Abstract
Intravaginal inoculation of rhesus macaques with varying doses of the CXCR4 (X4)-tropic SHIVSF33A isolate revealed a threshold inoculum for establishment of systemic virus infection, and a dose dependency in overall viral burden and CD4+ T cell depletion. While exposure to inoculum size of 1000 or greater 50% tissue infectious dose (TCID50) resulted in high viremia and precipitous CD4+ T cell loss, occult infection was observed in seven of eight macaques exposed to 500 TCID50 of the same virus. The latter was characterized by intermittent detection of low level virus with no evidence of seroconversion or CD4+ T cell decline, but with signs of an ongoing antiviral T cell immune response. Upon vaginal re-challenge with the same limiting dose 11-12 weeks after the first, classic pathogenic X4 SHIVSF33A infection was established in four of the seven previously exposed seronegative macaques, implying enhanced susceptibility to systemic infection with prior exposure. Pre-existing peripheral SIV gag-specific CD4+ T cells were more readily demonstrable in macaques that became systemically infected following re-exposure than those that were not. In contrast, early presence of circulating polyfunctional cytokine secreting CD8+ T cells, or strong virus-specific proliferative responses in draining lymph nodes and in the gut associated lymphoid tissue (GALT) following the first exposure was associated with protection from systemic re-infection. These studies identify the gut and lymphoid tissues proximal to the genital tract as sites of robust CD8 T lymphocyte responses that contribute to containment of virus spread following vaginal transmission.
Introduction
Exposure to the human immunodeficiency virus (HIV) results in a spectrum of clinical outcomes. The majority of individuals exposed to HIV are infected and develop antiviral antibody responses, eventually succumbing to AIDS over a mean of 10 years in the absence of therapeutic intervention. However, it is increasingly clear that in every cohort at risk for HIV infection, individuals are present who resist infection despite multiple documented exposures to the virus. These individuals, however, have subtle signs of cellular immune recognition of HIV antigens in spite of being virus negative, suggesting that they may have acquired HIV infection but that the virus is either cleared or no longer detectable by routine methods (Haynes, Pantaleo, and Fauci, 1996; Kulkarni, Butera, and Duerr, 2003; Rowland-Jones and McMichael, 1995; Shearer and Clerici, 1996). Occult or silent infection, therefore, has been proposed to account for the findings in these exposed seronegative (ES) cases. Further, naturally acquired cellular immunity found in these individuals was hypothesized to have a protective role in the prevention from HIV re-infection (Beattie, Rowland-Jones, and Kaul, 2002; Clerici and Shearer, 1996).
Occult or silent infection, characterized by an undetectable antibody response and the ability to isolate or amplify virus from PBMC obtained at only a few time points, has also been reported after experimental simian immunodeficiency virus (SIV) challenge, a model commonly used for studying HIV-1 transmission and pathogenesis. Such infections occur following inoculation of non-human primates with low doses of virus, and are more frequently associated with mucosal (intravaginal, intrarectal or oral)(Miller et al., 1994; Pauza et al., 1993; Trivedi et al., 1996; Van Rompay et al., 1998) than intravenous routes of challenge (Petry et al., 1997; Polacino et al., 1999). Similar to findings in HIV-1 infection, virus-specific T cell immunity, such as proliferation and IFN-γ responses, has been detected (Ma et al., 2004; McChesney et al., 1998; McDermott et al., 2004; Murphey-Corb et al., 1999; Petry et al., 1997). An understanding of the mechanisms that account for the control of infection in these ES individuals and macaques should contribute greatly to the development of an effective vaccine.
We and others have used infection of macaques with pathogenic CXCR4 (X4)- or CCR5 (R5)-tropic simian/human immunodeficiency virus (SHIV) to study HIV-1 transmission and pathogenesis in vivo (Bogers, Cheng-Mayer, and Montelaro, 2000). Infection of macaques with pathogenic X4 SHIVs such as SHIVSF33A is accompanied by rapid and severe depletion of peripheral blood and lymph node CD4+ T lymphocytes, mirroring the clinical pattern in patients infected with X4 isolates that frequently emerge in late-stage disease (Harouse et al., 1998; Rowland-Jones, 2003). These infections, however, were often conducted with high doses of viral inoculum to achieve high infection rates, raising questions regarding their relevance to HIV-1 heterosexual transmission in humans, which is typically inefficient (Gray et al., 2001). To establish a nonhuman primate model that more closely mimics human sexual transmission, titration experiments with X4 SHIVSF33A were performed in rhesus macaques (RM) to identify a minimum dose that resulted in consistent virus transmission and establishment of systemic infection following intravaginal (IVAG) challenges. These studies led to the identification of an inoculum threshold that is required for establishment of a generalized infection by vaginal challenge with this virus, and showed that macaques exposed to sub-threshold doses are silently infected. Our findings identify the gut and lymphoid tissues in proximity to the genital tract as sites for robust T cell antiviral immunity that may contain virus replication locally and suggest a role for the balance of CD8 and CD4 T cell mediated responses in protection from systemic re-infection after IVAG challenge.
RESULTS
A threshold requirement for vaginal infection with X4 SHIVSF33A
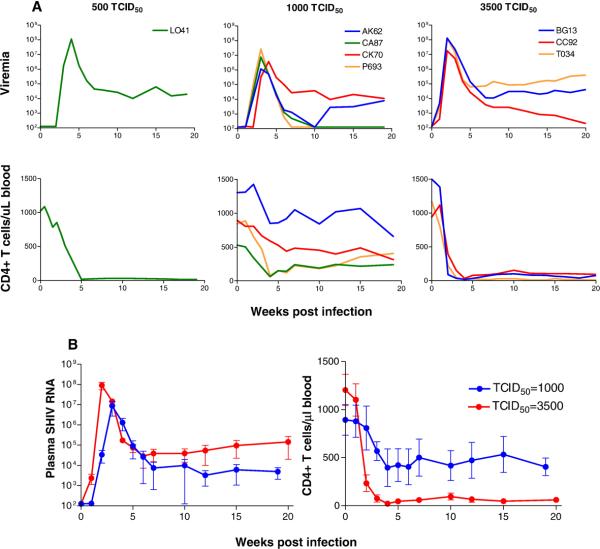
To examine the impact of inoculum dose on mucosal transmission, RM were exposed intravaginally (IVAG) to varying doses of SHIVSF33A. Six were inoculated with 50 TCID50 of the virus and four each with 500, 1000 and 3500 TCID50. Results are summarized in Table 1. None of the four macaques challenged with 50 or 500 TCID50 was systemically infected. In contrast, all four macaques inoculated once with 1000 TCID50 and three of four monkeys exposed to 3500 TCID50 of the virus were infected. Peak viremia of 106 – 108 RNA copies per ml plasma and peripheral CD4+ T cell decline were seen in all systemically infected animals (Fig. 1A). However, differences in virologic and immunologic status in macaques infected with 1000 (n=4) and 3500 (n=3) TCID50 doses were noted. There was a delay in virus replication, a 1 log unit less in mean peak viral load, viral set-point and greater absolute peripheral CD4+ T cell numbers in macaques receiving the 1000 vs the 3500 TCID50 challenge dose (Fig. 1B).
Table 1.
Infection status in macaques challenged intravaginally with varying doses of X4 SHIVsf33a. (I) and (II) designate the two groups of macaques that were challenged with 500 TCID50 of the virus.
| Dose (TCID50) |
Systemic infection (# infected/# challenged) |
|---|---|
| 50 | 0/6 |
| 500 (I) | 0/4 |
| 1000 | 4/4 |
| 3500 | 3/4 |
| 500 (II) | 1/4 |
Figure 1.
