Abstract
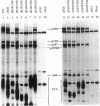
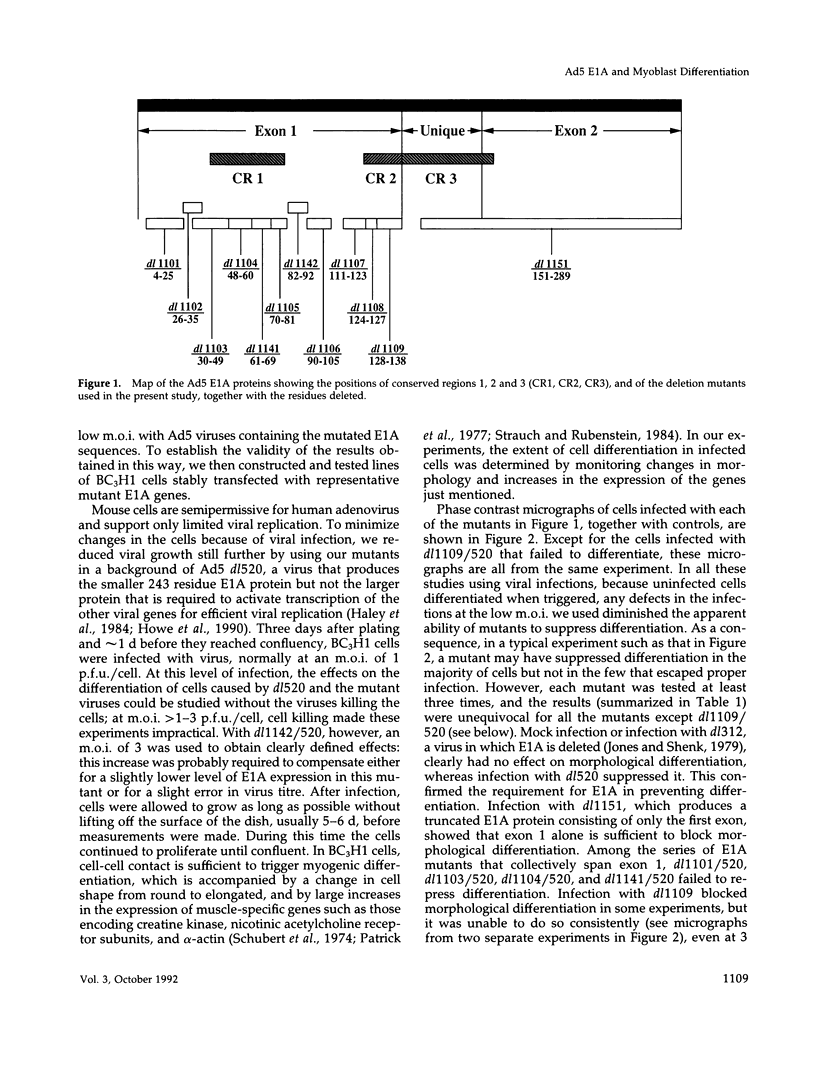
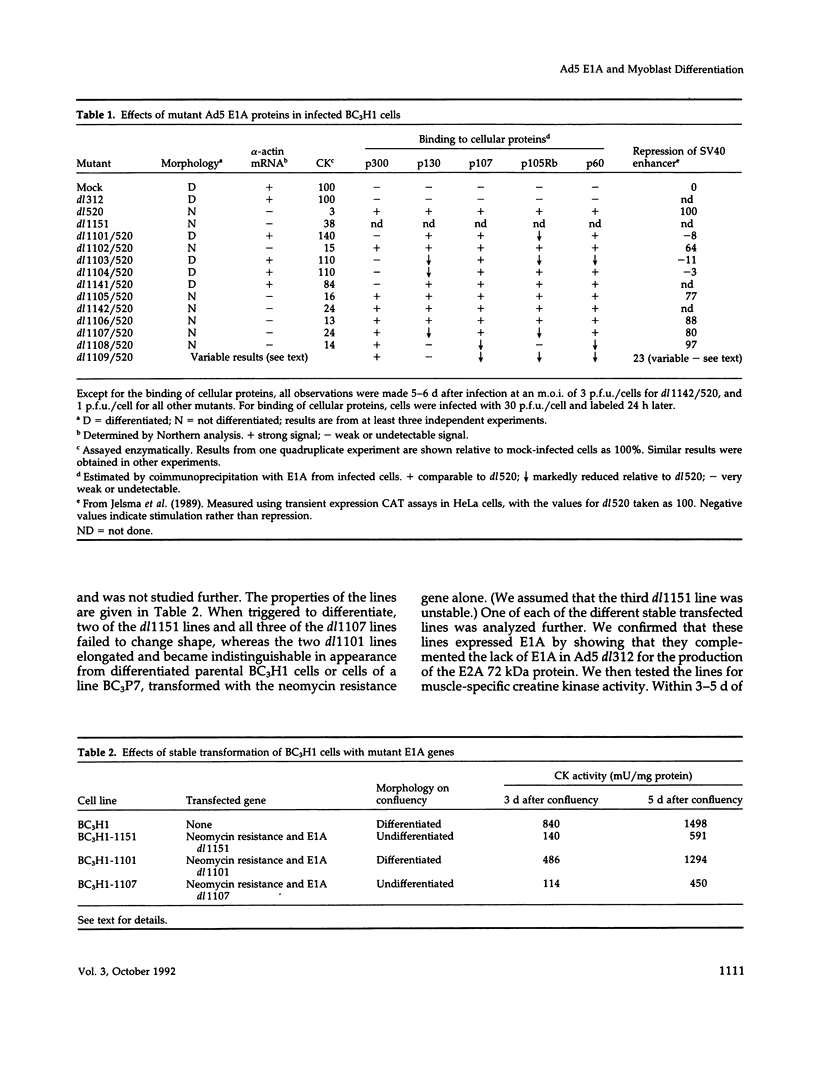
We have used deletion mutants to define the regions in Ad5 E1A proteins necessary to suppress differentiation of mouse BC3H1 myoblasts. We examined the differentiation of cells infected at a low multiplicity with viruses containing the E1A deletions and constructed so as to produce only the smaller of the two major E1A proteins. Only four of the mutant viruses containing deletions within the N-terminal 69 residues failed to suppress differentiation as judged by changes in morphology and in levels of muscle-specific alpha-actin mRNA and creatine kinase activity. The results were confirmed by analyses of lines of cells stably transfected with representative E1A mutants. The mouse cellular proteins to which mutant E1A proteins bound were identified by immunoprecipitating E1A proteins specifically from infected BC3H1 cells and by analyzing the precipitates on denaturing gels. Bands of proteins of 300, 130, 107, 105 (the retinoblastoma product), and 60 kDa (cyclin A) were distinguished. Failure to suppress differentiation correlated with loss of binding to the 300-kDa protein but not to any of the others. The regions of E1A defined in this way have been shown to be required for several other activities, including enhancer repression and transformation. One function of the 300-kDa protein appears to be to facilitate the action of transcriptional enhancers of differentiation-specific genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautista D. S., Hitt M., McGrory J., Graham F. L. Isolation and characterization of insertion mutants in E1A of adenovirus type 5. Virology. 1991 Jun;182(2):578–596. doi: 10.1016/0042-6822(91)90599-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Functions of adenovirus E1A. Cancer Surv. 1986;5(2):367–387. [PubMed] [Google Scholar]
- Braun T., Bober E., Arnold H. H. Inhibition of muscle differentiation by the adenovirus E1a protein: repression of the transcriptional activating function of the HLH protein Myf-5. Genes Dev. 1992 May;6(5):888–902. doi: 10.1101/gad.6.5.888. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkemann S. A., Konieczny S. F., Taparowsky E. J. Adenovirus 5 E1A represses muscle-specific enhancers and inhibits expression of the myogenic regulatory factor genes, MyoD1 and myogenin. Cell Growth Differ. 1990 Aug;1(8):375–382. [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Glaser L., Wice B. Control of differentiation in BC3H1 muscle cells. Environ Health Perspect. 1989 Mar;80:143–148. doi: 10.1289/ehp.8980143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Haley K. P., Overhauser J., Babiss L. E., Ginsberg H. S., Jones N. C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley L. E., Benedict S., Gleavy J., Johnson G. L. Requirement of the adenovirus E1A transformation domain 1 for inhibition of PC12 cell neuronal differentiation. Cell Regul. 1991 Jun;2(6):479–489. doi: 10.1091/mbc.2.