Abstract
We report on the mechanism of a series of ZnII-activated magnetic resonance contrast agents that modulate the access of water to a paramagnetic GdIII ion to create an increase in relaxivity upon binding of ZnII. In the absence and presence of ZnII, the coordination at the GdIII center is modulated by appended ZnII binding groups. These groups were systematically varied to optimize the change in coordination upon ZnII binding. We observe that at least one appended aminoacetate must be present as a coordinating group to bind GdIII and effectively inhibit access of water. At least two binding groups are required to efficiently bind ZnII, creating an unsaturated complex and allowing access of water. 13C isotopic labeling of the acetate binding groups for NMR spectroscopy provides evidence of a change in the metal coordination of these groups upon the addition of ZnII supporting our proposed mechanism of activation as presented.
Introduction
Magnetic resonance imaging (MRI) is a noninvasive technique capable of producing three-dimensional images of opaque organisms with excellent spatial and temporal resolution.1 MRI measures the 1H NMR signal of water, where the signal intensity is proportional to the relaxation rates of the nuclear spins. Variation in the water concentration and local environment contributes to the vivid contrast observed in an acquired image. Intrinsic contrast can be augmented by employing paramagnetic contrast agents that are designed to accelerate the relaxation of water protons.1,2 Typically, GdIII chelates are used as a result of having seven unpaired electrons and a symmetrical S ground state. The efficiency of these complexes to decrease the T1 of water protons is reported by the relaxivity value, r1 (mM−1 s−1).1,2
The Solomon–Bloembergen–Morgan theory describes several variables that can be manipulated to produce changes in the relaxivity of a GdIII chelate and include the hydration number (q), the mean residence lifetime of coordinated waters (τm), and the rotational correlation time (τr).3,4 In order to image biological processes in vivo, our laboratory pioneered the development of a series of activated contrast agents sensitive to enzymatic activity and secondary messengers.5–10 These activated contrast agents exploit the modulation of coordinated water molecules to produce distinct relaxation states in response to a physiological event. Ideally, a q-modulated MRI contrast agent has two distinct states, a low relaxivity state (q = 0) before activation and a high relaxivity state (q = 1 or 2) after activation, to produce an increase in the observed MRI signal intensity.
ZnII plays a critical role in cellular physiology and is involved in structural stability, catalytic activity, and signal transduction processes.11–13 The release of high concentrations of ZnII (200–300 μM) from neuronal synaptic vessels into the extracellular fluids of the brain has been implicated in a variety of pathological pathways.14 For example, ZnII release has been implicated in the precipitation of β-amyloid plaques found in Alzheimer’s disease.15,16 The development of ZnII-activated MRI contrast agents could provide a valuable tool in the study of in vivo ZnII activity without using optical agents whose detection is limited by light scattering and photobleaching.17–19
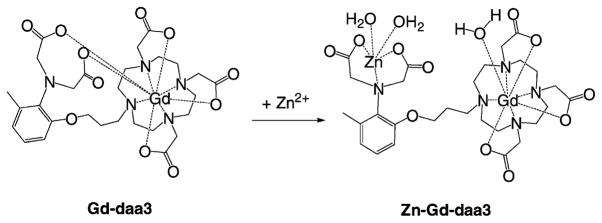
Recently, we reported a ZnII-activated MRI contrast agent where the relaxivity increases upon ZnII binding.20 Our proposed mechanism suggests that a pair of appended diaminoacetate arms bind to the GdIII ion to create a coordinatively saturated complex (q = 0; Figure 1). In the presence of ZnII, the diaminoacetates preferentially bind ZnII and produce a change in the coordination geometry around the GdIII center, producing an unsaturated complex and resulting in an increase in relaxivity. On the basis of a reported crystal structure of a diaminoacetate ZnII-coordination derivative,21 a distorted square-based pyramidal structure for ZnII binding to the contrast agent is proposed. Coordination of ZnII results in the formation of two five-membered rings sharing the Zn–N bond and can be described as an intermediate between square-based pyramidal and trigonal-bipyramidal geometries. The internal rearrangement of the diaminoacetate binding groups upon binding of ZnII results in an increase of over 100% in the observed relaxivity of the agent.
Figure 1.
Proposed mechanism of the ZnII-activated MRI contrast agent Gd-daa3.
Gd-daa3 has seven coordinating atoms available from the macrocyclic chelate and two aminoacetate ligands to bind GdIII, resulting in a coordination number of nine. Considering Zn-Gd-daa3, it is possible for one or both aminoacetates to bind ZnII. If both aminoacetate arms participate in ZnII binding, two water molecules are expected to bind to the GdIII ion. However, the experimentally determined q for Zn-Gd-daa3 is 1.20 This result raised the question concerning the coordination geometry of the aminoacetate pendant arms of Gd-daa3 in the presence and absence of ZnII.
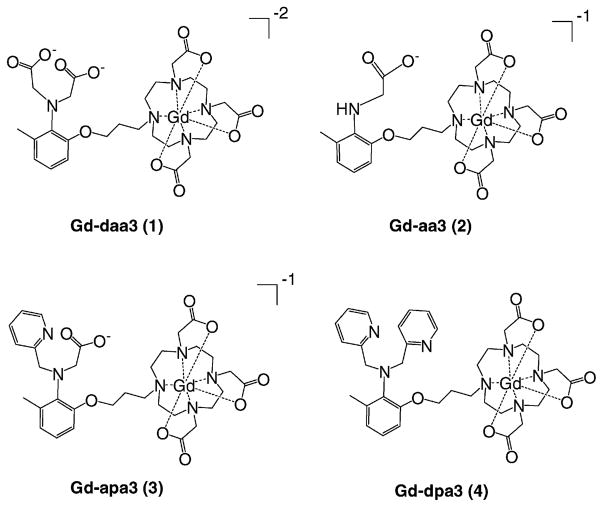
Here, we investigate the coordinating groups of a series of ZnII-activated agents to determine the role of binding and to prepare agents with a range of ZnII binding (Figure 2). The systematic variation of GdIII-coordinating aminoacetate groups and noncoordinating pyridyl groups allowed an investigation of the coordination geometry of Gd-daa3. We have discovered that while only one aminoacetate arm is necessary to efficiently block water to create a coordinatively saturated GdIII complex, at least two binding groups are necessary for the binding of ZnII. Therefore, it is possible to vary one of the aminoacetate arms to effectively tune the contrast agent to increase the selectivity and sensitivity for ZnII activation.
Figure 2.