(A) Viral load and absolute CD4+ T cells counts in macaques systemically infected following IVAG exposure to 500, 1000 and 3500 TCID50 of pathogenic X4 SHIVSF33A. The viral load detection threshold is 125 RNA copies/ml plasma. (B) Comparison of the kinetics and magnitude of virus replication and peripheral CD4+ T cell loss in macaques exposed IVAG to 1000 and 3500 TCID50 of X4 SHIVSF33A. Standard deviation of the means for macaques in the 1000 TCID50 (n=4) and 3500 TCID50 (n=3) are shown.
To confirm that 500 TCID50 represents a limiting dose of the X4 SHIVSF33A inoculum, four more macaques were challenged [500 (II), Table 1]. Data showed that one of four exposed macaques (L041) was systemically infected, with peak viremia of ~108 RNA copies/ml plasma and precipitous CD4+ T cell loss (Fig. 1A). Compared to those receiving higher inoculum doses (1000 and 3500 TCID50), the rate of reaching peak viremia was slower in L041, but caution is necessary in interpreting viral load and CD4+ T cell data from only one infected monkey in this experimental group. Nevertheless, these findings suggest a threshold requirement (≥ 500 TCID50) for vaginal infection with X4 SHIVSF33A. Additionally, there appears to be a dose dependency in the extent of virus replication and CD4+ T cell loss following mucosal transmission of this virus.
Silent infection in RM exposed to a limiting dose of X4 SHIVSF33A
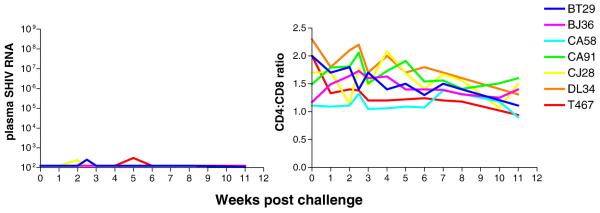
Systemic viremia was undetectable and CD4:CD8 T cell ratio measurements were within normal limits for a period of 11-12 weeks in seven of eight macaques exposed to a 500 TCID50 dose of X4 SHIVSF33A (Fig. 2). However, transient and very low level viremia of 200-300 RNA copies/ml, slightly above the detection limit of 125 copies/ml, was detected in peripheral blood of macaques BT29, CJ28 and T467 at single time points (wk2 for BT29, CJ28, and wk5 for T467). Furthermore, cell-associated SHIV DNA could be detected by PCR in PBMC of BT29, DL34 at wk5 and 7 post-exposure respectively, and in macaque T467 at two different time points sampled (wk3 and 11)(Table 2). All the animals, however, failed to seroconvert as determined by a commercially available strip immunoblot assay. SHIV DNA positivity and intermittent viremia in BT29, CJ28, DL34 and T467 signify virus replication, but level was apparently too low to sustain systemic spread of the infection. The remaining three seronegative macaques that were exposed to a limiting dose of X4 SHIVSF33A, however, appear to be largely virus-negative.
Figure 2.
Plasma viremia (RNA copies/ml) and CD4:CD8 ratios in X4 SHIVSF33A ES macaques. Macaques were exposed IVAG to 500 TCID50 X4 SHIVSF33A and monitored for virus load and CD4+ T cell loss.
Table 2.
PCR analysis of peripheral blood samples from seronegative macaques that were exposed IVAG to 500 TCID50 X4-SHIVsf33a
| Wks post- exposure |
Animal | ||||||
|---|---|---|---|---|---|---|---|
| BT29* | BJ36 | CA58 | CA91 | CJ28* | DL34 | T467* | |
| 0 | − | − | − | − | − | − | − |
| 3 | − | − | − | − | − | − | + |
| 4 | − | − | − | − | − | − | − |
| 5 | + | − | − | − | − | − | − |
| 7 | − | − | − | − | NA | + | − |
| 11 or 12 | − | − | − | NA | − | − | + |
Wks, weeks; NA, not available; + denotes detection of SHIV DNA by semi-nested Env PCR;
indicates macaques with viral blips at wk2 (BT29, CJ28) and wk5 (T467) post-exposure.
Presence of virus-specific cellular proliferative and immune responses in X4 SHIVSF33A exposed seronegative (ES) macaques
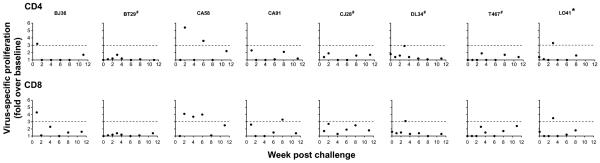
Exposed virus-negative cases of HIV and SIV infection have previously been reported and were shown to be associated with the presence of virus-specific cellular immune responses. Characterization of peripheral T cell immunity in the seven seronegative macaques exposed IVAG to 500 TCID50 of X4 SHIVSF33A was therefore undertaken. To quantitate antigen specific proliferation, PBMCs were stimulated with viral peptides and stained for Ki67, an intracellular marker that has been used for measuring proliferative responses. Using a stringent cutoff of three-fold above background (medium with DMSO alone), SIV gag-specific peripheral CD4+ T cell proliferative response could be detected in macaques BJ36, CA58 and DL34 within the first three weeks post-exposure, but not in the other ones at any of the time points examined (Fig. 3). The response was robust in CA58 while those in BJ36 and DL34 were comparable to that seen in L041, the infected monkey. Virus-specific peripheral CD8+ T cell proliferative responses, similar to L041, were intermittently detected in four out of seven ES macaques as well, three of which are the CD4-responding monkeys. The exception was CA91, which had CD8+ but not CD4+ T cell proliferative response.
Figure 3.
Virus-specific CD4+ and CD8+ T cell proliferative responses in exposed seronegative (ES) macaques. PBMCs from the seven ES macaques were stimulated with peptides spanning SIV gag (●), and then stained for cell surface markers and Ki67. Cells from L041, the infected macaque (*), were also analyzed for comparison. Dashed line denotes cutoff point of three-fold above background (medium with DMSO alone), the upper limit of background proliferation induced by the peptides in four naïve control macaques analyzed. Baseline data are available for the group II macaques (BT29, DL34, T467, L041) and are shown. # indicates animals with viral blips or intermittent proviral SHIV PCR positivity.
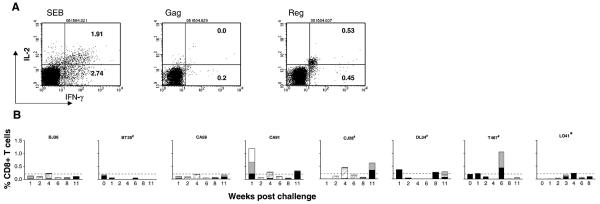
To determine whether peripheral CD8+ T cells of ES animals secrete cytokines in response to viral peptide stimulation, intracellular staining for IFN-γ and IL-2 was performed. PBMC collected over time from all seven ES animals responded with cytokine production to SEB stimulation (Fig. 4A). Upon viral peptide stimulation, intermittent production of IFN-γ and IL-2 in the peripheral CD8+ T lymphocyte population was detected in four (CA91, CJ28, DL34, T467) of the seven ES animals (Fig. 4B). Little or no circulating virus-specific cytokine secreting CD8+ T cells could be detected in the infected macaque L041, consistent with reports of adverse effects of virus infection in CD8+ T cell effector functions (Champagne et al., 2001; Goepfert et al., 2000; Shankar et al., 2000) . For CA91, virus-specific cytokine producing cells were present very early post-exposure (wk1), and were mainly in response to SIV/HIV regulatory antigen stimulation (Fig. 4A). Furthermore, about half of the responding cells were polyfunctional cells that produce IL-2 as well as IFN-γ. With time, however, the responses shifted to SIV Gag, with both mono (IFN-γ secretion only) and polyfunctional CD8+ T cells were detected (Fig. 4B). IFN-γ/IL-2 secreting CD8+ T cells were also present in CJ28, but at significant levels only at later time points post-exposure (wk4), and were mainly in response to SIV Gag stimulation. Although measurable, responses were generally weak for DL34 and T467, except for a single time point in the latter animal at wk6 post-exposure where over 1% of CD8+ T cells secrete IFNγ in response to gag and regulatory peptide stimulation. Notably, this rise in response follows a viral “blip” in this macaque at wk5 post-exposure. Collectively, the data support occult infection in macaques exposed to a limiting dose of X4 SHIVSF33A, as defined by absence or transient viremia with no evidence of seroconversion but measurable virus-specific T cell responses in the periphery. They further suggest that virus replication levels sufficient for induction of cell-mediated immunity (CMI) had apparently occurred in the ES macaques.
