Abstract
Human chymase is a highly efficient angiotensin II-generating serine peptidase expressed by the MCTC subset of mast cells. When secreted from degranulating cells, it can interact with a variety of circulating anti-peptidases, but is mostly captured by α2-macroglobulin, which sequesters peptidases in a cage-like structure that precludes interactions with large protein substrates and inhibitors, like serpins. The present work shows that α2-macroglobulin-bound chymase remains accessible to small substrates, including angiotensin I, with activity in serum that is stable with prolonged incubation. We used α2-macroglobulin capture to develop a sensitive, microtiter plate-based assay for serum chymase, assisted by a novel substrate synthesized based on results of combinatorial screening of peptide substrates. The substrate has low background hydrolysis in serum and is chymase-selective, with minimal cleavage by the chymotryptic peptidases cathepsin G and chymotrypsin. The assay detects activity in chymase-spiked serum with a threshold of ~1 pM (30 pg/ml), and reveals native chymase activity in serum of most subjects with systemic mastocytosis. α2-Macroglobulin-bound chymase generates angiotensin II in chymase-spiked serum, and appears in native serum as chymostatin-inhibited activity, which can exceed activity of captopril-sensitive angiotensin converting enzyme. These findings suggest that chymase bound to α2-macroglobulin is active, that the circulating complex is an angiotensin-converting enzyme inhibitor-resistant reservoir of angiotensin II-generating activity, and that α2-macroglobulin capture may be exploited in assessing systemic release of secreted peptidases.
Introduction
Human mast cell chymase cleaves angiotensin I selectively at Phe8 to generate bioactive angiotensin II (1–4). Indeed, chymase appears to be more efficient in this regard than angiotensin converting enzyme (ACE4), based on comparisons of kinetics of hydrolysis by purified enzymes (1). Chymase is not inactivated by pharmaceutical inhibitors of ACE, so it is potentially responsible for angiotensin II generated in humans treated with ACE inhibitors for hypertension. Generation of angiotensin II by chymase may explain the greater anti-hypertensive effect of ACE inhibitors combined with angiotensin II receptor blockers compared with ACE inhibitors alone (5). However, chymase and ACE belong to different enzyme classes and are made by different cells. ACE is an ecto-metallo-dipeptidase with few if any native inactivators, and is expressed mainly on the lumenal surface of vascular endothelium. On the other hand, chymase is a chymotryptic serine endopeptidase that is stored in mast cell secretory granules and potentially inactivated shortly after exocytosis by any of several inhibitors present in vast molar excess in extracellular fluids (6, 7). Because chymase is sequestered inside cells and soon encounters inhibitors when released outside, a major role in generating bioactive angiotensin may be considered unlikely. Nonetheless, pharmacological and genetic evidence in animal models suggest that generation of angiotensin II by non-ACE, chymase-like pathways are important in vasomotor dysfunction (8), vascular proliferation and stenosis (9, 10), angiogenesis (11, 12), ventricular remodeling and infarction (13, 14), aneurysm formation (15) and regulation of blood pressure (16). Currently, pharmaceutical efforts are underway to develop therapeutic inhibitors of the chymase pathway for generating angiotensin II and to inhibit effects of chymase that may relate to targets other than angiotensin I. The present studies were undertaken to explore the potential for chymase to generate angiotensin II in the bloodstream.
Initially chymase was proposed to be inactivated mainly by serpin-class inhibitors (6). However, subsequent work revealed that serpins, including α1-antichymotrypsin, are better substrates for human chymase than they are inactivators, and that much or most of chymase added to serum is inactivated by α2-macroglobulin (α2M), with which the association rate constants are highly favorable (7). This contrasts with the fate of certain other mast and leukocyte serine peptidases, including tryptase, which is too large to enter the α2M cage, and cathepsin G and neutrophil elastase, which are more rapidly inactivated by plasma serpins than by α2M (17). α2M is a major blood protein and differs from other circulating antipeptidases in key ways. It is non-specific with regard to peptidase class (serine, aspartyl, thiol, metallo) and attracts peptidases with a broad range of peptide target preferences (18). Although it attaches covalently to peptidases via a thiol ester that becomes reactive after cleavage of a target region in α2M, this connection is made with a lysine on the surface of the peptidase and does not involve the canonical antipeptidase mechanism of occupying the substrate binding site (19). Instead, α2M traps the peptidase in a cage-like structure, which is inaccessible to protein targets of the peptidase but may allow access by small substrates to the trapped peptidase. The present findings suggest that human chymase circulates bound to α2M, where it is active and can be assayed in serum using a selective, newly developed substrate. The findings further reveal that chymase captured by α2M generates angiotensin II. This suggests that chymase, after secretion by mast cells, remains active longer than once thought and may circulate bound to α2M, in which form it can generate angiotensin II.
Materials and Methods
Materials
Recombinant human prochymase was expressed in Trichoplusia ni cells and purified as previously described (20). Mature human chymase was activated from recombinant prochymase and repurified as described (20, 21). Human cathepsin G and bovine α-chymotrypsin were purchased from MP Biomedicals (Solon, OH) and Sigma (St. Louis, MO), respectively. Peptidase substrate succinyl-L-Ala-Ala-Pro-Phe-4-nitroanilide (AAPF-4NA) was from Sigma and succinyl-L-Ala-Glu-Pro-Phe-4-nitroanilide (AEPF-4NA) was from Bachem (Torrance, CA); succinyl-L-Val-Pro-Phe-4-nitroanilide (VPF-4NA) was generously synthesized and provided by Dr. John Burnier of Genentech (South San Francisco, CA). Angiotensin I was purchased from Peninsula Labs (now Bachem). Circulating antipeptidases α2M and α1-antichymotrypsin (α1ACT) were from EMD/Calbiochem (San Diego, CA) and human serum used in assay development was from Sigma.
Design and synthesis of a colorimetric substrate of chymase
Screening of recombinant human chymase with a combinatorial library of tetrapeptide substrates (21) identified Arg-Glu-Thr-Tyr or Arg-Glu-Thr-Phe as being highly favored in the P4-P1 positions on the N-terminal side of the site of hydrolysis. A synthetic inhibitor, Nα-benzoxycarbonyl-L-Arg-Glu-Thr-PheP-phosphonate synthesized based on these sequences inhibited chymase selectively in comparison with cathepsin G (21), which encouraged us to design an assay substrate, acetyl-L-Arg-Glu-Thr-Phe-4-nitroanilide (RETF-4NA) based on chymase-selective tetrapeptide sequence. RETF-4NA was custom-synthesized by Anaspec (San Jose, CA).
Assessment of concentration of active enzyme
Chymase activity was measured by addition of enzyme to buffer containing 1 mM AAPF-4NA, 45 mM Tris-HCl (pH 8.0), 1.8 M NaCl and 9% DMSO. Cathepsin G activity was measured by addition of enzyme to assay buffer containing 1 mM VPF-4NA, 0.1 M Hepes (pH 7.5), 0.5 M NaCl and 10% DMSO. Chymotrypsin activity was measured in buffer containing 1 mM AAPF-4NA, 0.1 M Hepes (pH 7.5), 0.5 M NaCl and 10% DMSO. Change in A410 nm was monitored at 25°C. The concentration of active enzyme in each preparation was determined by referencing observed activity under these conditions to reported specific activity, which is 2.1 × 106, 2.4 × 107 and 1.7 × 107 A410 nm/min/M for human cathepsin G (22), human chymase (6) and bovine chymotrypsin (23), respectively.