Complexes 1–4 with systematic variation of the aminoacetate and pyridyl ZnII-coordinating groups.
Experimental Methods
Materials
GdCl3·6H2O, EuCl3·6H2O, TbCl3·6H2O, and 1,4,7,10-tetraazacyclododecane (cyclen) were purchased and used as is from Strem Chemicals (Newburyport, MA). Isotopically labeled 1,2-13C-ethyl bromoacetate was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). All other chemicals were purchased and used as is from Sigma-Aldrich. CH2Cl2, tetrahydrofuran (THF), and MeCN were dried using a solvent system purchased from Glass Contour, San Diego, CA. Water was purified using a Millipore Milli-Q synthesis water system. NMR spectra were recorded on either Varian (Palo Alto, CA) Inova 400 MHz or Varian Inova 500 MHz instruments with deuterated chloroform or water as the solvent. Electrospray ionization mass spectrometry (ESI-MS) spectra were obtained on a Varian 1200 L single-quadrupole mass spectrometer. Differences in the calculated and found mass spectrometry data for some complexes are a result of the isotopic patterns of the GdIII complexes and the 13C-labeled complexes. To confirm the purity of the final complexes, elemental analysis was performed by Desert Analytics Laboratory (Tucson, AZ).
Synthesis
The synthesis and characterization of Gd-daa3 was accomplished as previously described.20 Gd-aa3 is a side product from the synthesis of Gd-daa3 that is collected from the semi-preparatory high-performance liquid chromatography (HPLC). The synthetic procedure and characterization for Gd-dpa3 and Gd-apa3 are described. DO3A-tris-tert-butyl ester was synthesized following literature procedures.22
1-(3-Bromopropoxy)-3-methyl-2-nitrobenzene (5)
To a solution of 3-methyl-2-nitrophenol (3.0 g, 19.6 mmol) in 200 mL of dry acetonitrile under nitrogen was added 9.12 g (66.0 mmol) of anhydrous potassium carbonate. After the reaction turned bright red because of the deprotonation of the phenol (10 min), 5.96 mL (58.7 mmol) of 1,3-dibromopropane was added, and the reaction was allowed to proceed at reflux overnight. After cooling to room temperature, the reaction was filtered, and the filtrate was washed with ethyl acetate and concentrated via rotary evaporation. The oily product was brought up in 100 mL of ethyl acetate and washed once with water followed by brine. The organic layer was dried over Na2SO4, filtered, and concentrated. The crude product was purified on a silica gel column, eluting with 5% ethyl acetate in hexanes to yield 5 as a light-yellow oil (5.1 g, 95% yield). 1H NMR (400 MHz, CDCl3w/TMS): δ 7.23 (t, J = 8 Hz, 1H), 6.85 (d, J = 8 Hz, 1H), 6.81 (d, J = 7.6 Hz, 1H), 4.12 (t, J = 5.6 Hz, 2H), 3.48 (t, J = 6.8 Hz, 2H), 2.22 (s, 3H), 2.21 (m, 2H). 13C NMR (400 MHz, CDCl3 w/TMS): δ 149.9, 142.3, 130.9, 130.8, 123.1, 111.2, 66.8, 32.1, 29.0, 17.1. MS (ESI+). Calcd for (M + H+): m/z 273.0. Found: m/z 273.9.
{4,7-Bis[(tert-butoxycarbonyl)methyl]-10-[3-(3-methyl-2-nitro-phenoxy)propyl]-1,4,7,10-tetraazacyclododec-1-yl}acetic Acid tert-Butyl Ester (6)
In a 250 mL round-bottomed flask, 2.0 g (7.3 mmol) of 5 was dissolved in 50 mL of dry acetonitrile. DO3A-tris-tert-butyl ester (2.5 g, 4.9 mmol) and anhydrous potassium carbonate (3.4 g, 24.3 mmol) were then added, and the resulting mixture was refluxed for 2 days. The reaction was cooled to room temperature and filtered. The crude product was absorbed onto silica and purified on a silica gel column, eluting with 2% methanol in dichloromethane. A 66% yield (2.3 g, 3.2 mmol) of 6 was obtained as a light-yellow oil. 1H NMR (500 MHz, CDCl3 w/TMS): δ 7.24 (t, J = 8 Hz, 1H), 6.83 (d, J = 8 Hz, 1H), 6.81 (d, J = 8 Hz, 1H), 4.01 (t, J = 6 Hz, 2H), 3.58–2.32 (multiplicity, 24H), 2.23 (s, 3H), 1.92 (m, 2H), 1.38 (s, 27H). 13C NMR (500 MHz, CDCl3 w/TMS): δ 170.6, 170.3, 149.6, 142.0, 131.2, 130.9, 123.2, 111.5, 82.0, 81.8, 66.4, 56.8, 55.5, 53.8, 53.4, 52.6, 50.0, 49.8, 48.0, 28.1, 22.9, 17.0. MS (ESI+). Calcd for (M + H+): m/z 707.5. Found: m/z 708.5. Calcd for (M + Na+): m/z 730.4. Found: m/z 730.5.
{2-Methyl-6-[3-(4,7,10-tris[(tert-butoxycarbonyl)methyl]-1,4,7,10-tetraazacyclododec-1-yl)propoxy]phenylamino}acetic Acid tert-Butyl Ester (7)
Product 6 (1.5 g, 2.1 mmol) was dissolved in methanol and added to a flask preloaded with 10% palladium on carbon (wet) in catalytic conditions. The reaction was equipped with a hydrogen reactor at 3.0 bar. After 48 h, the reaction was removed from the hydrogen reactor and filtered through celite, rinsing several times with methanol. Reduction of the nitro group to the amine was confirmed by MS [ESI+; m/z 678.5 (M + H+)]. The resulting orange oil was dissolved in 25 mL of dry acetonitrile. To this was added anhydrous potassium carbonate (0.85 g, 6.2 mmol) followed by 0.7 mL (4.5 mmol) of tert-butyl bromoacetate. The reaction was refluxed for 4 days and followed by thin-layer chromatography (TLC) to monitor the reaction progression. After a new spot was observed on the TLC due to the addition of the second acetate arm, the reaction was cooled to room temperature and filtered. The crude product was absorbed onto silica and purified on a silica gel column, eluting with 2% methanol in dichloromethane. 7 was obtained as an orange oil in 25% yield. 1H NMR (400 MHz, CDCl3 w/TMS): δ 6.66 (Ar, 3H), 3.89 (t, J = 6 Hz, 2H), 3.71 (s, 2H), 3.66 (s, 1H, NH), 3.39–2.26 (multiplicity, 24H), 2.22 (s, 3H), 1.91 (m, 2H), 1.38 (s, 27H), 1.32 (s, 9H). 13C NMR (400 MHz, CDCl3 w/TMS): δ 173.8, 172.8, 171.4, 170.7, 149.4, 137.1, 128.0, 124.0, 120.7, 109.8, 82.9, 82.6, 81.7, 80.6, 67.1, 57.3, 56.7, 53.9, 51.9, 50.5, 28.1, 26.6, 18.9. MS (ESI+). Calcd for (M + H+): m/z 791.5. Found: m/z 792.5. Calcd for (M + Na+): m/z 814.5. Found: m/z 814.5.