Figure 4.
Cytokine (IFN-γ and IL-2) production in response to antigen stimulation in CD8+ T cells of ES macaques. 4-color FACS analyses were performed on PBMCs stained for CD3, CD8, IFN-γ and IL-2. (A) Flow cytometry dot plots of cytokine production in response to superantigen SEB, Gag and HIV/SIV Reg peptide stimulation in macaque CA91 at wk1 post-exposure. (B) Percentage of CD8+ T cells that secrete IFN-γ in response to SIV Gag (■) or HIV/SIV Reg ( ), or both IFN-γ and IL-2 to SIV Gag (
), or both IFN-γ and IL-2 to SIV Gag ( ) and HIV/SIV Reg (□) of all seven ES macaques. Dashed line denotes a 0.2% response level, the upper limit of cumulative responding cells to viral peptides in six naïve macaques analyzed. Baseline values available for two of the macaques (BT29 and T467) as well as data for L041 are also shown. * indicates systemic infection and # indicates animals with viral blips or intermittent proviral SHIV PCR positivity.
) and HIV/SIV Reg (□) of all seven ES macaques. Dashed line denotes a 0.2% response level, the upper limit of cumulative responding cells to viral peptides in six naïve macaques analyzed. Baseline values available for two of the macaques (BT29 and T467) as well as data for L041 are also shown. * indicates systemic infection and # indicates animals with viral blips or intermittent proviral SHIV PCR positivity.
Proliferative and cytokine responses in lymphoid tissues and the gut of ES macaques
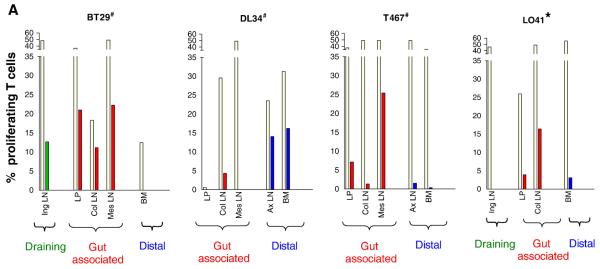
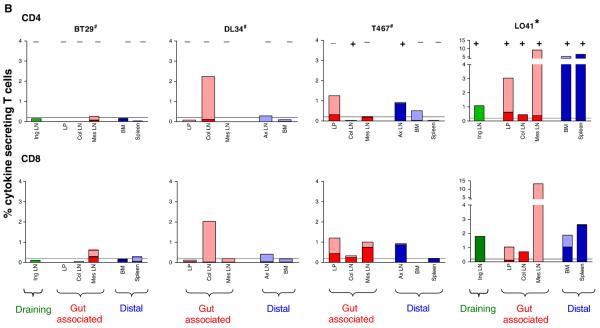
The availability of samples from surgery performed at wk3 post-exposure on one set of four animals that were exposed to 500 TCID50 of SHIVSF33A, three of which (BT29, DL34 and T467) remained seronegative, provided an opportunity to examine the development of antiviral immunity at various anatomical sites in ES macaques. These included the Iliac and Inguinal (Ing) lymph nodes (LNs) that drain the genital tract, gut-associated colonic (Col) and mesenteric (Mes) LNs that are proximal to the site of virus inoculation as well as the jejunum. Fluorescent dye 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) and intracellular cytokine staining were therefore performed with lymphocytes isolated from these tissues to examine for their ability to proliferate and secrete cytokines in response to antigen stimulation respectively. Splenic and bone marrow lymphocytes, and cells from distal LNs such as the axillary LN were tested from a subset of animals to assess systemic immune response. Results showed particularly robust SIV gag-specific T cell proliferation (10-20%) in the draining Ing LN, the gut-associated Col and Mes LNs, and in the jejunal laminar propria (LP) of BT29, but not at a distal site such as the bone marrow (BM)(Fig. 5A). Both CD4+ and CD8+ T cells proliferated, with similar magnitude (data not shown). Robust T cell proliferation was also seen in the Mes LN of T467, but the response was more modest in the LP (~5%) and weak (<5%) in the Col and the distal axillary (Ax) LN and BM. In contrast, T cell proliferation was either weak or absent in the Col and Mes LNs of macaque DL34, but strong in the peripheral Ax LN and BM. For comparison, >15% of cells in the Col LN of L041, the infected macaque proliferated, but responses in other tissue sites examined (Ing LN, LP and BM) were weak. The remarkable intensity of the virus-specific proliferative tissue responses contrasts with the very weak or undetectable viral load seen in these ES macaques.
Figure 5.
Antigen specific proliferative (A) and cytokine (B) responses in tissues of ES macaques. Surgery was performed in macaques BT29, DL34 and T467 at 3 weeks-post-exposure to 500 TCID50 X4 SHIVSF33A and tissue samples were collected. Cells prepared from various tissues were (A) labeled with CFSE and stimulated with either SEB (beige bars) or SIV gag peptides (other colored bars) for 6 days. Percentage of proliferating T cells is shown. (B) Tissue cells were stimulated with SHIV antigens and the percentage of CD4+ and CD8+ T cells that secrete IFN-γ in response to SIV Gag (■) or HIV/SIV Reg ( ), or both IFN-γ and IL-2 to SIV Gag (
), or both IFN-γ and IL-2 to SIV Gag ( ) and HIV/SIV Reg (□) is shown. For both sets of data, the colors designate three broadly classified tissue sites: green, draining lymph nodes; red, gut associated lymphoid tissue; purple, distal or secondary lymphoid tissues. # indicates animals with viral blips or intermittent proviral SHIV PCR positivity, and − /+ indicates the absence or presence respectively of SHIV specific DNA in the various tissue compartments as determined by semi-nested env PCR.
) and HIV/SIV Reg (□) is shown. For both sets of data, the colors designate three broadly classified tissue sites: green, draining lymph nodes; red, gut associated lymphoid tissue; purple, distal or secondary lymphoid tissues. # indicates animals with viral blips or intermittent proviral SHIV PCR positivity, and − /+ indicates the absence or presence respectively of SHIV specific DNA in the various tissue compartments as determined by semi-nested env PCR.