Kinetic comparisons of peptide-based colorimetric substrates
Hydrolysis of substrates was compared using recombinant human chymase, human cathepsin G, and bovine chymotrypsin in the presence and absence of α2M. For experiments involving α2M, each enzyme was incubated with 1000-fold molar excess of α2M in PBS (pH 7.4) at 25°C for 30 min, followed by incubation for 30 min with two-fold molar excess of α1ACT to inactivate any residual free enzyme. To assess relative sensitivity and specificity for chymase free in solution and when bound to α2M, we compared kinetic attributes of the novel substrate RETF-4NA with those of AAPF-4NA, AEPF-4NA and VPF-4NA. Substrates were dissolved in PBS (pH 7.4), containing 0.05% DMSO and 0.01% Triton X-100. Reactions were initiated by addition of free or α2M-bound enzyme. The reaction mixture was pipetted in triplicate in 180-μl aliquots into wells of a Costar 3320 flat bottom 96-well plate (Corning Life Sciences, Lowell, MA), which then was sealed with TempPlate RT Optical Film (USA Scientific, Ocala, FL) to minimize evaporation. Initial rates of nitroaniline release were measured spectrophotometrically at 410 nm and 25°C using a temperature-controlled, kinetic microplate reader (Synergy 2; BioTek, Winooski, VT). Turnover number kcat and Michaelis constant Km were determined from observed initial rates of hydrolysis over a range of substrate concentrations by nonlinear regression analysis as implemented by Prism 4 software (GraphPad, La Jolla, CA). Active enzyme concentrations used in calculating kcat from predicted maximum rates of hydrolysis were determined in separate assays under standard assay conditions for which specific activity values were available, as specified in the preceding paragraph.
Measurement of chymase activity in chymase-spiked serum
Pilot studies compared performance of substrates in serum that had been spiked with human chymase, cathepsin G, and chymotrypsin. Further pilot studies examined influence of assay duration and temperature of incubation, salt concentration, inclusion of DMSO and detergent, substrate selection (e.g., AEPF-4NA versus RETF-4NA), and mode of measurement (1-ml cuvette versus microtiter plate). Conditions were optimized for assay of native chymase activity in serum using the sealed, 96-well microtiter plate format described in the preceding paragraph. Briefly, 20 μl of serum were diluted 10-fold in 20 mM Tris-HCl (pH 7.9) containing 2 M NaCl, 0.05% DMSO, 0.01% Triton X-100 and 1 mM RETF-4NA. Change in A410 nm was measured serially in duplicate at 37°C for up to 3 h. In additional experiments, activity of chymase, cathepsin G and chymotrypsin spiked into human serum (Sigma) at a final concentration of 10 pM were compared using similar assay conditions, except that change in A410 nm was measured in 1-ml cuvettes in a Genesys 5 Spectro-photometer (Thermo Fisher Scientific, Waltham, MA).
Stability of chymase and cathepsin G in serum
Activity was compared in PBS and in enzyme-spiked serum during 8 h of incubation at 37°C. Aliquots were withdrawn at intervals to measure residual chymase and cathepsin G activity using AAPF-4NA and VPF-4NA, respectively, in 1-ml cuvettes. In additional experiments, stability to 5 cycles of freezing and thawing was examined in serum spiked with 10 ng/ml of active chymase.
Size-exclusion chromatography and immunoblotting of chymase-spiked serum
Normal human serum (100 μl) spiked with 340 ng of active human chymase or prochymase was chromatographed using an AKTA Purifier system (GE Healthcare, Piscataway, NJ) on a Superose 6 GL 10/300 size-exclusion column equilibrated with PBS. Outflow was monitored for absorbance at 280 nm. Aliquots of column fractions were assayed for amidolytic activity with RETF-4NA in 96-well format as noted for chymase-spiked serum. Aliquots from each fraction were divided into 6 pools covering distinct molecular weight regions. Portions of each pool were electrophoresed, electroblotted to a polyvinylidene difluoride membrane and probed with anti-human α2M monoclonal antibody 2D9 (Abcam, Cambridge, MA) and anti-human chymase (CC-1, Abcam). The column was calibrated with thyroglobulin (669 kDa), apoferritin (460 kDa), and bovine serum albumin (66 kDa). Human chymase was also chromatographed in PBS to establish elution behavior in the absence of α2M and other serum proteins.
Recruitment and pathological stratification of subjects with mastocytosis
Study participants were evaluated at the National Institutes of Health (Bethesda, MD) as part of Institutional Review Board-approved research protocols exploring the pathogenesis of mastocytosis. Twenty-five patients who met World Health Organization criteria for mastocytosis between 2003 and 2008 were included (24). The 15 adult subjects were classified as follows: 13 with indolent systemic mastocytosis and 2 with aggressive systemic mastocytosis. Of the 10 pediatric subjects, 7 were classified as indolent systemic mastocytosis and 3 ascutaneous mastocytosis.
Measurement of immunoreactive tryptase in subjects with mastocytosis
As part of establishing World Health Organization diagnostic criteria for systemic mastocytosis, a serum total tryptase level was obtained for all participants. Patient serum was collected at the National Institutes of Health and frozen to −20°C. It was subsequently shipped to the Mayo Medical Labs (Rochester, MN), where a serum total tryptase level was measured via fluorescence enzyme immunoassay, with normal level of <11.5 ng/ml, according to the laboratory. Serum for the chymase experiments was handled and mailed to the San Francisco Veterans Affairs Medical Center in a similar manner.
Measurement of chymase activity in subjects with mastocytosis
Serum from subjects with mastocytosis was assayed in duplicate for RETF-4NA-hydrolyzing activity in sealed microtiter plates as described for assays of chymase-spiked serum. RETF-4NA-hydrolyzing activity was measured in duplicate in separate aliquots of the same serum samples after pre-incubation with 100 μM chymostatin, a chymase inhibitor. Activity observed in the presence of chymostatin was considered background. The difference in ΔA410 nm measured with and without chymostatin was considered to be chymase-like activity. Concentration of active chymase in native samples was determined by extrapolation from standard curves generated using serum spiked with known concentrations of recombinant active chymase.
Assessment of angiotensin II-generating activity in chymase bound to inhibitors
Active chymase (1 pmol) was incubated in 7 μl of PBS at 37°C for 15 min with 5 pmol of human α2M or 5 pmol of human α1ACT. To verify the reaction of chymase with α2M, 1 pmol of chymase was first incubated in PBS at 37°C for 15 min with 5 pmol of α2M and then for 15 min with 5 pmol of α1ACT. Following these incubations, 1 μl of the resulting mixtures (containing 170 fmol of chymase) was incubated with 1 nmol of angiotensin I in 50 μl of PBS for 30 min at 37°C. Reactions were terminated by addition of 1 μl of 12 N HCl, diluted with 60 μl of an aqueous solution of 10% acetonitrile/0.1% trifluoroacetic acid, and injected onto a 2.1 × 250 mm BioBasic C-18 column (Thermo Scientific, Waltham, MA) equilibrated in 10% acetonitrile/0.1% trifluoroacetic acid on the AKTA purifier system (GE Healthcare). Angiotensin I and cleavage products were eluted with a linear gradient of 10–40% acetonitrile over 2.7 ml (3 column volumes). Outflow was monitored for A280 nm. Chromatograms were analyzed using Unicorn 5.0 software (GE Healthcare).