({2-Methyl-6-[3-(4,7,10-tris[(tert-butoxycarbonyl)methyl]-1,4,7,10-tetraazacyclododec-1-yl)propoxy]phenyl}pyridin-2-ylmethylamino)acetic Acid tert-Butyl Ester (8)
In 50 mL of dry acetonitrile, 0.4 g (0.5 mmol) of 8 was added followed by anhydrous potassium carbonate (0.28 g, 2.0 mmol) and 2-(bromomethyl)pyridine hydrobromide (0.26 g, 1.0 mmol). The reaction was refluxed overnight and then cooled to room temperature, filtered, and concentrated via rotary evaporation. The crude product was absorbed onto silica and purified on a silica gel column, eluting with 3% methanol in dichloromethane. 8 was collected in 30% yield (0.13 g, 0.15 mmol) as an orange oil. MS (ESI+). Calcd for (M + H+): m/z 882.6. Found: m/z 883.2. Calcd for (M + Na+): m/z 905.6. Found: m/z 905.1.
{4-{3-[2-[Bis(pyridin-2-ylmethyl)amino]-3-methylphenoxy]propyl}-7,10-bis[(tert-butoxycarbonyl)methyl]-1,4,7,10-tetraazacyclododec-1-yl}acetic Acid tert-Butyl Ester (9)
Product 6 (0.35 g, 0.5 mmol) was dissolved in methanol and added to a flask preloaded with 10% palladium on carbon (wet) in catalytic conditions. The reaction was equipped with a hydrogen reactor at 3.0 bar. After 48 h, the reaction was removed from the hydrogen reactor and filtered through celite, rinsing several times with methanol. Reduction of the nitro group to the amine was confirmed with MS [ESI+; m/z = 678.5 (M + H+)]. The product was concentrated and brought up in 30 mL of dry acetonitrile. To this solution was added anhydrous potassium carbonate (0.17 g, 1.3 mmol) followed by 2-picoyl chloride (0.15 g, 0.9 mmol). The reaction was refluxed for 5 days to ensure that both picoyl groups were added. After filtering and rinsing with ethyl acetate and methanol, the crude product was absorbed onto silica for column purification, eluting with 5% methanol in dichloromethane. 9 was obtained as a pale-orange oil (0.15 g, 37% yield). 1H NMR (500 MHz, CDCl3 w/TMS): δ 8.44 (d, J = 4.5 Hz, 2H), 7.54 (t, J = 7.5 Hz, 2H), 7.33 (d, J = 7.5 Hz, 2H), 7.08 (t, J = 7 Hz, 2H), 6.95 (t, J = 8 Hz, 1H), 6.68 (overlapping doublets, J = 8 Hz, 2H), 4.35 (s, 2H), 4.30 (s, 2H), 3.91 (t, J = 6 Hz, 2H), 3.2–2.2 (multiplicity, 24H), 2.17 (s, 3H), 1.95 (m, 2H), 1.42 (s, 27H). 13C NMR (500 MHz, CDCl3 w/TMS): δ 172.8, 160.0, 157.7, 148.9, 138.8, 137.6, 136.2, 126.2, 123.4, 123.0, 121.9, 109.8, 82.8, 82.5, 66.3, 60.2, 55.9, 51.6, 51.1–50.0 (overlapping cyclen peaks), 28.0, 25.6, 19.0. MS (ESI+). Calcd for (M + H+): m/z 859.6. Found: m/z 860.6. Calcd for (M + Na+): m/z 882.5. Found: m/z 882.6.
Metalation Procedure
A trifluoroacetic acid (TFA) solution, 95:2.5:2.5 (TFA/H2O/triisopropylsilane) was added to the protected ligands 8 and 9 for 12 h. After TFA was removed by purging the solution with air and 15 mL of diethyl ether was added, the precipitate was centrifuged and decanted. The diethyl ether wash was repeated twice, the final pellet was brought up in H2O, and the pH was adjusted to 6.5 with 1 M NaOH. A total of 1.1 equiv of GdCl3·6H2O was then added, and the resulting mixture was stirred at room temperature for several days. Unreacted GdIII precipitated as Gd(OH)3 after the addition of 1 M NaOH to a pH of 10 and pelleted. The crude mixture was purified by semipreprative HPLC on a reverse-phase column, eluting with acetonitrile and water using an isocratic ramp from 0% to 100% acetonitrile over 35 min. Analytical HPLC–MS was used to confirm the purity and identity of the collected fractions. Pure fractions were freeze-dried and stored in a desiccator. The same procedure was followed with TbCl3 · 6H2O to obtain the TbIII metal complexes.
Gd-daa3. Gadolinium(III) Carboxymethyl-{2-methyl-6-[3-[4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl]pro-poxy]phenylamino}acetic Acid (1)
Analytical LC–MS showed a single peak (ESI+). Calcd for (M + H+): m/z 778.2. Found: m/z 781.2. Anal. Calcd for C28H38GdN5O11 · 2H2O·2Na: C, 39.11; H, 4.93; N, 8.14. Found: C, 39.33; H, 4.93; N, 7.84. Tb-daa3. Analytical LC–MS showed a single peak (ESI+). Calcd for (M + H+): m/z 779.2. Found: m/z 779.2. Calcd for (M + Na+): m/z 802.2. Found: m/z 801.2.
Gd-aa3. Gadolinium(III) {2-Methyl-6[3-[4,7,10-tris(carboxy-methyl)-1,4,7,10-tetraazacyclododec-1-yl]propoxy]phenylamino}-acetic Acid (2)
Analytical LC–MS showed a single peak (ESI+). Calcd for (M + H+): m/z 721.1. Found: m/z 720.2. Anal. Calcd for C26H38GdN5O9 · H2O · Na: C, 40.94; H, 5.29; N, 9.18. Found: C, 40.81; H, 5.27; N, 9.02.