Tissue cells that secrete IFN-γ only were detectable but low in one ES animal (BT29), intermediate for the two others, and high in the systemically infected animal L041 (Fig. 5B). The difference in the magnitude of cytokine response between the ES and infected macaque was particularly striking for the distal sites, revealing the effect of a systemic spread on the localization of effector cells. Furthermore, the frequency of cytokine producing cells in lymphoid tissues, particularly those in proximity to the exposure site, was significantly higher in the three ES macaques as compared to that observed in the periphery blood. This also applied to L041, the infected monkey where tissue responses were dramatically higher than systemic blood responses. PCR analyses indicated the presence of cell-associated SHIV DNA in the Col and Ax LN of T467 as well as all tissues of the infected macaque L041 examined, but were not demonstrable in BT29 and DL34. The presence of virus-specific proliferative and cytokine responses in the distal sites of DL34 and T467 but not BT29 suggested that virus dissemination was more limited in the latter. Furthermore, the detection of SIV gag-specific proliferative responses and SHIV DNA in secondary lymphoid tissues of T467 at three weeks post-exposure indicates that the degree of virus infection and spread in this macaque is greater than that of DL34. Collectively, these findings identify the gut and lymphoid tissues proximal to the genital tract as sites of strong T cell immunity in ES macaques, and support the notion of limited virus dissemination as a result of local containment.
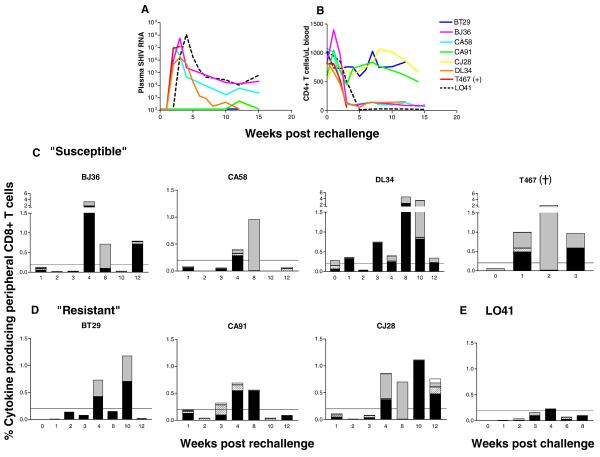
ES macaques are susceptible to systemic infection upon IVAG re-challenge with limiting dose of X4-SHIVSF33A
It has been suggested that occult infection could be a protective condition from HIV reinfection (Beattie, Rowland-Jones, and Kaul, 2002; Clerici and Shearer, 1996). Accordingly, the seven ES macaques were re-challenged IVAG with 500 TCID50 of X4 SHIVSF33A 11-12 weeks after the first exposure, with surgery performed at 3 weeks post-rechallenge on BT29, DL34 and T467, the monkeys from which pre-rechallenge tissue data are available. Results showed that BJ36, CA58, DL43 and T467 were susceptible to systemic re-infection whereas BT29, CA91 and CJ28 were resistant (Fig. 6). Peak viremia of 106 – 108 RNA copies/ml plasma was seen in the reinfected animals, with CA58 and T467 reaching peak levels of plasma SHIV RNA one week earlier than the other two (Fig. 6A). Viral loads dropped to levels of 103 - 105 RNA copies/ml plasma in three of the four susceptible macaques. T467, the other infected macaque, did not recover from surgery and was euthanized. Characteristic of classic X4 SHIVSF33A systemic infection of naïve animals, precipitous and sustained peripheral CD4+ T cell loss accompanied the rise in viremia (Fig. 6B). When compared to L041, the macaque that was infected following a single exposure to 500 TCID50 of the virus, the four animals that were systemically infected upon re-challenge with the same inoculum dose all displayed a replication kinetics that was faster (Fig. 6A), but with less severe CD4+ T cell depletion (Fig. 6B). Antibody responses could be detected in all four infected monkeys by 5 weeks post-rechallenge (data not shown), with significant levels of circulating virus-specific IFN-γ producing CD8+ T cells seen following systemic reinfection (Fig. 6C). This is in contrast to macaque L041 where peripheral SHIV-specific cytokine responses were either weak or absent (Fig. 6E). Since viral burden is comparable between the two groups of animals, the greater frequencies of cytokine-secreting cells in peripheral blood of macaques which were reinfected compared to that present following primary infection suggest anamnestic response with virus re-exposure.
Figure 6.
Viremia (A), CD4:CD8 ratios (B) and virus-specific cytokine producing CD8+ T cells in peripheral blood of macaques susceptible (C) and resistant (D) to systemic infection following virus rechallenge. ES macaques received a second IVAG challenge 11-12 weeks after the first with the same inoculum dose (500 TCID50) of X4-SHIVSF33A. Viral load (SHIV RNA copies per ml plasma), peripheral CD4+ T cell number and the percentage of peripheral CD8+ T cells that secrete IFN-γ in response to SIV Gag (■) or HIV/SIV Reg ( ), or both IFN-γ and IL-2 to SIV Gag (
), or both IFN-γ and IL-2 to SIV Gag ( ) and HIV/SIV Reg (□) were monitored at various time points post re-challenge. Virologic (A) and immunologic data (B, E) from L041, the macaque infected following a single exposure to 500 TCID50 of X4 SHIVSF33A were included for comparison. +, indicates death due to euthanasia.
) and HIV/SIV Reg (□) were monitored at various time points post re-challenge. Virologic (A) and immunologic data (B, E) from L041, the macaque infected following a single exposure to 500 TCID50 of X4 SHIVSF33A were included for comparison. +, indicates death due to euthanasia.
Plasma viremia remained below the detection limit in BT29 and CJ28, with a single viral blip of 540 RNA copies/ml detected in CA91 at wk12 post-rechallenge. The CD4:CD8 ratios were within the normal range for these resistant animals, and none developed antiviral antibody responses up to 12 weeks post-re-exposure. Interestingly, the magnitude of virus-specific CD8+ T lymphocyte cytokine responses in the blood of the resistant macaques was of the same order as that seen in the susceptible monkeys, while the plasma viral loads differed by at least six logs of magnitude. Furthermore, cells that secreted both IFN-γ and IL-2 present in CA91 and CJ28 prior to virus re-exposure (Fig. 4B) were also detectable post-rechallenge (Fig. 6D). These immunological findings indicate the maintenance of T cell memory responses in all ES macaques. Furthermore, the higher levels of peripheral response to SHIV antigens seen following re-challenge than primary exposure (Fig. 4) in the three resistant macaques suggests that they too could be re-infected, but control the virus from systemic spread.