Angiotensin generation by chymase in native human serum
One microliter of patient serum or serum spiked with various amounts of recombinant human chymase was incubated with 20 nmol of angiotensin I in PBS for 16 h at 37°C with or without 2 mM captopril, 0.4 mM chymostatin, or both inhibitors. Reactions were solid phase-extracted on PepClean C-18 spin columns (Thermo Scientific Pierce, Rockford, IL), eluted with 50% acetonitrile/0.1% trifluoroacetic acid, and dried by vacuum centrifugation. Pellets were resuspended in 110 μl of 10% acetonitrile/0.1% trifluoroacetic acid, and subjected to reverse-phase HPLC as described for angiotensin hydrolyzed in the presence of purified inhibitors.
Results
RETF-4NA is a sensitive and selective substrate for chymase when free or bound to α2M
As revealed in Fig. 1 and Table I, the kinetic performance of the colorimetric substrates compared in this study differ markedly. For chymotrypsin, the best substrate in terms of maximum hydrolytic rate is AAPF-4NA, which is much less rapidly hydrolyzed by cathepsin G and chymase, although this commercially available substrate has been used by investigators to assay all three peptidases. For cathepsin G, the best substrate by far was VPF-4NA, although this peptidase is weak overall compared with chymotrypsin and chymase (as revealed by kcat/Km specificity constants in Table I). Consequently, VPF-4NA is more efficiently hydrolyzed by chymotrypsin and chymase than by cathepsin G and it has comparatively little ability to discriminate among these enzymes. For chymase, VPF-4NA and RETF-4NA are the best of the substrates examined and yield similar specificity constants. However, as revealed by Fig. 1B and as hypothesized from results of combinatorial screening, our novel substrate RETF-4NA is substantially more selective than the other substrates for chymase in comparison with cathepsin G and chymotrypsin. When kcat/Km specificity constants are compared for enzymes incubated with substrate in PBS, the ratios for chymase, chymotrypsin and cathepsin G are 15: 8.5: 1 for AEPF-4NA and a much more-selective 55: 8.0: 1 for RETF-4NA. A selectivity advantage is also noted for AEPF-4NA incubated with α2M, as shown in Table I. Based on these sensitivity and selectivity profiles, AEPF-4NA and RETF-4NA were tested as candidate substrates with which to construct a serum-based, chymase-selective assay.
FIGURE 1.
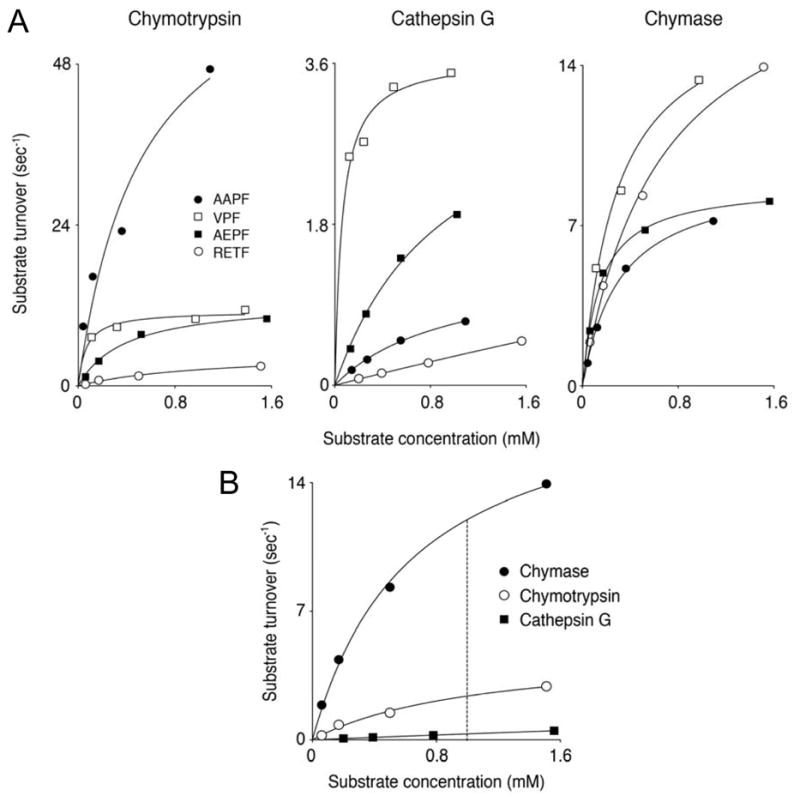
Screening of peptidyl nitroanilide substrates against chymase and related chymotryptic peptidases. Panel A reveals performance of chymotrypsin, cathepsin G and chymase versus standard substrates AAPF-4NA, VPF-4NA, and AEPF-4NA in comparison with that of novel substrate RETF-4NA, which was synthesized based on results of combinatorial screening of chymase with peptide substrates. To facilitate comparison between enzymes, results are normalized to substrate turnover (molecules of substrate hydrolyzed per second by each molecule of active enzyme). Panel B compares performance of the chymotryptic peptidases versus novel substrate RETF-4NA. The dashed vertical line indicates the concentration of RETF-4NA used in this work in assays of chymotryptic activity in serum.
Table I.
