accumbens and 8–10 for striatum. Bars over columns represent stress-related differences. * signifies greater than all other same gender groups. Outcomes of overall ANOVA: S=stress, G=gender, Pb=lead.
Frontal Cortex
Significant gender (G) × stress (S) differences in females were found for DOPAC and 5HIAA (169 and 146%; F(1,75)=7.68, p=0.007 and F(1,104)=3.94, p=0.0498, respectively) that reflected, in both cases, elevated levels in 150PS females; no differential changes occurred among male groups. Males exhibited slight but significantly higher levels of NE than females (F(1,103)=4.09, p=0.046). A significant overall Pb × gender interaction for 5HT (F(2,103)=3.105, p=0.049) reflected the higher levels across 150 ppm female NS, PS and OS groups than in either 0 ppm or 50 ppm groups, whereas no differences were found among male groups.
Nucleus Accumbens
Significant gender differences were found in nucleus accumbens for DA, DOPAC, NE, 5HT and 5HIAA (DA: F(1,103)=109.2, p<0.0001; DOPAC: F(1,102)=27.15, p<0.0001; NE: F(1,102)=13.67, p=0.0004; 5HT: F(1,102)=109.42, p<0.0001; 5HIAA: F(1,102)=15.19, p=0.0002), where levels in females were significantly greater than in males, and for DA TO (F(1,103)=51.37, p<0.0001), where DA turnover rates for females were notably lower than those of males.
Striatum
DOPAC and DA TO values in females were approximately twice those of males (DOPAC: F(1,104)=91.78, p<0.0001; DA TO: F(1,104)=43.42, p<0.0001), while 5HT levels of males exceeded those of females (F(1,106)=18.68, p<0.0001).
Neurochemical Profiles at Six Months of Age
Time Alone and Stress-Induced Changes
Effects of time alone (EM vs. NEM) and of stress conditions (0NS, 0PS and 0OS groups) on the trajectory of neurotransmitter changes are derived from 0 ppm group values and depicted as percent change from corresponding 2 mos group mean gender-based 0NS values in Figures 3–5 for frontal cortex, nucleus accumbens and striatum, respectively. Results are summarized in Table 3.
Table 3.
Changes in Levels of Neurotransmitters in 0 ppm EM Conditions Compared to 0 ppm NEM Conditions in Frontal Cortex, Nucleus Accumbens and Striatum and Associated Stress-Induced Modifications
| NEUROTRANSMITTER | |||||||
|---|---|---|---|---|---|---|---|
| REGION | DA | DOPAC | HVA | DA TO | NE | 5HT | 5HIAA |
| Frontal Cortex | |||||||
| Female1 | ↓ 58% | — | — | — | ↑ 57% | ↓ 45% | ↓ 54% |
| Interactions2 | NEM:>OS | ||||||
| Male1 | ↓ 45% | ↓ 77% | — | ↓ 27% | ↑ 138% | ↓ 100% | ↓ 119% |
| Interactions2 | |||||||
| Nucleus Accumbens | |||||||
| Female | NC | ↓ 29% | ↓ 39% | ↓ 12% | ↑ 22% | ↓ 65% | ↓ 53% |
| Interactions | NEM:>O | NEM:>OS | |||||
| Male | NC | ↓ 121% | ↓ 116% | ↓ 40% | NC | ↓ 77% | ↓ 56% |
| Interactions | NEM:>OS | ||||||
| Striatum | |||||||
| Female | ↑ 207% | NC | 129% | ↓ 15% | — | NC | ↑ EMxS |
| Interactions | NEM:<PS,OS | ||||||
| Male | ↑ EMxS | ↓ 179% | ↓ 76% | ↓ 4% | — | ↓ 125% | ↓ 167% |
| Interactions | ↓ NEM:PS, OS | NEM:>OS>PS | NEM:<OS | ||||
Symbols and abbreviations: ↓=decrease; ↑ = increase; DA=dopamine; DOPAC=dihydroxyphenylacetic acid; HVA=homovanillic acid; DA TO=dopamine turnover; NE=norepinephrine; 5HT=serotonin; 5HIAA=5hydroxyindole acetic acid; NC=no change; gray shading=comparable effects in males and females. Lower order ANOVA outcomes: EM=experimental manipulation; S=stress.
Changes in 0 ppm NS EM compared to 0 ppm NS NEM
Changes reflecting EMxS interactions
Frontal Cortex
Gender differences in overall statistical analyses were found for DA (EMxG: F(1,107)=4.81, p=0.03) and HIAA (EMxGxS, F(2,116)=3.59, p=0.031) and thus ANOVAs were subsequently carried out separately by gender for these neurotransmitters. Differential trajectories for EM vs. NEM conditions were found for every neurotransmitter measured in frontal cortex (EM: DA: F(1,107)=10.51, p=0.0016; NE: F(1,112)=68.2, p<0.0001; 5HT: F(1,116)=201.3, p<0.0001; 5HIAA: F(1,116)=499.6, p<0.0001), with an overall highly similar profile in both genders (Figure 3; Table 3).
NEM-associated increases in DA and DOPAC (measured in males only) of 104–209% at 6 mos were reduced by EM procedures to 54–115% or approximately back to, or slightly below, 2 mos time point levels (EM: F(1,58)=5.44, p=0.023, F(1,57)=4.1, p=0.048 and F(1,55)=26.56, p<0.0001 for female DA, male DA and male DOPAC, respectively). Similarly, the general increases in 5HT and 5HIAA (EM: F(1,58)=309.1, p<0.0001 and F(1,60)=163.9, p<0.0001 for males and females, respectively) of 65–177% associated with NEM conditions were markedly attenuated by EM procedures to values of 9–24% of 2 mos levels. DA TO values of males were reduced under NEM conditions, but further reduced by EM conditions (47–74 to 33–37%; F(1,56)=19.8, p=0.0001). In contrast, the modest increases in NE under NEM conditions (118–150%) in both males and females were actually significantly further enhanced to 207–365% by EM conditions.