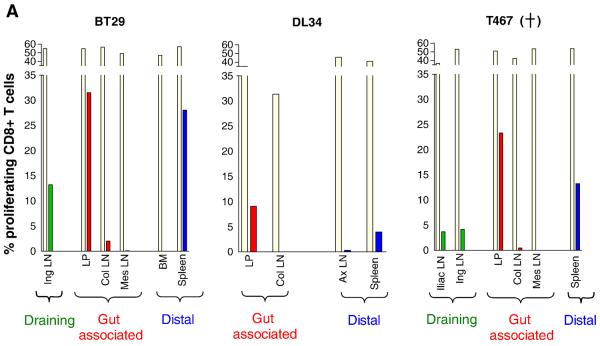
Protection from systemic vaginal re-infection is associated with maintenance of local virus-specific CD8+ T cell responses
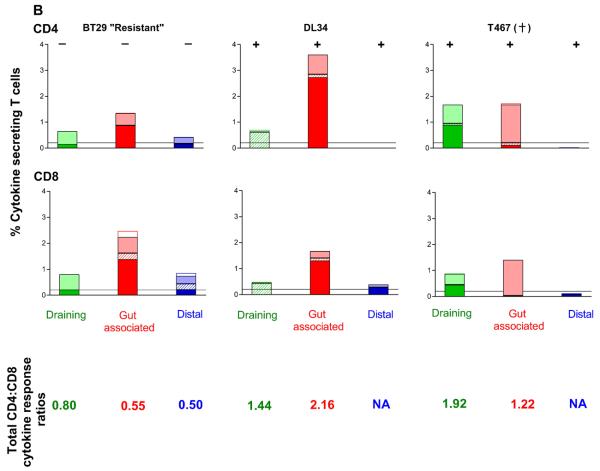
To gain a better understanding of the association of SHIV-specific T cell responses to susceptibility or resistance from systemic re-infection, the magnitude of T cell proliferation and cytokine production at tissue sites were examined in three macaques at 3 weeks post-rechallenge. This time point is the same as when surgery was performed following the first exposure, and was chosen such that tissue data generated after a primary and secondary virus exposure could be directly compared. Two of the animals were susceptible (DL34, T467), one was resistant (BT29). Cell-associated SHIV DNA could be readily detected by PCR in all tissues of the two infected macaques examined, but was absent in the resistant monkey (Fig. 7). CD4+ T cell proliferation as measured by CFSE staining was detectable in draining, gut and distal lymphoid tissues of the resistant animal BT29, but were largely absent in the two infected animals, possibly because of viral cytopathic effects (data not shown). 5-10% of CD8+ T cells in the LP of DL34 and T467, as well as the Ing and Iliac LN of T467 proliferated in response to SIV Gag antigen stimulation (Fig. 7A). In contrast, close to 30% of CD8+ T cells in the LP of the resistant macaque BT29 proliferated in response to SIV gag. Substantial proliferation (>10%) was also seen in the Ing LN and spleen of this animal, with the extent of proliferation being greater in the CD8+ than the CD4+ T cell subpopulation. For the susceptible macaque where both CD4+ and CD8+ proliferative responses could be measured (LP of DL34), however, the CD4+ T lymphocyte response is of greater magnitude than that of CD8+. The massive T cell proliferation seen at tissue sites of BT29 in spite of very low or undetectable viral load suggests that cellular immune response can be very persistent in occult infection.
Figure 7.
Antigen specific proliferative (A) and cytokine (B) responses in tissue compartments of rechallenged macaques. Surgery was performed in macaques BT29, DL34 and T467 at 3 weeks post-rechallenge and tissue samples were collected. Cells prepared from various tissues were (A) labeled with CFSE and stimulated with either SEB (beige bars) or SIV gag peptides (other colored bars) for 6 days. Percentage of CD8+ proliferating T cells is shown. (B) Tissue cells were stimulated with SHIV antigens and the percentage of CD4+ and CD8+ T cells that secrete IFN-γ in response to SIV Gag (■) or HIV/SIV Reg ( ), or both IFN-γ and IL-2 to SIV Gag (
), or both IFN-γ and IL-2 to SIV Gag ( ) and HIV/SIV Reg (□) is shown. Color designations are the same as those outlined in Figure 4. The ratio of total CD4 to CD8 cytokine producing T cells in the various tissue compartments of each macaque was generated and shown for comparison. − /+ indicate the absence or presence respectively of SHIV specific DNA as determined by semi-nested env PCR. +, indicates death due to euthanasia. NA, not applicable.
) and HIV/SIV Reg (□) is shown. Color designations are the same as those outlined in Figure 4. The ratio of total CD4 to CD8 cytokine producing T cells in the various tissue compartments of each macaque was generated and shown for comparison. − /+ indicate the absence or presence respectively of SHIV specific DNA as determined by semi-nested env PCR. +, indicates death due to euthanasia. NA, not applicable.
The ability of tissue cells to secrete IFN-γ and IL-2 was also examined post-rechallenge (Fig. 7B). Data showed that responses could be detected in the draining and gut-associated lymphoid tissues, but not at distal sites of the two susceptible macaques. Furthermore, responses were largely restricted to IFN-γ secretion only. And although both CD4+ and CD8+ responses were present, there was a tendency for greater percentage of CD4+ T cells to respond than CD8+, as indicated by a ratio of total CD4 to CD8 cytokine producing cells greater than 1.0. In contrast, a greater percentage of CD8+ than CD4+ T cells responded in the BT29, the resistant macaque (total CD4 to CD8 responding cell ratio <1.0) Furthermore, IFN-γ/IL-2 co-producing cells present in the gut-associated and distal lymphoid tissues of this animal were of the CD8 and not CD4 T cell lineage. Collectively, these results suggest protection from generalized infection following vaginal re-challenge in the resistant macaques is due to recall of robust antiviral CD8+ T cell immunity, especially in the gut and lymphoid sites proximal to the portal of virus entry.
Discussion
We report in this study that the virologic and immunologic outcome of vaginal exposures to the pathogenic X4 SHIVSF33A is dose dependent. We find that the kinetics and levels of peak viral RNA production, as well as the extent of CD4+ T cell loss, correlate with inoculum size (Fig. 1B). Furthermore, whereas seven of eight macaques exposed to > 500 TCID50 of X4 SHIVSF33A were systemically infected, only 1 of fourteen macaques exposed to doses equal to or less than 500 TCID50 was infected (p = 0.0004, Fisher's exact test)(Table 1). The sharp transition between susceptibility seen with 1000 TCID50 and resistance with 500 TCID50 challenge doses of the virus suggests a virus “threshold” for establishment of a generalized infection. In this regard, systemic vaginal HIV/SIV infection has been proposed to be caused by ordered virus dissemination and continuous seeding from the portal of entry to the intestine/local LN and then to distant organs (Haase, 2005; Miller et al., 2005). Our findings of an association between inoculum dose and kinetics of systemic SHIVSF33A replication, as well as a “threshold” requirement for a self-propagating infection following IVAG challenge are in support of this model of sequential stages in early HIV/SIV spread. However, it should be noted that the notion of a dose dependency for systemic infection and pathogenesis is not so clearly established for SIVmac infection (Daniel et al., 1987; Hirsch and Johnson, 1994; Holterman et al., 2000; Ma et al., 2004; McDermott et al., 2004; Miller et al., 1998; Neildez et al., 1998). Furthermore, whether the model of sequential stages of early HIV/SIV spread applies to infection via the intravenous route of challenge requires further investigation.
Consistent with observations made in people who remained virus-negative despite repeated exposures to HIV and in macaques challenged mucosally with low dose SIV, peripheral antiviral cellular immune responses could be detected in X4 SHIVSF33A ES macaques (Figs. 3 and 4). The presence of antiviral cell mediated immunity (CMI) in the periphery revealed that virus replication had indeed occurred in these animals, but at levels that although adequate for induction of CMI, were insufficient to establish a generalized systemic infection. Analysis of virus-specific T cell responses in LNs that drain the genital mucosa and in the gut of three ES macaques demonstrated induction of remarkably strong antiviral CMI responses at these local sites (Fig. 5). These data identify the sites of immune responses in ES macaques. They further support the notion that virus replication was contained locally in ES macaques due to the development of these localized T cell responses, so that sufficient virus did not reach the peripheral lymphoid tissue compartments through the blood stream to establish a self-propagating infection.
Exposure induced antiviral cellular immunity has been suggested to protect against subsequent re-exposure. Thus, we were surprised to find that whereas one of eight macaques was systemically infected following a single vaginal challenge with 500 TCID50 of the virus, four of seven macaques that were previously exposed became systemically infected upon vaginal re-challenge with the same inoculum dose. Furthermore, the rates of reaching the initial peak of plasma and CD4+ T cell decline were found to be faster in macaques that were systemically infected upon re-challenge than that following a single exposure to 500 TCID50 inoculum dose (Fig. 6). While the data in support of enhancement of transmission upon re-exposure did not reach significance, a trend was observed (p=0.12, Fisher's exact tests) that will need to be confirmed in a larger study.