Kinetic comparisons of peptidyl nitroanilide hydrolysis by chymotryptic peptidases
| AAPF-4NA | AEPF-4NA | RETF-4NA | VPF-4NA | ||
|---|---|---|---|---|---|
| Chymase | kcata | 9.3 ± 0.8 | 8.8 ± 0.2 | 19.7 ± 0.1 | 17.2 ± 0.4 |
| Kma | 0.31 ± 0.07 | 0.15 ± 0.01 | 0.64 ± 0.05 | 0.29 ± 0.04 | |
| kcat/Kma | 30 | 59 | 31 | 59 | |
| + α2M | kcat | 49 ± 2 | 51 ± 1 | 87 ± 1 | 8.9 ± 0.6 |
| Km | 0.28 ± 0.02 | 0.08 ± 0.08 | 0.32 ± 0.01 | 0.16 ± 0.03 | |
| kcat/Km | 170 | 640 | 270 | 55 | |
| Cathepsin G | kcat | 1.4 ± 0.1 | 3.7 ± 0.8 | 7.3 ± 11.9 | 13.8 ± 0.4 |
| Km | 0.97 ± 0.06 | 0.93 ± 0.08 | 13 ± 36 | 0.69 ± 0.07 | |
| kcat/Km | 1.4 | 3.9 | 0.56 | 20 | |
| + α2M | kcat | 0 ± 0 | 0.71 ± 0.26 | 0 ± 0 | 2.4 ± 2.1 |
| Km | - | 0.12 ± 0.15 | - | 0.8 ± 1.4 | |
| kcat/Km | - | 5.9 | - | 3.0 | |
| Chymotrypsin | kcat | 68 ± 8 | 12.8 ± 0.5 | 5.0 ± 0.4 | 11.8 ± 0.4 |
| Km | 0.52 ± 0.14 | 0.39 ± 0.04 | 1.1 ± 0.2 | 0.07 ± 0.01 | |
| kcat/Km | 130 | 33 | 4.5 | 170 | |
| + α2M | kcat | 48 ± 1 | 31 ± 1 | 3.9 ± 0.4 | 22 ± 1 |
| Km | 0.035 ± 0.003 | 0.17 ± 0.02 | 0.057 ± 0.026 | 0.037 ± 0.007 | |
| kcat/Km | 1400 | 180 | 68 | 59 | |
Units of turnover number kcat, Michaelis constant Km and specificity constant kcat/Km are s−1, mM, and s−1 mM−1, respectively
Chymase measured with high selectivity and sensitivity in chymase-spiked serum
In pilot experiments (not shown), background activity in serum was higher when using AEPF-4NA than when using RETF-4NA. We tested AEPF-4NA because it is commercially available and because our laboratory previously identified a preference by chymase for peptidic substrates with Glu in the P3 position, i.e., three residues on the N-terminal side of the site of hydrolysis (21). Indeed, as shown in Fig. 1 and Table I, AEPF-4NA is a better substrate for chymase than for the other peptidases. However, it is not as selective as our newly designed, custom-synthesized substrate RETF-4NA, which is fully optimized to match preferences identified by combinatorial substrate screening. To test selectivity of RETF-4NA in serum, equal amounts (final concentration 10 pM) of active chymase, chymotrypsin and cathepsin G were added separately to aliquots of low-background serum containing 1.4 mM RETF-4NA, which is well above the predicted Km of chymase and chymotrypsin but likely well below that of cathepsin G. At this concentration of substrate, the chymotrypsin activity was 10% that of chymase, and the activity of cathepsin G was undetectable. This was as predicted by kinetic screening of enzyme-substrate combinations in PBS and α2M (Table I), which revealed that rates of hydrolysis at substrate concentrations well above Km, as reflected by kcat values, are much higher for chymase than for chymotrypsin, and that hydrolysis of cathepsin G is nearly undetectable for cathepsin G when pre-incubated with α2M. More detailed kinetic evaluation of RETF-4NA hydrolysis by chymase in spiked serum revealed kcat of 9.6 ± 0.3 s−1 and Km of 0.48 ± 0.03 mM (yielding nominal kcat/Km of 20 s−1 mM−1). The kcat and kcat/Km estimates in this case are minimum values because they assume that all chymase added to serum remains active, which is likely not to be the case. The net effect is that chymase added to serum behaves similarly to the same concentration of chymase added to PBS, in terms of overall kinetic performance. The better performance of α2M-bound chymase in serum is probably offset by loss of a portion of the pool of active enzyme due to inhibition by other antipeptidases.
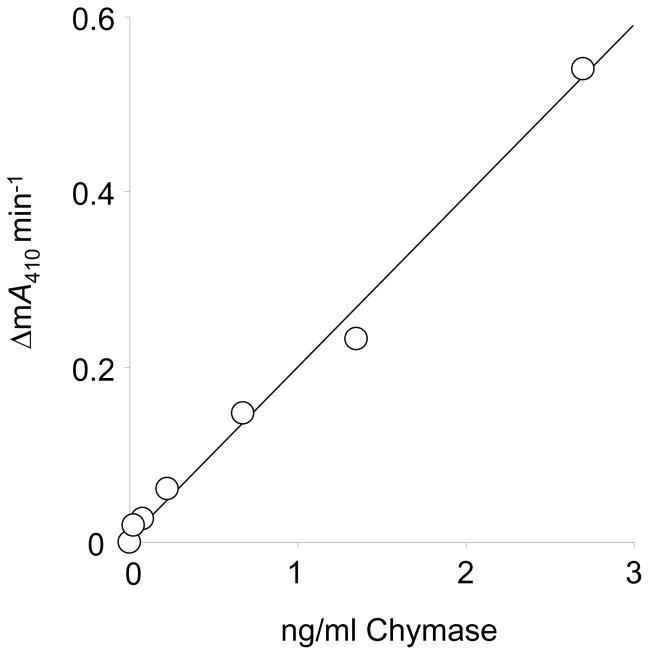
As shown in Fig. 2, chymase activity can be measured in serum over a large range of enzyme concentrations without evidence of a plateau effect or deviation from linearity. This is evidence that the assay has a wide dynamic range, likely reflecting the large molar excess of α2M available to engage chymase. The assay also is sensitive, being capable of detecting active chymase in serum at or above ~1 pM.
FIGURE 2.
RETF-4NA hydrolyzing activity in chymase-spiked serum. Human serum was spiked with recombinant human chymase over the range of concentrations of enzyme indicated by the x-axis. There is a strong linear correlation between concentrations of chymase in spiked serum and observed rates of substrate hydrolysis, as reflected by change in milli-Absorbance (ΔmA410 nm) per minute. Standard curves based on this relationship allow determination of levels of chymase-like activity in native serum, as in Fig. 4.
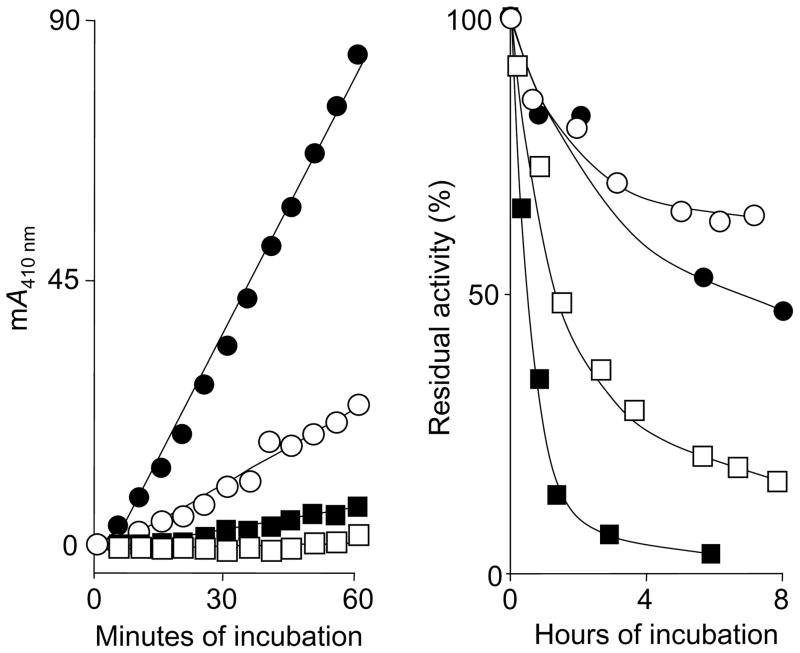
Serum stabilizes chymase and cathepsin G activity
As shown in Fig. 3, chymase activity in serum is remarkably stable to assay over time in serum, as compared with PBS. This stability contributes to the enhancement of assay sensitivity achieved by prolonged incubation.