The only stress-induced modification observed was for 5HIAA levels in females, where OS, under NEM conditions, actually increased 5HIAA levels above 2 mos time point values, while values were reduced for NS and PS NEM groups; the absence of corresponding differences under EM conditions indicated that all NEM effects had been reversed (post hoc EMxS: p=0.0001).
Nucleus Accumbens
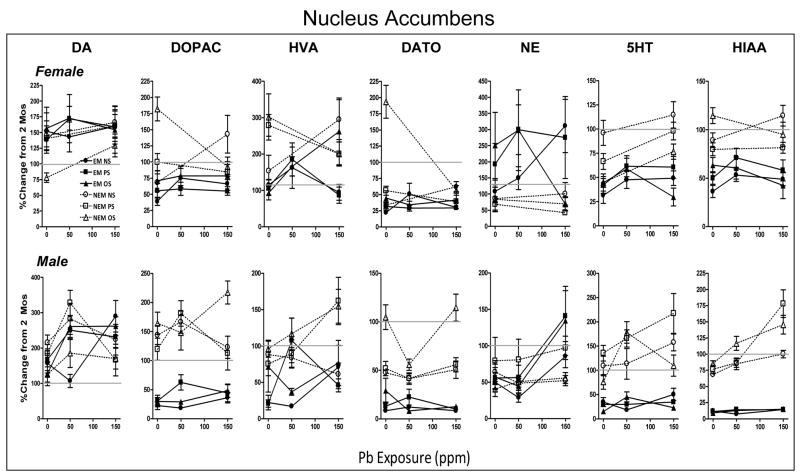
Gender-related differences were found for every neurotransmitter in the overall analyses (DA: G, F(1,115)=4.08, p=0.0475; DOPAC: GxS, F(1,115)=10.42, p=0.0016; NE: G, F(1,114)=8.03, p=0.0054; 5HT: GxEM, F(1,113)=14.45, p=0.0002; 5HIAA: G, F(1,115)=68.47, p<0.0001), consequently, separate ANOVAs were carried out for each gender and neurotransmitter to further clarify the nature of the effects. Differential trajectories of neurochemical changes under EM vs. NEM conditions at 0 ppm were observed in nucleus accumbens for all neurotransmitters except DA for both genders, and HVA and NE in males (Figure 4; FEMALES: DOPAC: F(1,59)=27.39, p<0.0001; HVA: F(1,58)=9.86, p=0.0027; DATO: F(1,59)=33.24, p<0.0001; NE: F(1,54)=4.01, p=0.049; 5HT: F(1,54)=16.71, p=0.001; 5HIAA: F(1,59)=54.75, p<0.0001; MALES: DOPAC: F(1,58)=96.48, p<0.0001; DATO: F(1,56)=62.03, p<0.0001; 5HT: F(1,57)=49.94, p<0.0001; 5HIAA: F(1,57)=420.46, p<0.0001).
Figure 4.
Group mean ± S.E. levels of DA, DOPAC, HVA, DA TO, NE, 5HT and 5HIAA in nucleus of females (top row) and males (bottom row) at 6 mos of age in relation to Pb exposure concentration (ppm) for NS, PS and OS groups in EM and NEM conditions, as indicated. Data are plotted as percent change from corresponding gender group mean 0NS values of littermates determined at 2 mos of age (Figure 1). Sample sizes are as described in Figure 3 legend. Outcomes from ANOVAs: EM= experimental manipulation, Pb=Pb exposure, S=stress. Horizontal line at 100% shown to facilitate visualization of direction and magnitude of change.
DA levels were comparably increased by EM and NEM conditions in both males and females (78–215%). The general increases relative to 2 mos levels in DOPAC in males (119–164%), and of HVA in females (154–301%) under NEM conditions were significantly attenuated by EM procedures (down to 22–30% for DOPAC: 93–115% for HVA). Under NEM conditions, levels of 5HT and 5HIAA did not change systematically relative to 2 mos values in either gender, but all were reduced by EM conditions (5HT: 15–45%; HIAA: 9–63%). In contrast, NE levels of females were reduced to 62–85% under NEM conditions, but increased by EM procedures (108–250%).
Stress-related modifications were found for DOPAC in females (EMxS: F(2,59)=5.08; p=0.009) and DA TO for both genders (EMxS: F(2,59)=16.63, p<0.0001 and F(2,58)=3.46, p=0.038 for females and males, respectively) under NEM conditions. OS significantly increased DOPAC levels in NEM females (+114%) and DA TO in both genders (+163% and +58% for females and males, respectively) above 2 mos time point values, while values in the corresponding 0NS and 0PS groups were generally reduced below 2 mos values (all post hoc p values<0.0001). Corresponding stress-related differences were not observed under EM conditions, where levels of DOPAC (39–71%) and DA TO (8–56%) were similarly attenuated across stress conditions, indicating the reversal of these effects by EM procedures.
Striatum
Gender-related differences were found for every neurotransmitter in the overall analyses, consequently, separate ANOVAs were carried out for each gender and neurotransmitter to further clarify the nature of the effects (DA: F(1,117)=69.02, p<0.0001; DOPAC: F(1,117)=77.72, p<0.0001; HVA: F(1,114)=188.06, p<0.0001; DA TO:F(1,119)=53.05, p<0.0001; 5HT:F(1,119)=52.62, p<0.0001; 5HIAA:F(1,119)=10.99, p<0.012). EM vs. NEM conditions differentially affected the trajectory of all neurotransmitters at 0 ppm in striatum except DOPAC, 5HT and 5HIAA in females (Figure 5; FEMALE: DA: F(1,60)=38.69, p<0.0001; HVA: F(1,60)=9.36, p=0.033; DA TO F(1,59)=53.76, p<0.0001; MALE: DA: F(1,60)=6.71, p=0.012; DOPAC: F(1,60)=172.44, p<0.0001; HVA: F(1,58)=120.08, p<0.0001; DA TO: F(1,59)=112.9, p<0.0001; 5HT: F(1,60)=114.9, p<0.0001; 5HIAA: F(1,60)=183.5, p<0.0001).