We sought to determine the relationship, if any, of host responses in the ES macaques and susceptibility or resistance to systemic vaginal re-infection to better understand the immunological correlates of protection. These analyses, as summarized in Table 4, suggest that induction (e.g. macaques CA91, CJ28, BT29) and recall (BT29) of robust virus-specific proliferative or multifunctional CD8+ T cell responses, especially in the gut and lymphoid tissues proximal to the site of virus entry, are linked to protection from systemic infection following vaginal re-exposure. In contrast, a common feature among 3 of the 4 macaques susceptible to systemic re-infection (BJ36, CA58, DL34) is the presence of gag-specific proliferating CD4+ T cells in the periphery prior to re-challenge. The exception was T467. This monkey, however, has the highest level of virus replication and seeding prior to re-exposure, which may have lowered the viral threshold required to establish a generalized infection. Thus, it is tempting to speculate that the presence of pre-existing virus-specific CD4+ T cells cellular responses in ES macaques may have rendered them more susceptibility to systemic infection when re-challenged with the same virus inoculum dose. In this regard, HIV-specific proliferating CD4+ T cells are preferred targets for virus infection (Demoustier et al., 2002; Douek et al., 2002; Harari et al., 2004; Harari et al., 2002), and enhanced SIV replication and accelerated progression to AIDS has been reported in macaques that are primed through vaccination to mount a predominantly CD4+ T cell response to the SIV envelope protein (Staprans et al., 2004). The fact that virus-specific CD4+ T cells serve both as immune effectors (Bevan, 2004) and targets for HIV infection indicates that attempts to appropriately balance the relative strengths of CD4 and CD8 T cell responses will be crucial to achieve maximum vaccine efficacy.
Table 4.
Summary of pre-existing antiviral cellular immunity in and susceptibility to reinfection in X4 SHIVsf33a ES macaques.
| Group | Animal | Peripheral Blood | Tissues | Susceptibility to virus re- infection |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Virus-specific proliferation |
CD8 cytokine production |
Virus-specific proliferation |
Cytokine Production | |||||||||
| CD4 | CD8 | Local | Distal | Local | Distal | |||||||
| CD4 | CD8 | CD4 | CD8 | |||||||||
| I | BJ36 | Yes | Yes | No | Yes | |||||||
| CA58 | Yes | Yes | No | Yes | ||||||||
| CA91 | No | Yes | Yes (p) | No | ||||||||
| CJ28 | No | No | Yes (p) | No | ||||||||
| II | BT29 | No | No | No | +++ | − | No | Yes ( m) | No | No | No | |
| DL34 | Yes | Yes | Yes (m) | + | +++ | Yes (m) | Yes (m) | No | No | Yes | ||
| T467 | No | No | Yes (m) | ++ | + | Yes (m) | Yes (m) | Yes (m) | Yes (m) | Yes | ||
Virus-specific proliferation in peripheral blood was determined by Ki67 staining with a stringent cutoff value of three-fold above background (medium alone). For cytokine production: m, monofunctional cells that secret IFNy only; p, polyfunctional cells that secrete both IFNy and IL-2. For tissue cell analysis, virus-specific proliferation was determined by CFSE staining, with local sites defined as the draining LN and gut associated lymphoid tissue, and distal sites as secondary lymphoid tissues such as the bone marrow, spleen and axillary LN. −, <2%; +, 2-5%; ++, 5-10% and +++, >10%. Susceptibility to re-infection is defined as the presence of detectable plasma viremia.
In summary, our findings extend previous observations made in the HIV and SIV systems (Kaul et al., 2000; Kaul et al., 2003; Murphey-Corb et al., 1999; Wilson et al., 2000) , identifying the sites of immune responses and virus replication in ES macaques. The potency and longevity of antiviral cell mediated immunity seen in the gut and lymphoid tissues in close proximity to the genital tract following IVAG inoculation with limiting dose of SHIVSF33A suggests efficient antigen-specific T-lymphocyte priming, as well as persistence of long-term memory by immunization via this mucosal route, even with very low antigenic load. While the small number of animals used does not provide definitive evidence, our data lend support for a role of local CD8+ T cell-mediated tissue responses in limiting virus dissemination, but also suggest that exposure induced virus-specific CD4+ T cell responses, in the absence of adequate antiviral CD8+ T cell responses, could facilitate susceptibility to systemic infection upon re-exposure via the same route. Further studies will be required to identify the virus-specific T lymphocyte subpopulations that are playing a central role in controlling virus infection. Regardless, our findings contribute to the understanding of the type of protective responses that should be induced by an effective anti-HIV vaccine, and emphasize the role of cellular immunity in the local mucosal and lymphoid environment for containing viral spread following vaginal transmission.
Materials and Methods
Animals, virus and challenge
All infections were carried out in Indian female adult RM (Macaca mulatta) individually housed at the Tulane National Primate Research Center (TNPRC) in compliance with the Guide for the Care and Use of Laboratory Animals, and the studies reviewed and approved by the Institutional Animal Care and Use Committee at TNPRC. Animals were confirmed to be serologically negative for simian type D retrovirus, SIV, and simian T-cell lymphotropic virus prior to inoculation. For all procedures, macaques were anesthetized with ketamine-HCL (10mg/kg) and intravaginal inoculations were performed by placing the virus inoculum atraumatically on the cervicovaginal mucosa. The animals were kept with their pelvis elevated for 20 minutes after virus exposures. Whole blood was collected in EDTA tubes (for viral load) or heparin tubes (for intracellular staining) at designated time intervals post-challenge. Physical examinations were performed by a veterinarian a minimum of once monthly and each animal was observed twice daily for appetite, activity, and stool consistency. Surgery was performed at indicated times and animals were euthanized at the end of the study by intravenous administration of ketamine-HCL followed by an overdose of sodium pentobarbital. Plasma virus was quantified by branched DNA analysis (Bayer Diagnostics, Emeryville, CA) and T cell subsets (CD3+, CD4+, and CD8+ T lymphocytes) were determined by Trucount according to the manufacturer's instructions (Becton Dickinson, San Jose, CA). Cell-free virus stock of X4 SHIVSF33A was propagated and tittered in 5.25M7 cells, a CEMx174 cell line that expressed CD4 and CXCR4, and is stably transduced with an HIV-1 long term repeat (LTR)-green fluorescent protein (GFP) and an HIV-1 LTR-luciferase reporter constructs, and with CCR5 (kindly provided by Ned Landau, Salk Institute, San Diego, CA). The in vitro titer of the challenge virus stock is 1.58 × 103 TCID50, and the RNA and Gag p27 antigen content are 1.1 × 109 copies.
Diagnostic PCR
To detect SHIV infection, blood, gut and lymph node (LN) from the colon, ileum, jejunum, as well as axilary, iliac, inguinal and mesenteric LN were collected at the time of surgery. Cell suspensions were prepared that were then stored in liquid nitrogen in 10% dimethyl sulfoxide-90% fetal bovine serum (FCS). Genomic DNA was extracted from lysates of 5-10 × 106 mucosal or lymphoid cells using QIAamp DNA Mini Kit (Qiagen, Valencia, CA) and analyzed for the presence of viral DNA. Briefly, a semi-nested PCR amplification using primers specific for HIV-1 env was performed, in duplicates per sample, using the outer primers V3-OS (5′gcacagtacaatgtacacatgg) and V3-OAS (5′cagtagaaaaattcccctccaca) in the first-round reaction and inner primers V3-S (5′ctgttaaatggcagtctagcaga) and V3-OAS in the second-round reaction. The first round amplification used 1.25U of HotStarTaq DNA polymerase (Qiagen) in 1.5mM MgCl2, 200μM concentrations of dNTPs and 2pmol concentrations of primers in a total volume of 50μl. After an initial hot start at 95°C for 15min, the cycling profile was 95°C for 15s, 58°C for 30s and 72°C for 40s for 40 cycles. Second-round amplification was performed using 2 μl of the first-round PCR under similar cycling profile for 30 cycles. Amplified products were analyzed by electrophoresis in 1% agarose gels. This assay can detect, in calibrated titrations based on validated Poisson statistics, single copy of cell-associated viral DNA, and the rate of false positives is negligible.