FIGURE 3.
Stability of chymase and cathepsin G activity in serum. The left panel shows examples of repeated measurements of A410 nm in single wells of a 96-well plate in a kinetic spectrophotometer at 37°C. Individual wells contained RETF-4NA substrate (0.5 mM) and spiked chymase concentrations as follows: ●, 2.1 ng/ml; ○, 0.68 ng/ml; ■, 0.23 ng/ml, and □, 0.08 ng/ml. The right panel shows results of long-term incubations of human cathepsin G and chymase in serum (○, cathepsin G; ●, chymase) and PBS (□, cathepsin G; ■, chymase). Enzyme spiked serum and PBS were incubated as stocks for 8 h. Aliquots were withdrawn at the indicated intervals and subjected to cuvette-based spectrophotometric assay of chymotryptic activity using AAPF-4NA for chymase and VPF-4NA for cathepsin G. Results are expressed as percentage of activity relative to activity measured at the start of incubation.
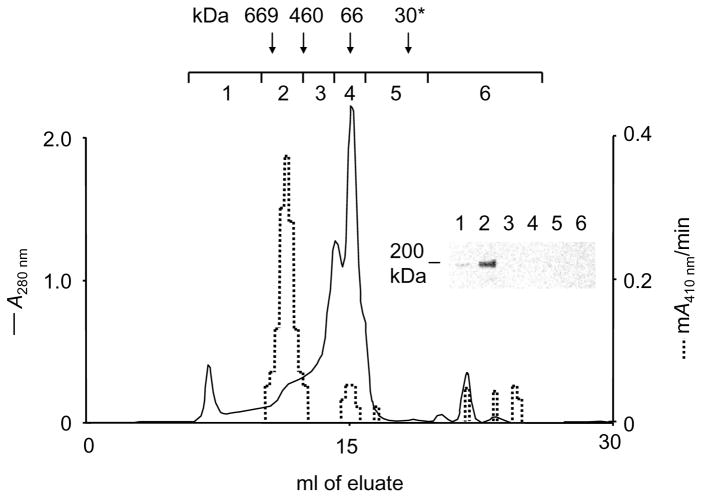
Chymase activity in serum co-elutes with α2M
As shown in Fig. 4, the vast majority of activity of chymase, when the enzyme is spiked into serum and size-fractionated by gel chromatography, elutes at an apparent Mr consistent with capture by α2M. Furthermore, α2M immunoreactivity of α2M in column fractions co-elutes with the peak of chymase activity, providing further evidence that chymase, when mixed with the complex mixture of peptidase inhibitors and other proteins in serum, binds mainly to α2M, which preserves its activity.
FIGURE 4.
Co-elution of chymase activity with α2M in serum. The chromatogram represented by the solid line shows absorbance of Superose 6 fractions generated by serum subjected to gel filtration chromatography in PBS. Downward arrows indicate elution positions of standard proteins of known size applied separately to the column in PBS. The asterisk indicates chymase (~30 kDa) applied to the column in PBS. The dashed line indicates levels of chymase-like (RETF-4NA-hydrolyzing) activity assessed in individual fractions of eluate generated by serum pre-mixed with active chymase. Fractions were also collected in six larger pools, as indicated, then concentrated and subjected to reducing SDS-PAGE and immunoblotting using antibodies recognizing α2M. Results, as revealed by the immunoblot, reveal strongest α2M-immunoreactivity in pool 2, which also contains most of the chymase activity.
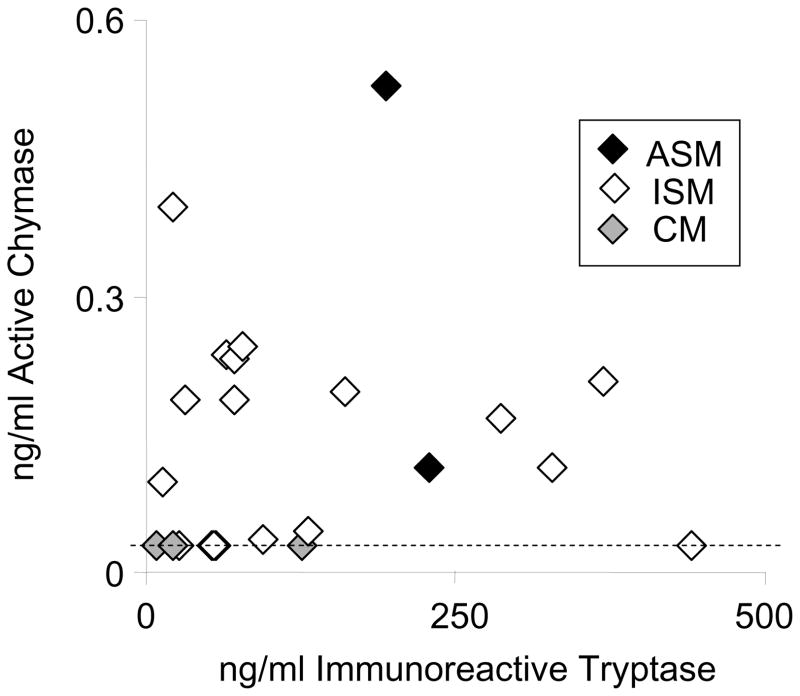
Detection of chymase activity in serum of subjects with mastocytosis
As shown in Fig. 5, the majority of subjects with systemic mastocytosis have detectable serum chymase-like activity, which correlates weakly with serum levels of immunoreactive total tryptase. Levels of active chymase in ng/ml are always lower than those of immunoreactive tryptase in paired samples. The highest chymase activity is in serum from a subject with aggressive systemic mastocytosis. Nearly all of the samples from subjects with indolent systemic mastocytosis have readily detectable chymase-like activity. However, all three of the subjects with cutaneous mastocytosis have low levels at or near the threshold for detection.
FIGURE 5.
Serum chymase activity in mastocytosis. Chymase activity and immunoreactive mast cell tryptase was measured in serum from subjects with various types of mastocytosis. Chymase activity was measured using the RETF-4NA microtiter plate assay. Total immunoreactive α plus βserum tryptase (protryptase plus mature tryptase) was measured by enzyme-linked immunosorbent assay. Each symbol represents data from a single subject and sample. The dashed line indicates the active chymase detection threshold (~0.03 ng/ml) of the assay. Mastocytosis abbreviations are as follows: ASM, aggressive systemic mastocytosis; ISM, indolent systemic mastocytosis; CM, cutaneous mastocytosis.