EM procedures further elevated the increases in DA in NEM conditions in both females and males from 92–217% to 157–430%. NEM conditions increased DOPAC levels in males (236–347%) and HVA levels of both genders (123–308%), all of which were reduced by EM procedures (47–218%). Relative to 2 mos values, DA TO declined under NEM conditions in females (30–69%), but increased in males (129–358%); both were reduced by EM procedures (15–42%. Levels of 5HT were comparably increased by EM and NEM conditions in females (148–315%), whereas NEM-induced increases in 5HT in males (113–176%) that were reduced by EM (51–64%). The increase in 5HIAA levels under NEM conditions in males (221–248%) was markedly attenuated by EM procedures (62–70%).
Stress-related modifications of these trajectories were observed for DA (EMxS: F(2,60)=8.71, p=0.0005), DA TO (EMxS: F(2,59)=11.65, p<0.0001), and 5HT (EMxS: F(2,60)=4.67, p= 0.013) in males. Specifically, DA levels were significantly lower in the NEM PS and OS groups than in EM counterparts, whereas no significant differences were found with EM vs. NEM NS groups. NEM conditions significantly increased DATO levels in PS and OS groups, but not in the NS group, resulting in a stress-related change OS>PS>NS. 5HT levels were increased in a reverse manner by NEM relative to EM values, with increases of 345%, 245% and 192% for the NS, PS and OS groups, respectively. In all 3 cases, stress-related differences were not found in the corresponding EM conditions, indicating reversal of these effects. For females, increases in 5HIAA in the 0NS NEM group were suppressed by both PS and OS (EMxS: F(2,58)=3.96, p=0.024; reduction to 46%;), but these effects were not found under EM conditions, consistent with their reversal.
Table 3 summarizes changes produced by time alone (0NS EM vs. 0NS NEM) and any stress-related interactions (EMxS) across brain regions for each gender. As it shows, EM generally attenuated levels of DA and metabolites and of 5HT and its metabolite 5HIAA across brain regions with similar effects in both genders, with a few exceptions (DA in striatum; striatal 5HIAA in females). Although less data was available, measurements of NE levels, in contrast, increased under the influence of EM procedures. Stress-related modifications of these effects were primarily related to OS, and observed only under NEM conditions, i.e., were reversed by EM procedures. Further, stress-induced modifications exhibited no systematic pattern, with the exception of the corresponding enhancements of DA TO in nucleus accumbens of both genders.
Pb and Pb-Stress-Induced Changes
Pb concentration effect curves for all NS, PS and OS EM and NEM groups are depicted as a percent change from corresponding gender group mean 2 mos 0NS values for frontal cortex, nucleus accumbens and striatum, respectively, in Figures 3–5; corresponding results are summarized in Table 4. For all female groups, analyses are based only on 0 and 150 ppm data due to lost samples at 50 ppm.
Table 4.
Pb and Pb-Stress Modifications of the Trajectory of Neurotransmitter Changes between 2 and 6 mos
| NEUROTRANSMITTER | |||||||
|---|---|---|---|---|---|---|---|
| DA | DOPAC | HVA | DA TO | NE | 5HT | 5HIAA | |
| FEMALE | |||||||
| EM-FC | ↑ | ↑ | — | ↑ | |||
| -NAC | — | — | PbxS | — | — | ↑ | — |
| -STR | ↑ | ↑ | PbxS | ↑+ | ↑ | ↑ | |
| NEM-FC | ↑ | ↑ | — | — | |||
| -NAC | — | PbxS | PbxS | PbxS | — | ↑ | PbxS |
| -STR | ↑ | — | ↑ | ↓ | ↑ | ↑ | |
| MALE | |||||||
| EM-FC | ↑+ | ↑* | — | ↑+ | — | ↑ | |
| -NAC | ↑ | — | — | — | — | — | — |
| -STR | ↑+ | ↑ | ↑* | — | ↑* | ↑* | |
| NEM-FC | ↓ | ↑ | ↑ | ↓ | — | — | |
| -NAC | ↑ | PbxS | PbxS | PbxS | — | ↑ | ↑ |
| -STR | ↓ | PbxS | — | ↑ | ↓ | ↓ | |
Symbols and abbreviations: ↓=decrease; ↑=increase; Lower order ANOVA outcomes: PbxS=Pb × stress interaction; FC=frontal cortex; NAC=nucleus accumbens; STR=striatum; DA=dopamine; DOPAC= dihydroxyphenylacetic acid; HVA=homovanillic acid; DA TO=dopamine turnover; NE=norepinephrine; 5HT = serotonin; 5HIAA = 5 hydroxyindole acetic acid; NC = no change. EM= experimental manipulation; NEM=no experimental manipulation; black shading = not measured; gray shading: reversal of NEM Pb effect by EM conditions;
=effect of Pb over-rides attenuating influence of EM;
=opposite effect of Pb in EM vs. NEM.
Frontal Cortex
Pb exposure influenced the trajectories of all neurotransmitters in frontal cortex except 5HT in both genders. However, the profiles of effects differed in males vs. females. In females, significant Pb-associated increases in DA (Pb: F(1,110)=11.63, p=0.0009) and NE levels (Pb: F(1,112)=18.73, p<0.0001) from 87–221 at 0 ppm to 143–405% at 150 ppm, and, correspondingly, from 139–365 to 202–459%, for DA and NE, respectively, were of comparable magnitude in EM and NEM conditions. Elevated levels of 5HIAA in the 0 ppm OS NEM group were not seen in the 150 ppm OS NEM group (PbxEMxS: F(2,113)=14.39, p<0.0001), whereas slight, but significant, Pb-related increases occurred in the EM condition (9–13 to 17–31%; post hoc p=0.022;).