Detection of antiviral antibody responses
Recombinant viral antigens derived from HIV-1SF2 p24 Gag, gp120/gp41 Env and p31 from the endonuclease portion of Pol, as well as p27 Gag from HIV-2UC1 were employed to analyse SHIV-specific antibody responses using the strip immunoblot assay, in accordance with the manufacturer's instructions (Novartis, Emeryville, CA, USA). Cross-reactivity of anti-SHIV antibodies in plasma with viral SIV and HIV-1 antigens allowed detection of the antiviral humoral response. Each independent assay was carried out with internal positive and negative plasma controls.
Peptide pools
Individual peptides consisting of 15 amino acid residues overlapping by 11 amino acids encompassing the SIV Gag/Nef, or HIV Tat/Rev proteins were obtained from the NIH AIDS Research and Reference Reagent Program (Germantown, MD, USA). The peptides were reconstituted in DMSO at 10 mg/ml, and combined so that each individual peptide was equally represented in the mixtures. The final concentration of each peptide in the pool was calculated to be 100 μg/ml, and the mix was aliquoted and stored at −80°C. The 125 peptides of SIVmac239Gag (Gag pool) were aliquoted as two separate pools, whereas the HIV-1 clade B consensus Tat and Rev (23 and 27 peptides respectively) were combined with the 64 peptides covering the SIVmac239 full-length Nef in a single pool (Reg pool). For stimulation, peptides were thawed and diluted in complete medium to a final concentration of 2 μg/ml.
Antibodies
The following antibodies (Abs) in various four-color combinations were used: FN-18(CD3; FITC-conjugated; Biosource International, Camarillo, CA, USA); SK3 (CD4 PE-conjugated; BD Biosciences); SK1 (CD8 PerCP-conjugated; BD Biosciences) and 2ST8.5h (CD8β PE-conjugated; Immunotech, Marseille Cedex, France) for surface staining. 4S.B3 (IFN-γ; PE- and PECy7-conjugated; BD Biosciences); MQ1-17H12 (IL-2; APC-conjugated; BD Biosciences) and B56 (Ki67; PE- conjugated; BD Biosciences) for intracellular staining. IgG isotype-matched controls were obtained both from BD Biosciences and eBioscience (San Diego, CA, USA).
Intracellular Antibody Staining
Peripheral blood mononuclear cells (PBMCs) isolated by ficoll gradient centrifugation from heparitinized blood or fresh cell suspensions prepared from mucosa, spleen and lymph node samples were resuspended in complete medium (RPMI 1640 supplemented with 10% FCS, 2mM L-glutamine, 100U/ml penicillin and 100μg/ml streptomycin) at a concentration of 2.5-10 × 106 cells/ml. One ml cell suspension was then seeded in each well of a 48 well plate, and incubated with peptide pools for 2 h at 37°C. Activation with Staphylococcus enterotoxin B (SEB, 5μg/ml; Sigma, St. Louis, MO) and complete medium with DMSO alone served as positive and negative control, respectively. Brefeldin A (10 μg/ml) was added and incubation continued for an additional 5 h. After activation was completed, cells were left overnight at 18°C. The following day, 100μl of PBS/20mM EDTA was added to the wells for 15min and the activated cells were pipetted vigorously to detach them from the sides of the plate. Cells were then transferred to staining tubes, washed with staining buffer (PBS with 1% FCS and 0.09% w/v sodium azide) and surface stained for 30-40 min at room temperature in the dark. Cells were subsequently washed, fixed and permeabilized using the GOLGI PLUG Kit according to protocol provided (BD Biosciences; Franklin Lakes, NJ, USA). Permeabilized cells were then stained, for 1h at room temperature in the dark with antibodies directed against intracellular cytokines. After the final wash the cells were resuspended in staining buffer. About 100,000 small lymphocytes, gated through forward and side scatter parameters that were chosen to be large enough to include lymphocyte blasts, were acquired on a FACScalibur (BD Biosciences) and analyzed with Cellquest software (BD Biosciences). Isotype controls were processed in parallel for each sample and used to set the gate for intracellular staining. In some experiments, surface staining was performed immediately after cell activation. The cells were then fixed with PBS-2% paraformaldehyde (PFA) and left overnight at 4°C. Intracellular staining for 2 h at 4°C was performed the following day. Specific cytokine responses were determined by subtracting background staining seen in negative control wells, and in the case of SIVmac239Gag, the data represent the sum of the percentages of responses to each of the two peptide pools. For Ki67 staining, fold difference in proliferation in response to Gag peptides and medium with DMSO alone was calculated, and results represent the higher of the fold differences seen with the two separate pools.
CFSE assay
5-10 × 106 lymphocytes prepared from tissue samples were incubated in 1ml of a 2 μM fluorescent dye 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) solution for 10 min at room temperature. After the addition of an equal volume of fetal calf serum (FCS) to stop the reaction and washing with PBS, the cells were resuspended in complete medium (RPMI 1640/10%FCS/20U IL-2) at a concentration of 5 × 106 cells per ml. Cultures were left in either medium alone (negative), medium with 3 μg/ml SEB (positive) or medium containing 15mer peptide mixes encompassing SIV Gag (2 μg/peptide/test). Stimulated and unstimulated cells were collected on day 6, surface stained with CD4-PE and CD8-PerCP (BD Biosciences), washed once with PBS, and fixed with PFA. Staining with appropriate isotype-matched control antibodies was performed to confirm the staining specificity and to set instrument compensation. Lymphocytes were gated by forward and side light scatter, and data acquired by flow cytometry using a FacsCalibur flow cytometer and Cell Quest software (BD Immunocytometry System). Only samples with a positive response to SEB were included in the analysis. The mean background proliferation was calculated based on the proliferating fractions in cells grown in media alone. The antigen-specific proportion of proliferating cells was calculated by subtracting the proportion of proliferating cells in unstimulated samples from the proliferating fraction measured in response to antigen. This assay proved to be more sensitive than Ki67 staining, especially when tissue cells were used.
Statistical analysis
Fisher's exact tests were used. A P value of <0.05 was considered significant.
Table 3.
PCR analysis of peripheral blood samples from ES macaques that are resistant to generalized IVAG re-infection with 500 TCID50 X4-SHIVsf33a.
| Wks post- exposure |
Animal | ||
|---|---|---|---|
| BT29 | CA91 | CJ28 | |
| 0 | − | NA | − |
| 1 | − | − | − |
| 3 | − | − | − |
| 7 | − | − | − |
| 12 | + | − | − |
Wk, weeks; wk 0 indicates day of re-challenge. NA, Not available
Acknowledgements
This work was supported by grants from the NIH (RO1A46980, R37AI41945), and from a subproject (MSA-03-362) provided by CONRAD, Eastern Virginia Medical School under a Cooperative Agreement (HRN-A-00-98-00020-00) with the United States Agency for International Development (USAID). The authors thank Lili Shek for performing the immunoblot assays and Peter Lopez for help with FACS analyses. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: 15-mer peptides encompassing the SIV Gag/Nef, or HIV Tat/Rev proteins.