Chymase captured and protected from serpins by α2M generates angiotensin II
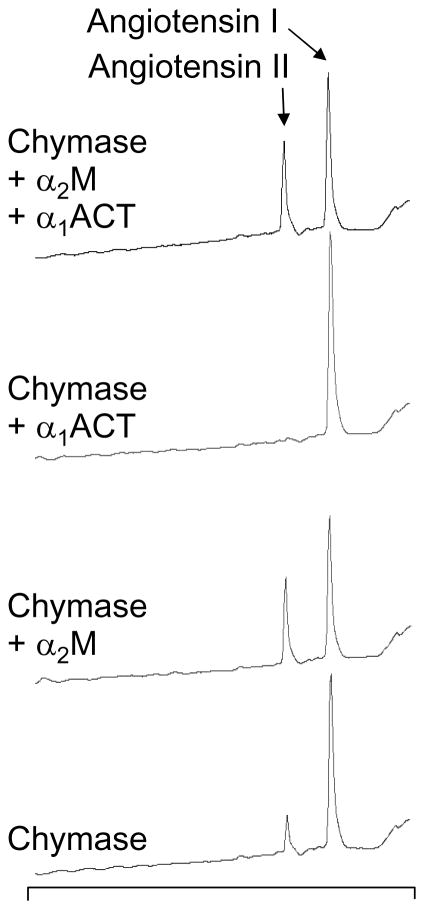
As revealed in the examples of chromatograms in Fig. 6, chymase generates bioactive angiotensin II from angiotensin I when preincubated with α2M, but has no detectable converting activity when preincubated with the serpin α1ACT. However, when preincubated with α2M and α1ACT together, chymase’s angiotensin II-generating capacity is preserved, consistent with chymase reacting more slowly with α1ACT than with α2M, and gaining protection within the α2M “cage” from inhibition by the serpin. This finding also reveals that the size of the cage in chymase-bound α2M is sufficiently large to admit the decapeptide angiotensin I, which is more than twice the length and mass of any of the tri- and tetra-peptide nitroanilide substrates used in this work to develop the serum chymase assay. The results shown in Fig. 6 also suggest that rates of hydrolysis by α2M-bound chymase are, if anything, greater than for chymase free in solution.
FIGURE 6.
Generation of angiotensin II by α2M-captured chymase. These HPLC chromatograms reveal products resulting from incubation of angiotensin I with chymase alone, chymase plus α1ACT, chymase plus α2M, or chymase plus the combination of α1ACT and α2M. Absorbance of the eluate was monitored continuously at 260 nm. Incubation time and chymase concentration were the same in each reaction and were selected so that digestion would allow visualization of parent as well as product peptides. Elution positions of angiotensin I and the bioactive product angiotensin II are as noted.
Chymase generates angiotensin II in serum
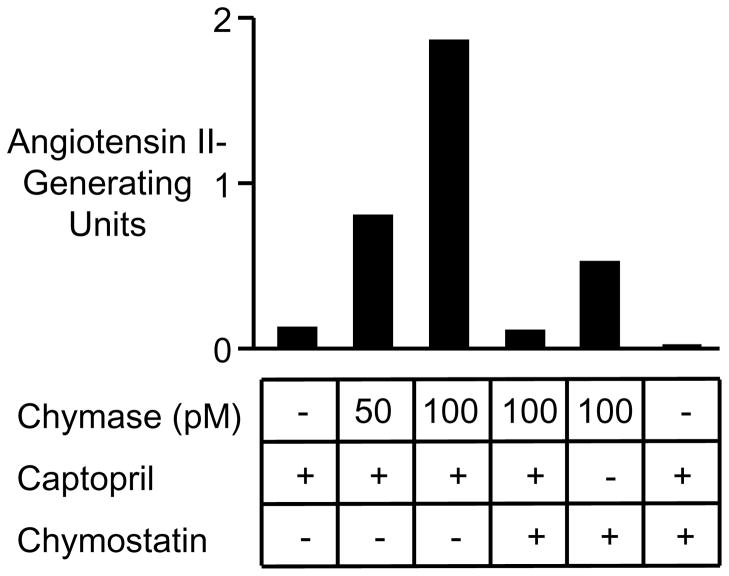
As shown in Fig. 7, the combination of chymase and captopril almost fully ablates angiotensin-generating capacity of native and chymase-spiked serum alike. The sample of serum used in the studies in Fig. 7 was chosen for its low baseline chymase-like (i.e., chymostatin-sensitive) activity to allow exploration of concentration-responsiveness to chymase in spiking experiments. Addition of chymase to serum increases chymostatin-sensitive angiotensin II-generating activity in proportion to the concentration of added chymase. These findings establish that chymase can generate angiotensin II in serum. In the sample of serum used in the Fig. 7 studies, native ACE-like (captopril-sensitive) activity is ~4-fold greater than that of native chymase-like (chymostatin-sensitive) activity, as reflected by relative angiotensin II-generating capacity revealed in the first, fourth and fifth bars of the graph. In other samples (not shown), native chymase-like angiotensin II-generating activity exceeds ACE-like activity. These findings suggest that chymase can generate angiotensin II in native serum and that its contribution can be similar to or even greater than that of soluble ACE.
FIGURE 7.
Generation of angiotensin II by serum chymase. The graph shows results of measurement of angiotensin II-generating capacity of native serum and of the same sample spiked with recombinant human chymase (50 or 100 pM). Samples were pre-incubated with an ACE inhibitor (captopril), a chymase inhibitor (chymostatin), or with both inhibitors, as indicated.
Discussion
This work reveals that an active form of human chymase circulates in the bloodstream bound to α2M, where it can cleave small peptide substrates, including angiotensin I. We exploited α2M binding to develop a sensitive and specific assay for chymase activity in the serum of subjects with mastocytosis. These studies reveal that chymase, after secretion by mast cells and capture by α2M, can cleave small peptides for a longer time than once thought possible. Chymase captured and protected by α2M, which affords protection from serpins and other fully inactivating inhibitors, may be an important source of non-ACE-generated angiotensin II near tissue sites of mast cell degranulation. The portion of α2M-caged chymase making its way to the bloodstream provides the basis of our activity-based serum assay, and may be a mobile source of active chymase that generates angiotensin in blood and in tissue locations remote from original sites of mast cell degranulation.
Under the optimized conditions of our assay, the activity of chymase bound to α2M in serum is remarkably stable, especially compared to stability of pure chymase in PBS or of chymase combined with serpins. This stability, which allows prolonged incubations with chymase substrates, was exploited to increase the sensitivity of the serum assay. The half-life of chymase-α2M complexes in vivo is not known, but is likely to be much shorter than that of α2M unattached to a peptidase. This is because α2M-peptidase complexes cleaved in the bait region undergo a conformational change, which is recognized by receptor proteins in the liver and elsewhere, which remove the complex from the circulation.
The assay we report in this work is an alternative to the development and application of immunoassay-based assays. Although antibodies raised against human chymase work well in immunohistochemical applications and in blotting of purified chymase, they are less unsuccessful as components of immunoassays for detecting chymase in complex biological fluids, including serum (24). This may be because most chymase released from mast cells becomes covalently linked to (and caged by) α2M, in which form its major antibody-binding epitopes may be shielded from interacting productively with antibodies. To our knowledge, the only reported successful use of an immunoassay to detect chymase in human serum was in post-mortem specimens in cases of anaphylaxis (25). However, in the vast majority of cases the level of chymase determined by immunoassay is below the level of detection (24, 25). Unlike activity-based assays, immunoassays have the potential to detect prochymase, denatured chymase, and other proteolytically inactive forms, which are not expected to be captured by α2M because they are unable to cleave the bait region.