Gd-apa3. Gadolinium(III) {{2-Methyl-6-[3-[4,7,10-tris(carboxym-ethyl)-1,4,7,10-tetraazacyclododec-1-yl]propoxy]phenyl}pyridin-2-ylmethylamino}acetic Acid (3)
Analytical LC–MS showed a single peak (ESI+). Calcd for (M + H+): m/z 812.2. Found: m/z 813.0. Anal. Calcd for C32H42GdN6O9 · 2H2O: C, 45.32; H, 5.47; N, 9.91. Found: C, 45.37; H, 5.79; N, 10.32. Tb-apa3. Analytical LC–MS showed a single peak (ESI+). Calcd for (M + H+): m/z 813.2. Found: m/z 814.2.
Gd-dpa3. Gadolinium(III) {4-{3-[2-[Bis(pyridin-2-ylmethyl)-amino]-3-methylphenoxy]propyl}-7,10-bis(carboxymethyl)-1,4,7,10-tetraaza-cyclododec-1-yl}acetic Acid (4)
Analytical LC–MS showed a single peak (ESI+). Calcd for (M + H+): m/z 846.3. Found: m/z 847.2. Anal. Calcd for C36H47GdN7O7: C, 51.05; H, 5.59; N, 11.58. Found: C, 51.15; H, 5.61; N, 11.22. Tb-dpa3. Analytical LC–MS showed a single peak (ESI+). Calcd for (M + H+): m/z 847.3. Found: m/z 848.3.
tert-Butyldimethyl-[3-(3-methyl-2-nitrophenoxy)propoxy]si-lane (10)
To a solution of 3-methyl-2-nitrophenol (5.0 g, 32.6 mmol) in dry acetonitrile (300 mL) under nitrogen was added K2CO3 (11.26 g, 81.5 mmol). After the reaction had turned a bright-red color because of the deprotonated state of the phenol (about 10 min), (3-bromopropoxy)-tert-butyldimethylsilane (9.04 mL, 39.2 mmol) was added. The reaction was refluxed at 70 °C until it was a pale-yellow color, cooled to room temperature, and filtered. After evaporation of the solvent, the mixture was brought up in 100 mL of ethyl acetate and washed once with an aqueous saturated sodium bicarbonate solution and once with brine. The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified on a silica gel column, eluting with 1% ethyl acetate in hexanes, yielding 10 as light-yellow crystals after drying (10.11 g, 94% yield). 1H NMR (500 MHz, CDCl3 w/TMS): δ 7.27 (t, J = 8 Hz, 1H), 6.89 (d, J = 8.5 Hz, 1H), 6.84 (d, J = 8 Hz, 1H), 4.15 (t, J = 6 Hz, 2H), 3.75 (t, J = 6 Hz, 2H), 2.30 (s, 3H), 1.96 (m, J = 5.5 Hz, 2H), 0.88 (s, 9H), 0.04 (s, 6H). 13C NMR (500 MHz, CDCl3 w/TMS): δ 150.44, 142.43, 131.06, 130.76, 122.54, 111.01, 65.89, 59.15, 32.23, 26.11, 18.49, 17.17, −5.13, −5.41. MS (ESI+). Calcd for (M + H+): m/z 325.2. Found: m/z 326.2. Calcd for (M + Na+): m/z 348.2. Found: m/z 348.1.
{{2-[3-(tert-Butyldimethylsilanyloxy)propoxy]-6-methylphen-yl}ethoxycarbonylmethylamino}acetic Acid Ethyl Ester (11)
In 30 mL of methanol, 1.0 g of 10 (3.07 mmol) was dissolved and added to a flask preloaded with 10% palladium on carbon (wet) in catalytic conditions. The reaction was equipped with a hydrogen reactor at 3.0 bar for 24 h. Upon completion of reduction, the reaction was filtered over celite, rinsed several times with 50 mL methanol, and concentrated via rotary evaporation of the solvent. The resulting oil was transferred to a 100 mL round-bottomed flask and dissolved in dry acetonitrile (30 mL). Proton sponge (2.79 g, 13.0 mmol) was dissolved in the reaction mixture before the addition of 1.0 g (5.92 mmol) of 1,2-13C-ethyl bromoacetate followed by sodium iodide (1.95 g, 13.0 mmol). After 2 days of refluxing, the reaction was filtered, rinsed with ethyl acetate, and concentrated via rotary evaporation. The crude product was absorbed onto silica and purified on a silica gel column, eluting with a slow gradient of 2% ethyl acetate in hexanes to 5% ethyl acetate. The desired product 11 was collected in 75% yield as a light-yellow oil. 1H NMR (500 MHz, CDCl3 w/TMS): δ 7.00 (t, J = 8 Hz, 1H), 6.79 (d, J = 7.5 Hz, 1H), 6.72 (d, J = 7.5 Hz, 1H), 4.12 (m, 4H), 4.05 (t, J = 6.5 Hz, 2H), 3.91 (s, broad, 2H), 3.85 (t, J = 6 Hz, 2H), 3.74 (s, broad, 2H), 2.47 (s, 3H), 2.04 (m, 2H), 1.24 (t, J = 7 Hz, 6H), 0.91 (s, 9H), 0.07 (s, 6H). 13C NMR (500 MHz, CDCl3 w/TMS): δ 172.5, 157.0, 139.3, 137.7, 126.4, 122.9, 109.7, 64.8, 60.5, 56.6, 49.8, 32.9, 26.2, 21.3, 18.6, 14.4, −5.1. MS (ESI+). Calcd for (M + H+): m/z 469.3. Found: m/z 472.3. Calcd for (M + Na+): m/z 492.2. Found: m/z 494.3.