In males, Pb exposure decreased DA (PbxEM: F(2,172)=15.94, p<0,0001; 104–181 to 84–101%) and NE (PbxEM: F(2,178)=11.95, p<0.0001; 128–147 to 107–125%) under NEM conditions, but increased levels (106–115 to 151–243% and from 277–320 to 383–495%, respectively) under EM conditions, with EM elevations even further enhanced by OS at 50 ppm for DA (+93%) and NE (+181%), and at 150 ppm for DA (+128%). Pb increased DOPAC levels (PbxEM: F(2,182)=3.49, p=0.032) in both EM (53–63 to 121–162%) and NEM (93–127 to 135–197%) conditions, with a more pronounced effect in the 50PS NEM group (+143%) relative to the 0PS NEM group (post hoc PbxS: p=0.009). Notably, while DOPAC levels were lower at 0 ppm under EM than NEM conditions, the increases at 150 ppm in EM groups reached levels comparable to those of NEM groups; thus, Pb exposure over-rode the attenuating influence of EM. Pb-induced increases in DA TO (PbxEM: F(2,175)=8.62, p=0.0003; 47–73 to 120–205%) and 5HIAA (PbxEM: F(2,185)=11.34, p<0.0001; 128–140 to 168–235%) under NEM conditions were generally reversed by EM procedures.
Nucleus Accumbens
Pb influences were found for all neurotransmitters in nucleus accumbens with the exception of DA and 5HT in females and NE in both genders. Effects in females were variable and generally not remarkable. They included variable but significant Pb-related increases in 5HT (Pb: F(1,111)=10.09, p=0.0019), as well as markedly elevated DOPAC (PbxSxEM: F(2,114)=5.32, p=0.0062) and DA TO (PbxSxEM: F(2,114)=14.08, p<0.0001) levels at 0 ppm in the OS NEM group but no corresponding stress interaction at 150 ppm. PS resulted in modest but significantly lower 5HIAA levels in female NEM groups (PbxS: F(2,115)=5.54, p=0.005). Pb+stress differentially affected HVA levels in females (PbxSxEM: F(2,110)=6.02, p=0.003), due to increases in the 150 ppm OS EM group (post hoc PbxS: p=0.034), whereas the increases in NEM PS and OS groups at 0 ppm were not seen at 150 ppm (post hoc PbxS: p=0.042).
In males, Pb exposure increased DA levels (PbxEM: F(2,185)=5.24, p=0.006) in both EM (127–211 to 231–290%) and NEM (125–215 to 166–224%) conditions. In the EM condition, more pronounced increases with PS (+143%) and OS (+154%) were found than in NS at 50 ppm (PbxS: F(4,185)=3.08, p=0.0047). DOPAC levels (EM: F(1,185)=177.65, p<0.0001) were increased under NEM conditions only for 150 ppm combined with OS (+64%; PbxS: F(4,185)=3.23, p=0.0137), a potentiated effect reversed by EM procedures. Similarly, potentiated Pb-induced increases in HVA (PbxSxEM: F(4,157)=4.06, p=0.0037) under NEM conditions were found at 150 ppm in PS (+66%) and OS (+59%) groups (post hoc PbxS: p=0.032), a Pb+stress additive effect reversed by EM procedures. Under NEM conditions, DA TO (PbxS: F(4,183)=4.29, p=0.0024; PbxEM: F(2,183)=6.77, p=0.0015) was markedly elevated by OS at 0 and 150 ppm, but not at 50 ppm (post hoc PbxS: p=0.010); these stress modifications were also eliminated by EM procedures. Pb exposure increased 5HT comparably in EM and NEM conditions (Pb: F(2,184)=4.04, p=0.019; 77–109 to 114–210%). Pb-associated increases in 5HIAA levels were seen only in NEM conditions (PbxEM: F(2,184)=18.66, p<0.0001; 69–85 to 85–191%). Potentiated increases in 5HIAA were produced in the NEM condition (post hoc PbxS: p=0.0005) by OS at 50 ppm (+32%) and at 150 ppm by PS (+93%) and OS (+60%), effects reversed by EM in both cases.
Striatum
Pb exposure influenced the trajectory of changes in striatum of all neurotransmitters measured in both genders. In females, Pb-induced increases in DA, 5HT and 5HIAA levels were of comparable magnitude under NEM and EM conditions (DA: F(1,113)=8.78, p=0.0037; 5HT: F(1,113)=14.4, p=0.0002; 5HIAA: F(1,110)=15.53, p=0.0001. In contrast, Pb-associated increases in HVA (PbxEM: F(1,113)=6.76, p=0.0106) under NEM conditions (290–309 to 341–387%) were reversed by EM procedures. The influence of Pb on DOPAC occurred under EM conditions (PbxSxEM: F(2,113)=3.33, p=0.0394), where increased levels at 0 ppm were conferred by OS, and higher levels at 50 ppm produced by NS (post hoc PbxS: p=0.008). For DA TO (PbxEM: F(1,110)=6.46, p=0.012; PbxS, F(2,110)=7.83, p=0.0007), OS elevated levels at both 0 and 150 ppm only under NEM conditions (post hoc PbxS; p=0.01019).