Investigations in mice suggest that little if any prochymase is stored by mast cells, except in the case of animals lacking the intracellular chymase-activating enzyme dipeptidylpeptidase I (26). In humans, it is not known whether there is constitutive release of prochymase from mast cells in tissues. However, if a major portion of chymase were released in the proenzyme form, one would expect greater ease in developing immunoassays, since prochymase is not captured byα2M. In contrast, the great majority of circulating immunoreactive β tryptases, which are produced by most human mast cells, is thought to be immature, inactive pro-enzyme (27, 28). This is also true of α tryptases in humans who possess α genes (29). However, levels of mature β tryptases can rise substantially in some subjects shortly after anaphylaxis (30), presumably because the active tryptase tetramer, which is much larger than monomeric chymase, is too big to be engulfed by the α2M cage (31). The weak correlation of active chymase levels with tryptase levels in our mastocytosis samples--as well as the major difference between tryptase and chymase in the range of measured concentrations--may relate to major disparity between tryptases and chymase in the extent to which mast cells release the two peptidase types as proenzymes. If human chymase, unlike tryptases, is released mainly from granules via a regulated pathway, then there is the potential that chymases are released acutely in larger amounts in settings of anaphylaxis, which is a possibly we are exploring. Nonetheless, it seems likely that chymase activity in serum is influenced by total body burden of mast cells, in that in vitro assays of mast cells suggest that chymase “leaks” from mast cell granules in the absence of specific stimulation at a low but steady rate (32). This hypothesis is consistent with our assay results in subjects with mastocytosis, in that chymase levels are much higher in systemic mastocytosis than in more localized cutaneous mastocytosis. Nonetheless, a few subjects with the indolent subtype of systemic mastocytosis had levels at or below the level of detection, which may reflect low mast cell burden or possibly variations in peptidase phenotype, because of mastocytosis cells can vary in relative expression of tryptases and chymase (33).
The present studies underscore the potential value of combinatorial peptide substrate screening in the development of selective substrates for specific peptidases. Our custom-developed substrate RETF-4NA was synthesized for the present studies based on results of a screen of ~160,000 potential peptide substrates varying in amino acid composition at positions P1 through P4 in relation to the site of hydrolysis (21). In the serum chymase assay, RETF-4NA was clearly superior to standard, available substrates of chymotryptic serine peptidases. Although the kcat/Km of RETF-4NA is similar to that of several other peptidyl nitroanilide substrates cleaved by chymase, RETF-4NA is substantially more chymase-selective than other substrates. This undoubtedly contributes to the low levels of non-specific RETF-4NA cleavage in serum, which in turn reduces the signal-to-noise ratio in the assay and provides an important boost to sensitivity. Intriguingly, RETF-4NA as well as certain of the other substrates are more avidly cleaved by chymase in its α2M-bound form than in its free, unbound form. Prior work with chymase and peptidyl nitroanilide substrates has shown that the amidolytic activity of human chymase is sensitive to salt and solvent effects. Perhaps the environment in the macroglobulin cage favors these types of interactions. Tight quarters in the α2M cage may favor interactions between substrate and cage walls, which may enhance substrate binding. However, the kinetic data summarized in Table I suggest that the effect on binding (as reflected by lowering of Km) is not as great as the effect on substrate turnover (as reflected by increases in kcat).
The observed serum chymase activity in this study is likely to have originated mainly from mast cells in extravascular sites, since mast and mastocytosis cells in mature, granulated form generally circulate in small numbers or not at all. The significance of angiotensin II generated by chymase in blood per se is unclear. Although we identified samples of serum in which chymase-like activity makes a greater contribution than ACE-like activity, most angiotensin II generation by ACE in vivo is thought to be contributed by membrane-bound ACE attached to the lumenal surface of endothelial cells, rather than by ACE shed into solution. Our data do not permit an estimate of the relative contributions of α2M-bound chymase versus endothelial cell-bound ACE, although we expect that the former is much greater than the latter. Perhaps the greatest significance of activity detected in serum is as a marker of chymase released in extravascular tissues. Local angiotensin II-generating capacity can be assumed to be much greater at sites of mast cell degranulation, before dilution in serum. Indeed, in tissues such as heart angiotensin II-generating machinery appears to be heavily compartmentalized, with ACE being largely responsible for intravascular production and chymase-like serine peptidases being responsible for extravascular (interstitial) production (34). In the absence of specific mast cell stimulation, baseline leak of chymase from resident mast cells, combined with α2M capture, could provide background production of angiotensin II, which could be responsible for proposed tonic effects on smooth muscle and stromal cells (16, 35, 36), potentially contributing to remodeling, including arteriopathy and fibrosis. In settings of acute mast cell degranulation, chymase-generated angiotensin II can be expected to spike, producing short-term effects, such as changes in caliber of skeletal muscle resistance vessels (8).
Footnotes
This work was supported in part by National Institutes of Health Grant HL024136.
Abbreviations used in this paper: ACE, angiotensin converting enzyme; α2M, α2-macroglobulin;
α1ACT, α1-antichymotrypsin; AAPF-4NA, succinyl-L-Ala-Ala-Pro-Phe-4-nitroanilide; AEPF-4NA, succinyl-L-Ala-Glu-Pro-Phe-4-nitroanilide; VPF-4NA, succinyl-L-Val-Pro-Phe-4-nitroanilide; RETF-4NA, acetyl-L-Arg-Glu-Thr-Phe-4-nitroanilide
Disclosures
The authors have no conflicts of interest.