{(Ethoxycarbonyl)methyl-[2-(3-hydroxypropoxy)-6-methyl-phenyl]amino}acetic Acid Ethyl Ester (12)
To a solution of 11 (1.1 g, 2.3 mmol) in THF (25 mL) was added tetrabutylammonium fluoride (1.5 g, 5.8 mmol). After 2 h at room temperature, the deprotection was completed as observed by TLC (1:3 EtOAc/hexanes). THF was removed via rotary evaporation. The crude product was brought up in ethyl acetate and washed once with water and then brine. The organic layer was dried over sodium sulfate, filtered, concentrated, and purified through a silica plug, eluting with 25% ethyl acetate in hexanes and yielding the desired product in 83% yield (0.68 g, 1.9 mmol). 1H NMR (500 MHz, CDCl3 w/TMS): δ 7.00 (t, J = 8 Hz, 1H), 6.80 (d, J = 8 Hz, 1H), 6.74 (d, J = 8.5 Hz, 1H), 4.14 (m, 6H, overlapping OCH2CH3 and OCH2CH2CH2OH), 4.06 (s, broad, 2H), 3.90 (t, J = 5.5 Hz, 2H), 3.79 (s, broad, 2H), 2.45 (s, 3H), 2.08 (m, 2H), 1.23 (t, J = 7 Hz, 6H). 13C NMR (500 MHz, CDCl3 w/TMS): δ 172.5, 156.4, 138.5, 137.3, 126.1, 123.4, 110.1, 65.7, 60.2, 49.6, 32.4, 18.7, 14.4. MS (ESI+). Calcd for (M + H+): m/z 355.2. Found: m/z 358.2. Calcd for (M + Na+): m/z 378.2. Found: m/z 380.1.
{[2-(3-Bromopropoxy)-6-methylphenyl]ethoxycarbonylmethyl-amino}acetic Acid Ethyl Ester (13)
To a solution of 12 (0.5 g, 1.4 mmol) in dry methylene chloride (15 mL) was added carbon tetrabromide (0.7 g, 2.1 mmol) followed by the slow addition of triphenylphosphine (0.7 g, 2.8 mmol). After 2 h at room termperature, the reaction was washed with water and brine, dried over sodium sulfate, filtered, and concentrated. The crude product was purified on a silica gel column, eluting with 5% ethyl acetate in hexanes and giving 13 in 94% yield as an orange oil. 1H NMR (500 MHz, CDCl3 w/TMS): δ 7.01 (t, J = 8 Hz, 1H), 6.81 (d, J = 7 Hz, 1H), 6.73 (d, J = 8.5 Hz, 1H), 4.11 (m, 6H, overlapping OCH2CH3 and OCH2CH2CH2Br), 4.00 (s, broad, 2H), 3.73 (s, broad, 2H), 3.67 (t, J = 6.5 Hz, 2H), 2.46 (s, 3H), 2.37 (m, 2H), 1.23 (t, J = 6.5 Hz, 6H). 13C NMR (500 MHz, CDCl3 w/TMS): δ 172.0, 156.5, 139.2, 137.6, 126.3, 123.5, 109.9, 65.7, 60.6, 56.3, 49.6, 32.7, 18.6, 14.5. MS (ESI+). Calcd for (M + H+): m/z 417.1. Found: m/z 420.0. Calcd for (M + Na+): m/z 440.1. Found: m/z 442.0.
{Ethoxycarbonylmethyl-{2-methyl-6-[3-[4,7,10-tris[(tert-bu-toxycarbonyl)methyl]-1,4,7,10-tetraazacyclododec-1-yl)propoxy]-phenyl]amino}acetic Acid Ethyl Ester (14)
To a solution of DO3A-tris-tert-butyl ester (0.55 g, 1.1 mmol) in 15 mL of dry acetonitrile was added anhydrous potassium carbonate (0.44 g, 3.2 mmol). After 5 min, a solution of 13 (0.55 g, 1.3 mmol) in 5 mL of dry acetonitrile was added to the reaction, and the mixture was refluxed overnight. The reaction was cooled and filtered, rinsing with acetonitrile followed by methanol. The solvents were removed by rotary evaporation, and the crude product was purified on a silica gel column, eluting with 3% MeOH in dichloromethane to yield 14 in 27% yield. After trituration with diethyl ether several times, a pale-yellow solid was obtained. 1H NMR (400 MHz, CDCl3 w/TMS): δ 6.91 (t, J = 8 Hz, 1H), 6.72 (d, J = 7.6 Hz, 1H), 6.62 (d, J = 8.4 Hz, 1H), 3.95–2.30 (multiplicity, 36H), 2.35 (s, 3H), 1.27 (s, 27H), 1.17 (m, 6H). 13C NMR (500 MHz, CDCl3 w/TMS): δ 172.6, 164.2, 156.4, 139.0,126.3, 123.3, 109.7, 82.9, 82.6, 66.5, 60.5, 55.8, 51.7, 50.7, 50.0, 49.8, 49.4, 49.2, 47.9, 28.1, 18.4, 14.4. MS (ESI+). Calcd for (M + Na+): m/z 874.5. Found: m/z 876.6.
Eu-daa3. Europium(III) Carboxymethyl-{2-methyl-6-[3-[4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl]propoxy]phenylamino}acetic Acid (15)
The protected ligand 14 was first reacted with 1.0 M NaOH for 24 h to deprotect the ethyl groups. After neutralization to a pH of 7, the crude product was freeze-dried and then brought up in a solution of TFA for 24 h. TFA was removed by purging the solution with air, and approximately 15 mL of diethyl ether was added. The precipitate was centrifuged and decanted. The diethyl ether wash was repeated two times, the final pellet was brought up in H2O, and the pH was adjusted to 6.5 with 1 M NaOH. A total of 1.1 equiv of EuCl3 · 6H2O was added and stirred at room temperature for several days. Unreacted EuIII precipitated as Eu(OH)3 after the addition of 1 M NaOH, and the crude mixture was purified by semipreprative HPLC, eluting with acetonitrile and water. Analytical HPLC–MS was used to confirm the purity and identity of the collected fractions and showed a single peak with MS (ESI+). Calcd for (M + H+): m/z 772.6. Found: m/z 772.5.
Relaxivity Measurements
A 1 mM solution of each GdIII complex was prepared in buffer containing 100 mM KCl/100 mM HEPES at pH 7.4. These were serially diluted four times to give 500 μL of five different sample concentrations at a [Gd]:[Zn] ratio of 1:0. Aliquots of a 5.0 mM ZnCl2 solution in HEPES were added to each of the samples to give a [Gd]:[Zn] ratio of 1:0.5. After 30 min of incubation at 37 °C, T1 measurements were performed on a Bruker mq60 minispec relaxometer with an inversion–recovery pulse sequence with the appropriate recycle delays. The spectrometer fits 10 data points to an exponential decay for each T1 measurement and calculates the standard deviation for each T1, representing an error of less than 1%. This titration was repeated until a 1:3 ([Gd]:[Zn]) ratio was reached.