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. A., Bayley S. T. Effects of Ad5 E1A mutant viruses on the cell cycle in relation to the binding of cellular proteins including the retinoblastoma protein and cyclin A. Virology. 1992 Jan;186(1):15–24. doi: 10.1016/0042-6822(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Howe J. A., Mymryk J. S., Egan C., Branton P. E., Bayley S. T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Evelegh C. M., Cunniff N. F., Skiadopoulos M. H., Floroff M. R., Denman J. E., Bayley S. T. Use of deletion and point mutants spanning the coding region of the adenovirus 5 E1A gene to define a domain that is essential for transcriptional activation. Virology. 1988 Apr;163(2):494–502. doi: 10.1016/0042-6822(88)90290-5. [DOI] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Patrick J., McMillan J., Wolfson H., O'Brien J. C. Acetylcholine receptor metabolism in a nonfusing muscle cell line. J Biol Chem. 1977 Mar 25;252(6):2143–2153. [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Fromental C., Chambon P. General repression of enhanson activity by the adenovirus-2 E1A proteins. Genes Dev. 1990 Jan;4(1):137–150. doi: 10.1101/gad.4.1.137. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard G., Connolly J. A. Membrane glycoproteins are involved in the differentiation of the BC3H1 muscle cell line. Exp Cell Res. 1987 Nov;173(1):144–155. doi: 10.1016/0014-4827(87)90340-5. [DOI] [PubMed] [Google Scholar]
- Stein R. W., Corrigan M., Yaciuk P., Whelan J., Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990 Sep;64(9):4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch A. R., Rubenstein P. A. Induction of vascular smooth muscle alpha-isoactin expression in BC3H1 cells. J Biol Chem. 1984 Mar 10;259(5):3152–3159. [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Nasr R. J., Chinnadurai G. An N-terminal region of adenovirus E1a essential for cell transformation and induction of an epithelial cell growth factor. Oncogene. 1988 Feb;2(2):105–112. [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a ras cooperation activity is separate from its positive and negative transcription regulatory functions. Mol Cell Biol. 1988 May;8(5):2177–2183. doi: 10.1128/mcb.8.5.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. G., Draetta G., Moran E. E1A induces phosphorylation of the retinoblastoma protein independently of direct physical association between the E1A and retinoblastoma products. Mol Cell Biol. 1991 Aug;11(8):4253–4265. doi: 10.1128/mcb.11.8.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K. A., Muscat G. E., Kedes L. Adenovirus E1A products suppress myogenic differentiation and inhibit transcription from muscle-specific promoters. Nature. 1988 Apr 7;332(6164):553–557. doi: 10.1038/332553a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yaciuk P., Moran E. Analysis with specific polyclonal antiserum indicates that the E1A-associated 300-kDa product is a stable nuclear phosphoprotein that undergoes cell cycle phase-specific modification. Mol Cell Biol. 1991 Nov;11(11):5389–5397. doi: 10.1128/mcb.11.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]