References
- 1.Reilly CF, Tewksbury DA, Schechter NB, Travis J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem. 1982;257:8619–8622. [PubMed] [Google Scholar]
- 2.Wintroub BU, Schechter NB, Lazarus GS, Kaempfer CE, Schwartz LB. Angiotensin I conversion by human and rat chymotryptic proteinases. J Invest Derm. 1984;83:336–339. doi: 10.1111/1523-1747.ep12264144. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekharan UM, Sanker S, Glynias MJ, Karnik SS, Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996;271:502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- 4.Muilenburg DJ, Raymond WW, Wolters PJ, Caughey GH. Lys40 but not Arg143 influences selectivity of angiotensin conversion by human a-chymase. Biochim Biophys Acta. 2002;1596:346–356. doi: 10.1016/s0167-4838(02)00224-8. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Liu K, Michalicek J, Angus JA, Hunt JE, Dell’Italia LJ, Feneley MP, Graham RM, Husain A. Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J Clin Invest. 2004;114:112–120. doi: 10.1172/JCI20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schechter NM, Sprows JL, Schoenberger OL, Lazarus GS, Cooperman BS, Rubin H. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989;264:21308–21315. [PubMed] [Google Scholar]
- 7.Walter M, Sutton RM, Schechter NM. Highly efficient inhibition of human chymase by α2-macroglobulin. Arch Biochem Biophys. 1999;368:276–284. doi: 10.1006/abbi.1999.1309. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Caughey GH, Gao XP, Rubinstein I. Mast cell chymase-like protease(s) modulates Escherichia coli lipopolysaccharide-induced vasomotor dysfunction in skeletal muscle in vivo. J Pharmacol Exp Therap. 1998;284:1156–1164. [PubMed] [Google Scholar]
- 9.Shiota N, Okunishi H, Takai S, Mikoshiba I, Sakonjo H, Shibata N, Miyazaki M. Tranilast suppresses vascular chymase expression and neointima formation in balloon-injured dog carotid artery. Circulation. 1999;99:1084–1090. doi: 10.1161/01.cir.99.8.1084. [DOI] [PubMed] [Google Scholar]
- 10.Nishimoto M, Takai S, Kim S, Jin D, Yuda A, Sakaguchi M, Yamada M, Sawada Y, Kondo K, Asada K, Iwao H, Sasaki S, Miyazaki M. Significance of chymase-dependent angiotensin II-forming pathway in the development of vascular proliferation. Circulation. 2001;104:1274–1279. doi: 10.1161/hc3601.094304. [DOI] [PubMed] [Google Scholar]
- 11.Muramatsu M, Katada J, Hayashi I, Majima M. Chymase as a proangiogenic factor. A possible involvement of chymase- angiotensin-dependent pathway in the hamster sponge angiogenesis model. J Biol Chem. 2000;275:5545–5552. doi: 10.1074/jbc.275.8.5545. [DOI] [PubMed] [Google Scholar]
- 12.Katada J, Muramatsu M, Hayashi I, Tsutsumi M, Konishi Y, Majima M. Significance of vascular endothelial cell growth factor up-regulation mediated via a chymase-angiotensin-dependent pathway during angiogenesis in hamster sponge granulomas. J Pharmacol Exp Ther. 2002;302:949–956. doi: 10.1124/jpet.102.034231. [DOI] [PubMed] [Google Scholar]
- 13.Chen LY, Li P, He Q, Jiang LQ, Cui CJ, Xu L, Liu LS. Transgenic study of the function of chymase in heart remodeling. J Hypertens. 2002;20:2047–2055. doi: 10.1097/00004872-200210000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Jin D, Takai S, Yamada M, Sakaguchi M, Kamoshita K, Ishida K, Sukenaga Y, Miyazaki M. Impact of chymase inhibitor on cardiac function and survival after myocardial infarction. Cardiovasc Res. 2003;60:413–420. doi: 10.1016/s0008-6363(03)00535-2. [DOI] [PubMed] [Google Scholar]
- 15.Furubayashi K, Takai S, Jin D, Muramatsu M, Ibaraki T, Nishimoto M, Fukumoto H, Katsumata T, Miyazaki M. The significance of chymase in the progression of abdominal aortic aneurysms in dogs. Hypertens Res. 2007;30:349–357. doi: 10.1291/hypres.30.349. [DOI] [PubMed] [Google Scholar]
- 16.Ju H, Gros R, You X, Tsang S, Husain M, Rabinovitch M. Conditional and targeted overexpression of vascular chymase causes hypertension in transgenic mice. Proc Natl Acad Sci U S A. 2001;98:7469–7474. doi: 10.1073/pnas.131147598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virca GD, Travis J. Kinetics of association of human proteinases with human α2-macroglobulin. J Biol Chem. 1984;259:8870–8874. [PubMed] [Google Scholar]
- 18.Borth W. α2-Macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 19.Kolodziej SJ, Wagenknecht T, Strickland DK, Stoops JK. The three-dimensional structure of the human α2-macroglobulin dimer reveals its structural organization in the tetrameric native and chymotrypsin α2-macroglobulin complexes. J Biol Chem. 2002;277:28031–28037. doi: 10.1074/jbc.M202714200. [DOI] [PubMed] [Google Scholar]
- 20.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 21.Raymond WW, Waugh Ruggles S, Craik CS, Caughey GH. Albumin is a substrate of human chymase: Prediction by combinatorial peptide screening and development of a selective inhibitor based on the albumin cleavage site. J Biol Chem. 2003;278:34517–34524. doi: 10.1074/jbc.M304087200. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Minematsu Y, Reilly CF, Travis J, Powers JC. Human leukocyte cathepsin G. Subsite mapping with 4-nitroanilides, chemical modification, and effect of possible cofactors. Biochemistry. 1985;24:2040–2047. doi: 10.1021/bi00329a036. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K, Powers JC, Ashe BM, Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the α1-protease inhibitor reactive site. J Biol Chem. 1979;254:4027–4032. [PubMed] [Google Scholar]
- 24.Satomura K, Shimizu S, Nagato T, Komeichi H, Osuga M, Katsuta Y, Aramaki T, Omoto Y. Establishment of an assay method for human mast cell chymase. Hepatol Res. 2002;24:361–367. doi: 10.1016/s1386-6346(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 25.Nishio H, Takai S, Miyazaki M, Horiuchi H, Osawa M, Uemura K, Yoshida K, Mukaida M, Ueno Y, Suzuki K. Usefulness of serum mast cell-specific chymase levels for postmortem diagnosis of anaphylaxis. Int J Legal Med. 2005;119:331–334. doi: 10.1007/s00414-005-0524-1. [DOI] [PubMed] [Google Scholar]
- 26.Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz LB, Min HK, Ren S, Xia HZ, Hu J, Zhao W, Moxley G, Fukuoka Y. Tryptase precursors are preferentially and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, Mono-Mac-6 cells, and human skin-derived mast cells. J Immunol. 2003;170:5667–5673. doi: 10.4049/jimmunol.170.11.5667. [DOI] [PubMed] [Google Scholar]
- 28.Min HK, Moxley G, Neale MC, Schwartz LB. Effect of sex and haplotype on plasma tryptase levels in healthy adults. J Allergy Clin Immunol. 2004;114:48–51. doi: 10.1016/j.jaci.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Akin C, Soto D, Brittain E, Chhabra A, Schwartz LB, Caughey GH, Metcalfe DD. Tryptase haplotype in mastocytosis: Relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. 2007;123:268–271. doi: 10.1016/j.clim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 31.Caughey GH. Tryptase genetics and anaphylaxis. J Allergy Clin Immunol. 2006;117:1411–1414. doi: 10.1016/j.jaci.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caughey GH, Lazarus SC, Viro NF, Gold WM, Nadel JA. Tryptase and chymase: comparison of extraction and release in two dog mastocytoma lines. Immunology. 1988;63:339–344. [PMC free article] [PubMed] [Google Scholar]
- 33.Horny HP, Greschniok A, Jordan JH, Menke DM, Valent P. Chymase expressing bone marrow mast cells in mastocytosis and myelodysplastic syndromes: an immunohistochemical and morphometric study. J Clin Pathol. 2003;56:103–106. doi: 10.1136/jcp.56.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dell’Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, Durand J, Hankes GH, Oparil S. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKay S, Jongste JCd, Saxena PR, Sharma HS. Angiotensin II induces hypertrophy of human airway smooth muscle cells: expression of transcription factors and transforming growth factor-β1. Am J Respir Cell Mol Biol. 1998;18:823–833. doi: 10.1165/ajrcmb.18.6.2924. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki M, Takai S, Jin D, Muramatsu M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. 2006;112:668–676. doi: 10.1016/j.pharmthera.2006.05.008. [DOI] [PubMed] [Google Scholar]