Luminescence Lifetime Measurements
The fluorescence decay rates of the terbium analogues of 1, 3, and 4 in buffered H2O and D2O were measured on a Hitachi F4500 fluorometer monitoring the emission at 544 nm with an excitation of 254 nm. Aliquots of HEPES buffer and ZnCl2 in HEPES were freeze-dried before being brought up in D2O to ensure there was no water present. A 200 μM solution of the terbium complex in HEPES buffer was measured in the presence of 300 μM ZnCl2 and without ZnCl2. A total of 25 scans were averaged and fit to a monoexponential decay with an r2 value of 0.99.
Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
The concentration of each sample for relaxivity was determined by ICP-AES (Varian). A 10 μL aliquot of each sample was digested in 90 μL of nitric acid. Each sample was diluted with water to the appropriate concentration for ICP-AES analysis. Gadolinium concentrations were determined from a standardized curve of six standards ranging from 0 to 500 ppb GdIII, measuring the emission of GdIII at 335.048, 336.224, and 342.246 nm.
Results
Synthesis
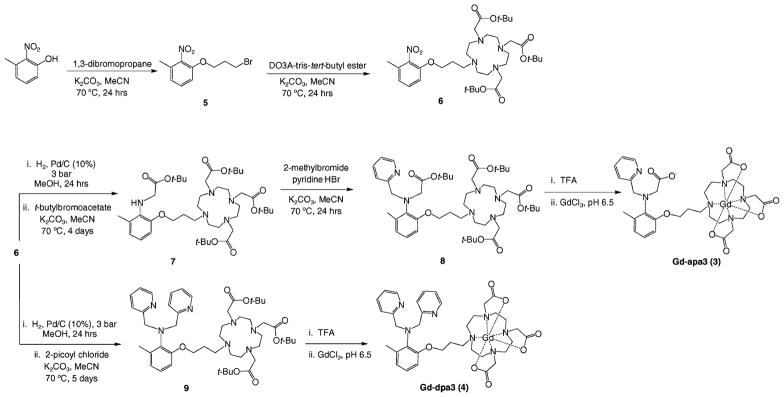
The syntheses of 3 and 4 are outlined in Scheme 1. Starting with 3-methyl-2-nitrophenol, 1,3-dibro-mopropane was added using potassium carbonate in dry acetonitrile. DO3A-tris-tert-butyl ester was synthesized following literature procedures22 and combined with 5 under basic conditions to yield 6. The nitro group of 6 was then reduced under standard palladium-catalyzed hydrogenation conditions and reacted with either tert-butyl bromoacetate to yield 7 or with 2-picoyl chloride to yield 9. The final pyridyl binding group was added to 7 using 2-bromopyridine hydrobromide to yield the final tert-butyl-protected ligand 8. Both 8 and 9 were reacted with trifluoroacetic acid to remove the tert-butyl protecting groups and reacted with GdCl3 · 6H2O at a pH of 6.5 for 2 days. The final compounds 3 and 4 were purified by semipreparatory HPLC and characterized by LC–MS and elemental analysis.
Scheme 1.
Synthesis of Gd-apa3 (3) and Gd-dpa3 (4)
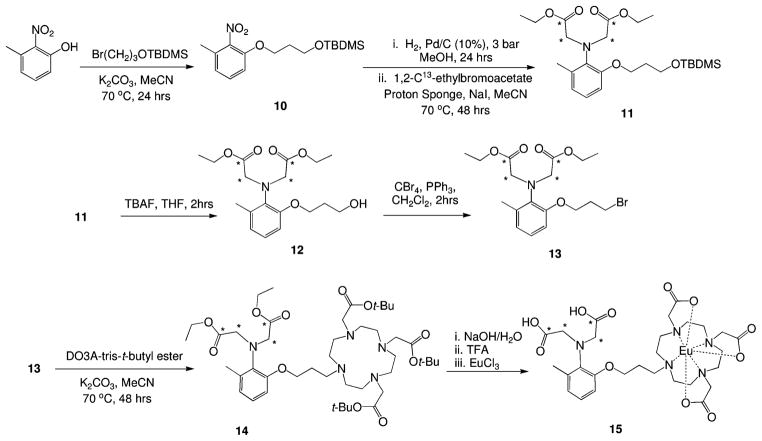
The 13C isotopic labeling of the aminocarboxylates of Gd-daa3 was synthesized following previously published procedures with some modifications outlined in Scheme 2.20 The synthesis begins with alkylation of the commercially available 3-methyl-2-nitrophenol with the tert-butyldimethylsilyl-protected alcohol to give 10 in 95% yield. After standard hydrogenation conditions, the two aminoacetate arms were added using 1,2-13C-ethyl bromoacetate in the presence of proton sponge and sodium iodide. Deprotection of the TBDMS protecting group was achieved with tetrabutylammonium fluoride to yield 12, which was brominated with carbon tetrabromide in the presence of triphenylphosphine to give 13. The addition of DO3A-tris-tert-butyl ester was achieved with potassium carbonate in acetonitrile under refluxing conditions to give the fully protected ligand 14. The ethyl groups were deprotected through the addition of 1 M NaOH at room temperature. After neutralization, the tert-butyl groups were deprotected with TFA before the addition of the metal with EuCl3 · 6H2O. The final compound was purified by semipreparatory HPLC and characterized by analytical LC–MS.
Scheme 2.
Synthesis of 13C-Isotopically Labeled Eu-daa3 (15; * = 13C)
Relaxivity
To evaluate the binding properties of the aminoacetate and pyridyl groups employed in complexes 1–4, relaxivities were first measured in the absence of ZnII at 60 MHz and 37 °C in HEPES buffer to determine the effectiveness in creating a coordinatively saturated GdIII complex with low relaxivity. As seen in Table 1, the complexes with one or two aminoacetates are binding to GdIII and thus create a low-relaxivity complex. The relaxivity of Gd-dpa3 of 7.5 mM−1 s−1 illustrates the inability of the pyridyl nitrogen atoms to bind with GdIII and therefore not reduce water access to the GdIII ion. The lowest relaxivity observed is for Gd-daa3, suggesting that both aminoacetates participate in the binding of GdIII, as depicted in Figure 1. The two complexes with only one aminoacetate, Gd-aa3 and Gd-apa3, do not have as low of a relaxivity as that observed for Gd-daa3; however, they still have the ability to bind GdIII and effectively reduce the access of water. This suggests that a coordination number of eight is sufficient in creating a coordinatively saturated GdIII complex. The lower relaxivity for Gd-apa3 compared to Gd-aa3 may be due to the added steric effects in reducing water access from the second pyridyl arm of Gd-apa3. While there is no direct evidence from these experiments of the effect on second-sphere solvation, the presence of the noncoordinating pyridyl ligand may allow for the reduction of second-sphere water molecules, resulting in an overall decrease in the observed relaxivity.