References
- Beattie T, Rowland-Jones S, Kaul R. HIV-1 and AIDS: what are protective immune responses? J HIV Ther. 2002;7(2):35–9. [PubMed] [Google Scholar]
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- Bogers WM, Cheng-Mayer C, Montelaro RC. Developments in preclinical AIDS vaccine efficacy models. AIDS. 2000;14(Suppl 3):S141–51. [PubMed] [Google Scholar]
- Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410(6824):106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- Clerici M, Shearer GM. Correlates of protection in HIV infection and the progression of HIV infection to AIDS. Immunol Lett. 1996;51(12):69–73. doi: 10.1016/0165-2478(96)02557-6. [DOI] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, Sehgal PK, Hunsmann G, Schmidt DK, King NW, Desrosiers RC. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987;68(Pt 12):3183–9. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Demoustier A, Gubler B, Lambotte O, de Goer MG, Wallon C, Goujard C, Delfraissy JF, Taoufik Y. In patients on prolonged HAART, a significant pool of HIV infected CD4 T cells are HIV-specific. AIDS. 2002;16(13):1749–54. doi: 10.1097/00002030-200209060-00006. [DOI] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Goepfert PA, Bansal A, Edwards BH, Ritter GD, Jr., Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74(21):10249–55. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5(10):783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103(3):966–72. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- Harari A, Rizzardi GP, Ellefsen K, Ciuffreda D, Champagne P, Bart PA, Kaufmann D, Telenti A, Sahli R, Tambussi G, Kaiser L, Lazzarin A, Perrin L, Pantaleo G. Analysis of HIV-1- and CMV-specific memory CD4 T-cell responses during primary and chronic infection. Blood. 2002;100(4):1381–7. doi: 10.1182/blood-2001-11-0080. [DOI] [PubMed] [Google Scholar]
- Harouse JM, Tan RC, Gettie A, Dailey P, Marx PA, Luciw PA, Cheng-Mayer C. Mucosal transmission of pathogenic CXCR4-utilizing SHIVSF33A variants in rhesus macaques. Virology. 1998;248(1):95–107. doi: 10.1006/viro.1998.9236. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Pantaleo G, Fauci AS. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271(5247):324–8. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32(2):183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Holterman L, Niphuis H, Koornstra W, Dubbes R, ten Haaft P, Heeney JL. The rate of progression to AIDS is independent of virus dose in simian immunodeficiency virus-infected macaques. J Gen Virol. 2000;81(Pt 7):1719–26. doi: 10.1099/0022-1317-81-7-1719. [DOI] [PubMed] [Google Scholar]
- Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, Njagi E, MacDonald KS, Bwayo JJ, McMichael AJ, Rowland-Jones SL. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164(3):1602–11. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- Kaul R, Thottingal P, Kimani J, Kiama P, Waigwa CW, Bwayo JJ, Plummer FA, Rowland-Jones SL. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS. 2003;17(8):1139–44. doi: 10.1097/00002030-200305230-00004. [DOI] [PubMed] [Google Scholar]
- Kulkarni PS, Butera ST, Duerr AC. Resistance to HIV-1 infection: lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev. 2003;5(2):87–103. [PubMed] [Google Scholar]
- Ma ZM, Abel K, Rourke T, Wang Y, Miller CJ. A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations. J Virol. 2004;78(24):14048–52. doi: 10.1128/JVI.78.24.14048-14052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney MB, Collins JR, Lu D, Lu X, Torten J, Ashley RL, Cloyd MW, Miller CJ. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4(+)-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J Virol. 1998;72(12):10029–35. doi: 10.1128/jvi.72.12.10029-10035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AB, Mitchen J, Piaskowski S, De Souza I, Yant LJ, Stephany J, Furlott J, Watkins DI. Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates. J Virol. 2004;78(6):3140–4. doi: 10.1128/JVI.78.6.3140-3144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79(14):9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Marthas M, Greenier J, Lu D, Dailey PJ, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72(4):3248–58. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Marthas M, Torten J, Alexander NJ, Moore JP, Doncel GF, Hendrickx AG. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68(10):6391–400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey-Corb M, Wilson LA, Trichel AM, Roberts DE, Xu K, Ohkawa S, Woodson B, Bohm R, Blanchard J. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J Immunol. 1999;162(1):540–9. [PubMed] [Google Scholar]
- Neildez O, Le Grand R, Caufour P, Vaslin B, Cheret A, Matheux F, Theodoro F, Roques P, Dormont D. Selective quasispecies transmission after systemic or mucosal exposure of macaques to simian immunodeficiency virus. Virology. 1998;243(1):12–20. doi: 10.1006/viro.1997.9026. [DOI] [PubMed] [Google Scholar]
- Pauza CD, Emau P, Salvato MS, Trivedi P, MacKenzie D, Malkovsky M, Uno H, Schultz KT. Pathogenesis of SIVmac251 after atraumatic inoculation of the rectal mucosa in rhesus monkeys. J Med Primatol. 1993;22(23):154–61. [PubMed] [Google Scholar]
- Petry H, Dittmer U, Stahl-Hennig C, Jurkiewicz E, Hunsmann G, Luke W. Silent infection of macaques inoculated with low-dose SIVmac251/spl. AIDS Res Hum Retroviruses. 1997;13(16):1355–6. doi: 10.1089/aid.1997.13.1355. [DOI] [PubMed] [Google Scholar]
- Polacino P, Stallard V, Klaniecki JE, Montefiori DC, Langlois AJ, Richardson BA, Overbaugh J, Morton WR, Benveniste RE, Hu SL. Limited breadth of the protective immunity elicited by simian immunodeficiency virus SIVmne gp160 vaccines in a combination immunization regimen. J Virol. 1999;73(1):618–30. doi: 10.1128/jvi.73.1.618-630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland-Jones SL. Timeline: AIDS pathogenesis: what have two decades of HIV research taught us? Nat Rev Immunol. 2003;3(4):343–8. doi: 10.1038/nri1058. [DOI] [PubMed] [Google Scholar]
- Rowland-Jones SL, McMichael A. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Curr Opin Immunol. 1995;7(4):448–55. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96(9):3094–101. [PubMed] [Google Scholar]
- Shearer GM, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17(1):21–4. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- Staprans SI, Barry AP, Silvestri G, Safrit JT, Kozyr N, Sumpter B, Nguyen H, McClure H, Montefiori D, Cohen JI, Feinberg MB. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci U S A. 2004;101(35):13026–31. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Horejsh D, Hinds SB, Hinds PW, II, Wu MS, Salvato MS, Pauza CD. Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: selective amplification and host responses to transient or persistent viremia. J Virol. 1996;70(10):6876–83. doi: 10.1128/jvi.70.10.6876-6883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay KK, Marthas ML, Lifson JD, Berardi CJ, Vasquez GM, Agatep E, Dehqanzada ZA, Cundy KC, Bischofberger N, Pedersen NC. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res Hum Retroviruses. 1998;14(9):761–73. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]
- Wilson LA, Murphey-Corb M, Martin LN, Harrison RM, Ratterree MS, Bohm RP. Identification of SIV env-specific CTL in the jejunal mucosa in vaginally exposed, seronegative rhesus macaques (Macaca mulatta) J Med Primatol. 2000;29(34):173–81. doi: 10.1034/j.1600-0684.2000.290311.x. [DOI] [PubMed] [Google Scholar]