In males, Pb reduced levels of DA (PbxEM: F(2,186)=19.75, p<0.0001; 92–182 to 49–110%), 5HT (PbxEM: F(2,189)=29.55, p<0.0001; 113–176 to 67–136%) and 5HIAA (PbxEM: F(2,188)=18.7, p<0.0001; 221–248 to 183–228%) under NEM conditions, but increased levels under EM conditions (142–185 to 271–324%, 51–64 to 102–141% and 62–70 to 124–163%, respectively). For all three neurotransmitters, EM effects were enhanced by OS, where peak levels were already attained at 50 ppm (post hoc PbxS: p=0.03, 0.0001 and <0.0001; +208, +85 and +96%, respectively). Furthermore, for both 5HT and 5HIAA, the increases at 150 ppm in EM groups essentially produced values consistent with NEM levels, indicating that 150 ppm Pb exposure over-rode the EM-associated attenuation of NEM effects. Similarly, Pb-associated increases in HVA (PbxEM: (F(2,184)=10.79, p<0.0001) in 50 ppm OS and 50 ppm PS EM groups, and in all 150 ppm EM groups reached levels associated with NEM conditions, consistent with a Pb exposure associated over-ride of the EM influence (47–70 to 115–246%). Pb-associated increases in DA TO (PbxSxEM: F(4,186)=5.05, p=0.0007) under NEM conditions (129–358 to 225–753%), were markedly enhanced (potentiated) by OS, but fully reversed by EM procedures. Modest Pb-associated increases in DOPAC occurred in both EM and NEM conditions (Pb: F(2,189)=3.02, p=0.05; SxEM: F(2,189)=7.4, p=0.0008; 57–80 to 52–151%), although 150 ppm OS further increased levels under NEM conditions (post hoc PbxS: p<0.0001), while 50 ppm levels were stress-related under EM conditions, with PS>OS>NS (post hoc PbxS: p=0.004).
Table 4 summarizes Pb and Pb+/-stress associated modifications of EM vs. NEM trajectories of neurotransmitter changes between 2 and 6 mos for each brain region for females and males. As it shows, Pb or Pb+/-stress modulation of 2 to 6 mos trajectories were found under both EM and NEM conditions in both genders, although a greater percent change was found under NEM conditions in both cases, especially for males (65 vs. 71% and 53 vs. 84%, for females and males, respectively). In females, Pb exposure generally increased neurotransmitter levels (except striatal DA TO under NEM conditions), whereas in males, increases occurred under EM conditions and both increases and decreases were found under NEM conditions.
EM attenuated some but not all effects of Pb and or Pb+/-stress, with reversals predominating in nucleus accumbens in both genders (gray shading Table 4; females: DOPAC, HVA and DA TO; males: DOPAC, HVA, DA TO, 5HT and 5HIAA; indicated by Pb or PbxS effect in NEM condition but no corresponding change in the EM condition) and NEM DA TO changes in all regions in males. Pb exposure over-rides of the attenuating influence of EM, i.e., where EM values reached NEM levels, were also found, but only in males, where they occurred with frontal cortex DOPAC, and striatal HVA, 5HT and 5HIAA as Pb exposure concentration increased. In some cases (specifically striatal HVA of females, frontal cortex DA and NE and striatal DA of males), Pb-induced changes were in opposite directions in EM vs. NEM conditions. Although not shown, conditions in which stress modified Pb effects were always driven by OS in females, but examples of both OS and PS modulation were observed for males.
DISCUSSION
Results from this study confirmed that neurotransmitter levels change over time alone (NEM conditions), even in normal (control: 0ppm NS) animals, between 2 and 6 mos of age and did so across multiple brain regions and monoamines. Moreover, EM conditions significantly modified the trajectories seen under NEM conditions, and with the exception of increases in NE and DA in striatum, EM conditions typically blunted the changes in monoamine levels that occurred with time alone. Pb+/stress modified the trajectory of monoamine changes in both EM and NEM conditions, but predominated under NEM conditions. While modifications induced by stress alone over time under NEM conditions were fully reversed by EM procedures, Pb+/-stress-associated modifications in NEM were far less responsive to reversal, and occurred primarily in nucleus accumbens. Indeed in some cases, Pb exposure effects over-rode the attenuating influence of EM, resulting in neurotransmitter levels associated with NEM conditions.
Time Alone (NEM Condition) Was Associated with Neurochemical Changes Between 2 and 6 mos of Age
Importantly, time alone resulted in often quite dramatic neurochemical changes both in control (0 ppm) and Pb+/-stress challenged offspring between the 2 and 6 mos time points, with differences as great as 300%. While the dynamic nature of neurochemical function over time is known, most comparisons involve early vs. aged stages of the life span [30, 31, 35, 36]. This study provides evidence of ongoing dynamics post-development of monoamine systems [58, 59] but well before age-related changes ensue, indicating their modulation even during the adult period. While a previous study reported reductions in amphetamine-stimulated striatal DA release at 2 vs. 6 mos of age in male, but not female mice, a difference attributed to the presence vs. absence of hormonal activities at earlier vs. later ages [34], the profile of changes with time here showed general gender comparability (Table 3), albeit differences in magnitude of change. Differences in findings could easily reflect the species and outcome measure used. The fact that time alone changes neurochemical function at this stage has particular implications for experimental design of intervention studies. First, the marked differences between the EM and NEM neurotransmitter profiles at the 6 mos time point indicate that neurochemical changes, and perhaps other biochemical changes, observed from animals not subjected to environmental manipulations may not be generalizable to, or explanatory for, those with specific environmental histories. Secondly, determinations of basal neurotransmitter changes prior to imposing intervention vs. no intervention conditions may allow a separation of the actual underlying neurochemical changes directly relevant to the intervention procedure per se separate from time-based changes.
Experimental Manipulations Modify the Trajectory of Neurotransmitter Changes Occurring Under NEM Conditions
EM conditions modified, sometimes dramatically and broadly across regions (frontal cortex, nucleus accumbens, and striatum) and neurotransmitters, the trajectory of changes observed in NEM conditions between 2 and 6 mos (Figures 3–5 and Table 3). Such behavioral plasticity was first proposed by Hebb [60], with subsequent studies demonstrating that differential experiences with problem solving, and later, differential housing environments could produce significant changes in both brain structure and neurochemical function [61, 62]. The differences in trajectories seen here between EM and NEM conditions are indeed consistent with such previous reports. It is notable that EM vs. NEM conditions here also resulted in significantly different trajectories of corticosterone changes in response to stress challenges, as we have previously reported [4,56].