Table 1.
Relaxivity in the Presence and Absence of ZnII at 60 MHz and 37 °C in HEPES Buffer
|
r1 (mM−1s−1) |
|||
|---|---|---|---|
| 0 equiv of ZnII | 1 equiv of ZnII | % change in r1 | |
| Gd-daa3 | 2.3 | 5.1 | + 121% |
| Gd-aa3 | 4.1 | 4.2 | + 2.4% |
| Gd-apa3 | 3.4 | 6.9 | + 103% |
| Gd-dpa3 | 7.5 | 7.2 | − 4.0% |
In the presence of 1 equiv of ZnCl2 in HEPES buffer at 60 MHz and 37 °C, the relaxivities of both Gd-daa3 and Gd-apa3 increase by 121% and 103%, respectively, while there was no significant change in the relaxivity for Gd-aa3 (Table 1). Importantly, Gd-aa3 exhibits no increase in r1 upon the addition of ZnII, implying that there is no change in the coordination environment. This is the only complex with only one available binding group and, therefore, it is reasonable to suggest that two binding groups are required for coordinating ZnII. Given that Gd-dpa3 was not able to create a coordinatively saturated structure when no ZnII was present, it was expected that there would be little change in its relaxivity with the addition of ZnII, as observed.
Luminescence
The relaxivity studies presented suggest that, in the absence of ZnII, complexes with at least one available aminoacetate arm to coordinate GdIII have low relaxivity values, as would be expected for a q = 0 complex. While the relaxivity measurements reflect the effect of both the inner-sphere water molecules and the second solvation shell, fluorescence lifetime measurements can directly report on the first solvation sphere. To determine if, in fact, an eight-coordinate complex could provide a coordinatively saturated complex, the hydration numbers (q) of the terbium analogues of 1, 3, and 4 were determined using time-based fluorescence microscopy measurements. The fluorescence decay rates of the TbIII analogues in water and D2O with and without ZnII present were measured to calculate q. Because of the efficient vibronic coupling of the TbIII excited state to the O–H oscillators compared to that of the O–D oscillators, a shorter luminescence lifetime is observed in H2O than in D2O.23 The number of coordinated water molecules is then calculated using eq 1.24,25 The fluorescence lifetimes and calculated q values are summarized in Table 2. These measurements confirm a change in the coordination environment for Gd-daa3 and Gd-apa3 from a q = 0 complex to a q = 1 complex. The hydration numbers for Gd-apa3 provide further evidence of an eight-coordination GdIII complex. In the absence of ZnII, the eight coordinating arms bind GdIII with no open site for water access, while in the presence of ZnII, there is one inner-sphere water molecule bound to GdIII along with the seven coordinating groups from the chelate. The hydration numbers calculated for Gd-dpa3 confirm that in the absence of ZnII the pyridyl groups do not bind GdIII. Therefore, the hydration number in the absence and presence of ZnII remains unchanged.
Table 2.
Fluorescence Decay Lifetimes of 1, 3, and 4 in H2O and D2O and Their Calculated q Values
| τH2O (ms) | τD2O (ms) | q | τH2O (ms) + ZnII | τD2O (ms) + ZnII | q + ZnII | |
|---|---|---|---|---|---|---|
| 1 | 1.97 | 2.71 | 0.3 | 1.46 | 2.65 | 1.0 |
| 3 | 1.76 | 2.21 | 0.2 | 1.63 | 2.67 | 0.8 |
| 4 | 1.21 | 2.33 | 1.4 | 1.18 | 2.33 | 1.5 |
| (1) |
Europium 13C NMR Spectroscopy
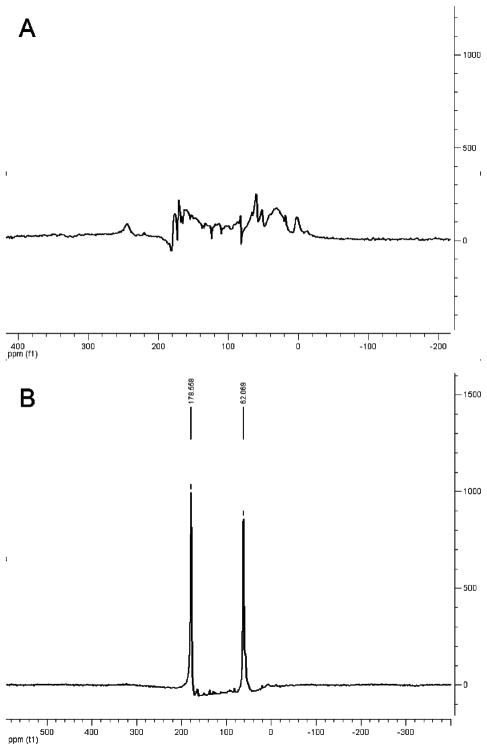
In order to investigate the ZnII binding capability of the aminoacetate arms, the EuIII analogue of Gd-daa3 was synthesized with 13C isotopic labeling of the aminoacetate groups. In the absence of ZnII, no carbon shifts are visible because of the line-broadening effects of the paramagnetic EuIII metal center when the aminoacetate arms are bound to EuIII (Figure 3A). Upon the addition of ZnII to the same sample, two peaks are seen in the 13C NMR spectrum corresponding to the two carbon atoms on the aminocetates with shifts at 178 ppm for the carbonyl and 62 ppm for the methylene (Figure 3B). When bound to ZnII and not bound directly to the EuIII metal, the line broadening due to the paramagnetism is reduced, resulting in two carbon shifts from the 13C-labeled aminoacetates that were not previously seen. These results provide direct evidence of the interaction of the aminoacetates with the ZnII and EuIIImetal centers.
Figure 3.
(A) 13C NMR in the absence of ZnII of Eu-daa3 and (B) 13C NMR in the presence of 1 equiv of ZnII with Eu-daa3 providing evidence of the interaction of the aminoacetate groups with ZnII and EuIII.
Discussion
The four complexes investigated in this study provide evidence of the mechanism of activation for ZnII-activated contrast agents. The first of this series of ZnII-activated contrast agents, Gd-daa3, has two aminoacetate arms that are proposed to vary metal-binding coordination upon the addition of ZnII.20 Three new complexes were synthesized in which the ZnII binding groups are modified with one or two pyridyl groups (Gd-apa3 and Gd-dpa3, respectively) or with the complete removal of one of the coordinating aminoacetate arms (Gd-aa3). Investigation of the relaxivities and q values determined that two structural requirements were necessary for the preparation of ZnII-activated MRI agents. The first requirement is the presence of at least one aminoacetate to create a coordinatively saturated complex before binding of ZnII. The second requirement is the need for at least two ZnII coordination groups to effectively bind ZnII and create a high-relaxivity state when ZnII is present.
Evidence of the efficient reduction of water access with only one aminoacetate coordinating arm present is demonstrated by the low-relaxivity values of complexes 1–3 in the absence of ZnII. The hydration numbers of Tb-daa3 and Tb-apa3 before the addition of ZnII indicate a coordinatively saturated q = 0 complex. However, the high-relaxivity value of Gd-dpa3 in the absence of ZnII and the hydration number of Tb-dpa3 are indicative of a coordinatively unsaturated structure in which water can access the GdIII metal center, providing further evidence of the need for one aminoacetate to create a low-relaxivity complex. The slight decrease of relaxivity observed for Gd-dpa3 in the presence of ZnII may be due to a more rigid conformation that reduces the access of water to GdIII. While the hydration numbers remain statistically unchanged for Gd-dpa3 in the presence and absence of ZnII, the observed decrease in relaxivity could be interpreted as a change in the second-shell solvation environment.
The second criterion is that ZnII binding can only occur if there are at least two ZnII-coordinating arms present. The addition of ZnII increases the relaxivity by more than 100% for Gd-daa3 and Gd-apa3. In the case of Gd-aa3, only one coordinating arm is available for ZnII binding and can therefore not coordinate ZnII to create a change in relaxivity in the presence of ZnII. NMR spectroscopy of 13C-labeled aminoacetates of Eu-daa3 provides evidence of the ZnII coordination to the aminoacetates to support the mechanism of activation presented in Figure 1, where a change in the coordination number from nine to eight is observed upon ZnII binding.
A thorough understanding of the mechanism of ZnII activation of the MRI contrast agent Gd-daa3 described in this work is important for the development of new agents with a range of binding constants for ZnII. We have determined that one of the aminoacetate arms can be modified with a variety of functional groups while still maintaining the ability to modulate q, which is necessary for activation of the contrast agent.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering Grant 1 R01 EB005866-01 and the Nanomaterials for Cancer Diagnostics and Therapeutics under Grant 5 U54 CA1193 41-02. J.L.M. is a Scholar of the Chicago Chapter of the ARCS (Achievement Rewards for College Scientists) Foundation. R.M.B. received an NSEC undergraduate research fellowship for support of this work.
References
- 1.Merbach A, Toth E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. John Wiley & Sons, Ltd; New York: 2001. [Google Scholar]
- 2.Caravan P, Ellison JJ, McMurry TJ, Laufer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 3.Solomon I. Phys Rev. 1955;99:559–565. [Google Scholar]
- 4.Bloembergen N, Morgan LO. J Chem Phys. 1961;34:842–850. [Google Scholar]
- 5.Li WH, Fraser SE, Meade TJ. J Am Chem Soc. 1999;121:1413–1414. [Google Scholar]
- 6.Li WH, Parigi G, Fragai M, Luchinat C, Meade TJ. Inorg Chem. 2002;41:4018–4024. doi: 10.1021/ic0200390. [DOI] [PubMed] [Google Scholar]
- 7.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 8.Duimstra JA, Femia FJ, Meade TJ. J Am Chem Soc. 2005;127:12847–12855. doi: 10.1021/ja042162r. [DOI] [PubMed] [Google Scholar]
- 9.Urbanczyk-Pearson LM, Femia FJ, Smith J, Parigi G, Duimstra JA, Eckermann AL, Luchinat C, Meade TJ. Inorg Chem. 2008;47:56–68. doi: 10.1021/ic700888w. [DOI] [PubMed] [Google Scholar]
- 10.Urbanczyk-Pearson LM, Meade TJ. Nat Protoc. 2008;3:341–350. doi: 10.1038/nprot.2007.529. [DOI] [PubMed] [Google Scholar]
- 11.Takeda A. Brain Res Rev. 2000;34:137–148. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- 12.Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Arch Toxicol. 2006;80:1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- 13.Frederickson CJ, Koh JY, Bush AI. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 14.Vallee BL, Falchuk KH. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Frederickson CJ, Cuajungco MP, Frederickson CJJ. Alzheimer’s Dis. 2005;8:155–160. doi: 10.3233/jad-2005-8208. [DOI] [PubMed] [Google Scholar]
- 16.Noy D, Solomonov I, Sinkevich O, Arad T, Kjaer K, Sagi I. J Am Chem Soc. 2008;130:1376–1383. doi: 10.1021/ja076282l. [DOI] [PubMed] [Google Scholar]
- 17.Hanaoka K, Kikuchi K, Urano Y, Nagano T. J Chem Soc, Perkin Trans. 2001;2:1840–1843. [Google Scholar]
- 18.Hanaoka K, Kikuchi K, Urano Y, Narazaki M, Yokawa T, Sakamoto S, Yamaguchi K, Nagano T. Chem Biol. 2002;9:1027–1032. doi: 10.1016/s1074-5521(02)00216-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X-a, Lovejoy KS, Jasanoff A, Lippard SJ. Proc Nat Acad Sci, USA. 2007;104:10780–10785. doi: 10.1073/pnas.0702393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Major JL, Parigi G, Luchinat C, Meade TJ. Proc Nat Acad Sci, USA. 2007;104:13881–13886. doi: 10.1073/pnas.0706247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hidalgo MA, Suarez-Varela J, Avila-Roson JC, Martin-Ramos JD, Romerosa A. Acta Crystallogr. 1996;C52:810–812. [Google Scholar]
- 22.Dadabhoy A, Faulkner S, Sammes PG. J Chem Soc, Perkin Trans. 2002;2:348–357. [Google Scholar]
- 23.Kropp JL, Windsor MW. J Chem Phys. 1965;42:1599–1608. [Google Scholar]
- 24.Horrocks WD, Sudnick DR. Acc Chem Res. 1981;14:384–392. [Google Scholar]
- 25.Quici S, Cavazzini M, Marzanni G, Accorsi G, Armaroli N, Ventura B, Barigelletti F. Inorg Chem. 2005;44:529–537. doi: 10.1021/ic0486466. [DOI] [PubMed] [Google Scholar]