What cannot be ascertained from the current experiment is which component(s) of the EM condition are responsible for the ameliorating influence of EM on neurotransmitter levels. EM here included daily handling, weighing, and transport, behavioral testing, reinforcement availability and physical activity involved in FI performance. Physical exercise as an intervention has been generally shown to be less efficacious as an intervention strategy than either enrichment or formal training, suggesting it might be less influential [62]. Handling during the early postnatal period has been previously reported to attenuate stress effects [63, 64]. While early handling (weighing and culling of litters) was comparable in EM and NEM offspring, differences could certainly reflect the more sustained handling of EM animals as a component of behavioral testing.
The role of the specific behavioral paradigm, as well as the use of reinforcement contingencies may also be critical and is a particularly intriguing question. Passive avoidance training of mice was associated with multiple neurochemical changes and increased plasma corticosterone levels, whereas exposure to the apparatus alone increased corticosterone levels but did not produce neurochemical changes, suggesting both that the behavioral contingencies were the critical influence, and that neurochemical changes may be dissociated from changes in corticosterone [65]. However, other studies have also reported increases in nucleus accumbens DOPAC levels of mice of both genders [66] and increases in HVA in nucleus accumbens in male mice [67] simply in response to exposure to a novel environment, suggesting reinforcement contingencies are not critical. Obviously the contradictory nature of these findings leaves open the question of the importance of reinforcement contingencies per se and their potential influence independently of other conditions of behavioral testing. It is likely that both alter neurochemical function, but in ways that differ according to the specific intervention.
If reinforcement contingencies per se are important in determining the nature of the changes in the neurochemical profile, then an additional question is how the nature of the contingency itself influences the expression of neurochemical changes. Dunn et al. [65] utilized passive avoidance, a negative reinforcement-based paradigm considered by them as a form of stress that has indeed been shown to increase corticosterone levels [68]. It will be important to determine the extent to which positive vs. negative reinforcement based behavioral experience influences both the profile of neurochemical changes, and performance in subsequent behavioral situations following early insults. A related question is whether the influence of the behavioral paradigm would differ depending upon its mediation or not by brain regions likewise impacted by Pb and or stress.
Importantly, the EM procedures used here are typical of those used in many experiments evaluating the consequences of developmental insults, and are often carried out in conjunction with measures of changes in neurochemical function. The current findings suggest that the neurochemical changes seen post-behavioral testing may not be the same as those operative before testing, i.e., those presumably associated with the developmental insults per se, and therefore raise the question of which of such changes, or both, are important. That is, the potential confounds associated with behavioral experience leave many residual questions about whether effects attributed to early developmental insults reflect the insult itself, or the subsequent sequence of environmental influences as they interact with the developmental insult [69]. Nevertheless, it is ultimately an understanding of these interactions of developmental insults, such as Pb+/-stress, with different behavioral contexts that will be critical to elaborating the development of dysfunction or psychopathology and for devising effective behavioral therapeutic strategies.
Stress, but not Pb+/-Stress-Associated Changes in NEM Neurotransmitter Trajectories Were Reversed by EM Procedures
It is notable that stress-induced modifications of neurochemical changes were actually minimal at the 2 mos time point, restricted to frontal cortex DOPAC and 5HIAA in females but, instead, emerged over time in both genders primarily in OS groups, i.e., those that had sustained PS followed by OS challenges. Since corresponding changes were not found with PS alone, OS-induced neurotransmitter changes could reflect cumulative stress, the unmasking of PS effects by later stress challenges, or OS itself. An OS only group was not included in this experiment given the extent to which it would have expanded the effort, so the latter possibility cannot yet be evaluated. But in relation to the former two possibilities, it is interesting to note a report that acoustic startle responding was increased in offspring of dams exposed either to chronic mild stress or to dexamethasone only if they had also experienced immobilization stress for blood collection [70]. Similarly, PS was associated with changes in heart rate, temperature and physical activity not under basal conditions, but only under conditions of stress challenge [71]. Collectively such studies support the potential for cumulative or unmasked stress effects and support the use of stress challenges in adulthood as a useful method to unmask such effects.
Interestingly, all stress-associated changes in neurotransmitter levels under NEM conditions were reversed by EM procedures, findings consistent with other reports describing the ability of environmental interventions to reverse stress-associated effects [23]. Frequently such reversals have been achieved through ‘enriched’ housing [24, 25, 72] or handling during the postnatal period [5]. While daily handling occurred in the course of behavioral testing and weighing, it did not begin until offspring were already 2–3 mos of age, well past the period handling is typically applied in such studies [2] or even later in periadolescence [26]. Thus, the current findings extend the set of conditions under which stress-related effects, at least some of the neurotransmitter changes, can be ameliorated; behavioral differences were not ameliorated [8,56].
In contrast to the reversal of OS-associated changes in neurotransmitter levels under NEM conditions by EM procedures, reversals of Pb+/-stress associated neurochemical changes were generally restricted to nucleus accumbens where they were seen in both male and female offspring. In prior studies, enriched housing was shown to reverse learning deficits in the water maze, glutamatergic NR1 subunit mRNA deficits and neurotrophic factor gene expression changes in hippocampus [27, 29], suggesting a broad efficacy. The restriction of the ameliorative effects of EM to nucleus accumbens in this study is particularly notable given the critical role of this region in mediating response rates on the FI schedule as we have previously demonstrated [13, 73, 74], and therefore raises the possibility that behavioral procedures evoking sustained activity in a region may contribute to overcoming Pb+/-stress effects in that region. The current findings, which extend the set of conditions under which Pb effects can be reversed, are reminiscent of those suggesting that reversals of Pb-induced toxicity may be both intervention and task dependent [28].
In addition to the fact that reversals of Pb+/stress-induced neurotransmitter changes were restricted to nucleus accumbens, Pb exposure under EM conditions also actually increased levels of frontal cortex DA, and striatal HVA, 5HT and 5HIAA of males to values associated with NEM conditions, indicating its ability to over-ride the ameliorating influence of EM. In that regard, and related to the potential for effective intervention strategies to be both intervention and task-dependent, it is notable that EM conditions also actually increased frontal cortical NE levels in females relative to NEM conditions, and produced a profile of Pb-stress related NE changes with marked similarity to Pb-stress effects on fixed interval response rates, that were Pb-stress additive [8]. In fact, in corresponding principal component analyses that included all neurotransmitters and fixed interval behavioral performance, frontal cortex NE levels were among the strongest predictors of behavioral outcome. The latter observation again underscores the need to understand how various environmental conditions act on the altered CNS milieu arising from early developmental insults.
Acknowledgments
Supported by NIH grant ES05017 (D. Cory-Slechta, PI) and ES01247 (T. Gasiewicz, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koehl M, Lemaire V, Vallee M, Abrous N, Piazza PV, Mayo W, Maccari S, Le Moal M. Long term neurodevelopmental and behavioral effects of perinatal life events in rats. Neurotox Res. 2001;3:65–83. doi: 10.1007/BF03033231. [DOI] [PubMed] [Google Scholar]
- 3.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 4.Rossi-George A, Virgolini M, Weston D, Cory-Slechta DA. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicol Appl Pharmacol. 2009;234:117–127. doi: 10.1016/j.taap.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallee M, Maccari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur J Neurosci. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 6.White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Kuhlmann AC, McGlothan JL, Guilarte TR. Developmental lead exposure causes spatial learning deficits in adult rats. Neurosci Lett. 1997;233:101–104. doi: 10.1016/s0304-3940(97)00633-2. [DOI] [PubMed] [Google Scholar]
- 8.Virgolini MB, Rossi-George A, Lisek R, Weston D, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicol. 2008;29:812–827. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Ma Y, Ni L, Zhao S, Li L, Zhang J, Fan M, Liang C, Cao J, Xu L. Lead exposure through gestation-only caused long-term learning/memory deficits in young adult offspring. Exp Neurol. 2003;184:489–495. doi: 10.1016/s0014-4886(03)00272-3. [DOI] [PubMed] [Google Scholar]
- 10.Rice DC, Gilbert SG. Sensitive periods for lead-induced behavioral impairment (nonspatial discrimination reversal) in monkeys. Toxicol Appl Pharmacol. 1990;102:101–109. doi: 10.1016/0041-008x(90)90087-b. [DOI] [PubMed] [Google Scholar]
- 11.Rice DC. Lead-induced changes in learning: Evidence for behavioral mechanisms from experimental animal studies. Neurotoxicol. 1993;14:167–178. [PubMed] [Google Scholar]
- 12.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 13.Cory-Slechta DA, O’Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- 14.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowy M, Gault L, Yammamato B. Adrenalectomy attenuates stress induced elevation in extracellular glutamate concentration in hippocampus. J Neurosci. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 16.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 17.Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pokora MJ, Richfield EK, Cory-Slechta DA. Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: Time course of effects and interactions with chronic dopamine agonist treatments. J Neurochem. 1996;67:1540–1550. doi: 10.1046/j.1471-4159.1996.67041540.x. [DOI] [PubMed] [Google Scholar]
- 19.Anderson NB, Armstead CA. Toward understanding the asociation of socioeconomic status and health: A new challenge for the biopsychosocial approach. Psychosom Med. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal S, Muntner P, Batuman V, Rabito FA. Estimated burden of blood lead levels 5mug/dl in 1999–2002 and declines from 1988 to 1994. Environ Res. 2008 doi: 10.1016/j.envres.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 23.Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Francis Dd, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laviola G, Rea M, Morley-Fletcher S, Di Carlo S, Bacosi A, De Simone R, Bertini M, Pacifici R. Beneficial effects of enriched environment on adolescent rats from stressed pregnancies. Eur J Neurosci. 2004;20:1655–1664. doi: 10.1111/j.1460-9568.2004.03597.x. [DOI] [PubMed] [Google Scholar]
- 26.Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci. 2003;18:3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- 27.Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol. 2003;53:50–56. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- 28.Petit TL, Alfano DP. Differential experience following developmental lead exposure: Effects on brain and behavior. Pharmacology Biochemistry & Behavior. 1979;11:165–171. doi: 10.1016/0091-3057(79)90009-1. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JS, Lee MH, Anderson DW, Zuck L, Lidsky TI. Enriched environment during development is protective against lead-induced neurotoxicity. Brain Res. 2001;896:48–55. doi: 10.1016/s0006-8993(00)03249-2. [DOI] [PubMed] [Google Scholar]
- 30.Dorce VA, Palermo-Neto J. Behavioral and neurochemical changes induced by aging in dopaminergic systems of male and female rats. Physiol Behav. 1994;56:1015–1019. doi: 10.1016/0031-9384(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Ruiz J, De Miguel R, Hernandez ML, Cebeira M, Ramos JA. Comparisons between brain dopaminergic neurons of juvenile and aged rats: sex-related differences. Mech Ageing Dev. 1992;63:45–55. doi: 10.1016/0047-6374(92)90015-6. [DOI] [PubMed] [Google Scholar]
- 32.Friedemann M, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–332. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- 33.Gerhardt GA, Maloney RE., Jr Microdialysis studies of basal levels and stimulus-evoked overflow of dopamine and metabolites in the striatum of young and aged Fischer 344 rats. Brain Res. 1999;816:68–77. doi: 10.1016/s0006-8993(98)01095-6. [DOI] [PubMed] [Google Scholar]
- 34.McDermott JL, Dluzen DE. Aging and sex differences in striatal dopaminergic function. Neuroscience. 2007;149:401–408. doi: 10.1016/j.neuroscience.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 35.Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 37.Nader MA, Bowen CA. Effects of different food-reinforcement histories on cocaine self-administration by rhesus monkeys. Psychopharmacology (Berl) 1995;118:287–294. doi: 10.1007/BF02245957. [DOI] [PubMed] [Google Scholar]
- 38.Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology (Berl) 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- 39.Nader MA, Reboussin DM. The effects of behavioral history on cocaine self-administration by rhesus monkeys. Psychopharmacology (Berl) 1994;115:53–58. doi: 10.1007/BF02244751. [DOI] [PubMed] [Google Scholar]
- 40.Morgan D, Brebner K, Lynch WJ, Roberts DC. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol. 2002;13:389–396. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Holmes A, Rodgers RJ. Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci Biobehav Rev. 1999;23:971–980. doi: 10.1016/s0149-7634(99)00030-5. [DOI] [PubMed] [Google Scholar]
- 42.Holmes A, Iles JP, Mayell SJ, Rodgers RJ. Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behav Brain Res. 2001;122:159–167. doi: 10.1016/s0166-4328(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 43.Brockel BJ, Cory-Slechta DA. Lead, attention, and impulsive behavior: Changes in a fixed-ratio waiting-for-reward paradigm. Pharmacology Biochemistry and Behavior. 1998;60:545–552. doi: 10.1016/s0091-3057(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 44.Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- 45.Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Developmental Neuropsychology. 2004;26:513–540. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- 46.Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 ug per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cory-Slechta DA, Weiss B, Cox C. Mobilization and redistribution of lead over the course of calcium disodium ethylenediamine tetracetate chelation therapy. J Pharmacol Exp Ther. 1987;243:804–813. [PubMed] [Google Scholar]
- 48.Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- 49.Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 50.Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Igosheva N, Taylor PD, Poston L, Glover V. Prenatal stress in the rat results in increased blood pressure responsiveness to stress and enhanced arterial reactivity to neuropeptide Y in adulthood. J Physiol. 2007;582:665–674. doi: 10.1113/jphysiol.2007.130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- 53.Pallares ME, Scacchi Bernasconi PA, Feleder C, Cutrera RA. Effects of prenatal stress on motor performance and anxiety behavior in Swiss mice. Physiol Behav. 2007;92:951–956. doi: 10.1016/j.physbeh.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 54.Cory-Slechta DA. Comparative neurobehavioral toxicology of heavy metals. In: Suzuki T, editor. Toxicology of Metals. New York: CRC Press; 1996. pp. 537–560. [Google Scholar]
- 55.Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997;21:775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 56.Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicol. 2008;29:928–939. doi: 10.1016/j.neuro.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widzowski DV, Finkelstein JN, Pokora MJ, Cory-Slechta DA. Time course of postnatal lead-induced changes in dopamine receptors and their relationship to changes in dopamine sensitivity. Neurotoxicol. 1994;15:853–865. [PubMed] [Google Scholar]
- 58.Park M, Kitahama K, Geffard M, Maeda T. Postnatal development of the dopaminergic neurons in the rat mesencephalon. Brain Dev. 2000;22 (Suppl 1):S38–44. doi: 10.1016/s0387-7604(00)00145-5. [DOI] [PubMed] [Google Scholar]
- 59.Tepper JM, Damlama M, Trent F. Postnatal changes in the distribution and morphology of rat substantia nigra dopaminergic neurons. Neuroscience. 1994;60:469–477. doi: 10.1016/0306-4522(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 60.Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 61.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 62.Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002) Prog Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Cirulli F, Capone F, Bonsignore LT, Aloe L, Alleva E. Early behavioural enrichment in the form of handling renders mouse pups unresponsive to anxiolytic drugs and increases NGF levels in the hippocampus. Behav Brain Res. 2007;178:208–215. doi: 10.1016/j.bbr.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 64.Tejedor-Real P, Sahagun M, Biguet NF, Mallet J. Neonatal handling prevents the effects of phencyclidine in an animal model of negative symptoms of schizophrenia. Biol Psychiatry. 2007;61:865–872. doi: 10.1016/j.biopsych.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 65.Dunn AJ, Elfvin KL, Berridge CW. Changes in plasma corticosterone and cerebral biogenic amines and their catabolites during training and testing of mice in passive avoidance behavior. Behav Neural Biol. 1986;46:410–423. doi: 10.1016/s0163-1047(86)90422-x. [DOI] [PubMed] [Google Scholar]
- 66.Jones BC, Hou X, Cook MN. Effect of exposure to novelty on brain monoamines in C57BL/6 and DBA/2 mice. Physiol Behav. 1996;59:361–367. doi: 10.1016/0031-9384(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 67.Cabib S, Algeri S, Perego C, Puglisi-Allegra S. Behavioral and biochemical changes monitored in two inbred strains of mice during exploration of an unfamiliar environment. Physiol Behav. 1990;47:749–753. doi: 10.1016/0031-9384(90)90089-m. [DOI] [PubMed] [Google Scholar]
- 68.De Boer SF, Slangen JL, Van der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiol Behav. 1990;47:1089–1098. doi: 10.1016/0031-9384(90)90357-a. [DOI] [PubMed] [Google Scholar]
- 69.Carrasco C, Vicens P, Redolat R. Neuroprotective effects of behavioural training and nicotine on age-related deficits in spatial learning. Behav Pharmacol. 2006;17:441–452. doi: 10.1097/00008877-200609000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Hougaard KS, Andersen MB, Kjaer SL, Hansen AM, Werge T, Lund SP. Prenatal stress may increase vulnerability to life events: comparison with the effects of prenatal dexamethasone. Brain Res Dev Brain Res. 2005;159:55–63. doi: 10.1016/j.devbrainres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Mastorci F, Vicentini M, Viltart O, Manghi M, Graiani G, Quaini F, Meerlo P, Nalivaiko E, Maccari S, Sgoifo A. Long-term effects of prenatal stress: Changes in adult cardiovascular regulation and sensitivity to stress. Neurosci Biobehav Rev. 2009;33:191–203. doi: 10.1016/j.neubiorev.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Cory-Slechta DA, Pazmino R, Bare C. The critical role of the nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Res. 1997;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- 74.Cory-Slechta DA, Brockel BJ, O’Mara DJ. Lead exposure and dorsomedial striatum mediation of fixed interval schedule-controlled behavior. Neurotoxicol. 2002;23:313–327. doi: 10.1016/s0161-813x(02)00059-1. [DOI] [PubMed] [Google Scholar]