Abstract
Targeting death receptor-mediated apoptosis has emerged as an effective strategy for cancer therapy. However, certain types of cancer cells are intrinsically resistant to death receptor-mediated apoptosis. In an effort to identify agents that can sensitize cancer cells to death receptor-induced apoptosis, we have identified Honokiol, a natural product with anticancer activity, as demonstrated in various preclinical studies, as an effective sensitizer of death receptor-mediated apoptosis. Honokiol alone moderately inhibited the growth of human lung cancer cells; however, when combined with tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL), greater effects on decreasing cell survival and inducing apoptosis than TRAIL alone were observed, indicating that Honokiol cooperates with TRAIL to enhance apoptosis. This was also true to Fas-induced apoptosis when combined with Fas ligand or an agonistic anti-Fas antibody. Among several apoptosis-associated proteins tested, c-FLIP was the only one that was rapidly downregulated by Honokiol in all of the tested cell lines. The downregulation of c-FLIP by Honokiol could be prevented by the proteasome inhibitor MG132. Moreover, Honokiol increased c-FLIP ubiquitination. These results indicate that Honokiol downregulates c-FLIP by facilitating its degradation through a ubiquitin/proteasome-mediated mechanism. Enforced expression of ectopic c-FLIP abolished Honokiol’s ability to enhance TRAIL-induced apoptosis. Several Honokiol derivatives, which exhibited more potent effects on downregulation of c-FLIP than Honokiol, showed better efficacy than Honokiol in inhibiting the growth and enhancing TRAIL-induced apoptosis as well. Collectively, we conclude that c-FLIP downregulation is a key event for Honokiol to modulate the death receptor-induced apoptosis.
Keywords: Honokiol, c-FLIP, death receptors, TRAIL, apoptosis, lung cancer
Introduction
It is well known that cells can die of apoptosis primarily through the extrinsic death receptor-induced pathway and/or the intrinsic mitochondria-mediated pathway. Cross-talk between these two pathways is mediated by the truncated proapoptotic protein Bid (1). The activation of the extrinsic death receptor-mediated apoptotic pathway relies on binding of a death ligand (e.g., tumor necrosis factor-related apoptosis-inducing ligand; TRAIL) to its corresponding death receptor(s) or aggregation (e.g. trimerization) of death receptors, which induces for the formation of the death-inducing signaling complex (DISC) followed by the activating cleavage of caspase-8 in the DISC. Because Bid serves as a caspase-8 substrate, activation of the extrinsic death receptor apoptotic pathway also turns on the intrinsic apoptotic pathway.
The death ligand TRAIL has recently received much attention because it preferentially induces apoptosis in transformed or malignant cells, but not in most normal cells, demonstrating potential as a tumor-selective apoptosis-inducing cytokine for cancer treatment (2). Currently recombinant human TRAIL is being tested as an anticancer agent in phase I clinical trials. In addition, agonistic antibodies against DR4 and DR5, respectively, which directly activate the extrinsic apoptotic pathway, have been developed and tested in phase I or II trials with well tolerance (3). Thus, the death receptor-, particularly the TRAIL death receptor-, mediated apoptosis has been under intense research as a cancer therapeutic target (4). An important issue in this regard is the intrinsic resistance of certain cancer cells to TRAIL/death receptor-induced apoptosis.
Activation of the extrinsic death receptor-mediated apoptotic pathway is primarily inhibited by cellular FLICE-inhibitory protein (c-FLIP), which inhibits caspase-8 activation by preventing recruitment of caspase-8 to the DISC (5, 6). c-FLIP has multiple splice variants, however, only two of them have been well characterized at the protein levels: the 26 kDa short form (c-FLIPS) containing two death effector domains and the 55 kDa long form (c-FLIPL) containing an inactive caspase-like domain in addition to the two death effector domains (7, 8). The levels of c-FLIP, including both FLIPL and FLIPS are subject to regulation by ubiquitin/proteasome-mediated degradation (9–11). It has been well documented that elevated c-FLIP expression protects cells from death receptor-mediated apoptosis, whereas downregulation of c-FLIP by chemicals or siRNA sensitizes cells to death receptor-mediated apoptosis (7). Moreover, overexpression of c-FLIP also protects cells from apoptosis induced by cancer therapeutic agents such as etoposide and cisplatin (12–16).
Honokiol (HNK) (Fig. 1A) is an active component purified from magnolia, a plant used in traditional Chinese and Japanese medicine. It has been shown that HNK induces apoptosis and inhibits the growth of certain types of cancer cells (17–22) and has excellent in vivo antitumor activity against skin tumors (23), angiosarcoma (24), breast cancer (21) and bone metastasis of prostate cancer (25). However, the precise mechanism of growth inhibition and apoptosis by HNK is largely unknown although it appears to be associated with the inhibition of NF-κB (22) and downregulation of Bcl-XL and Mcl-1 (18, 19). In an effort to identify agents that sensitize cancer cells to the death receptor-mediated apoptosis, we have identified that HNK is a potent sensitizer of death receptor-induced apoptosis, primarily through inducing c-FLIP degradation. Thus, our findings highlight a novel mechanism by which HNK modulates apoptosis in human cancer cells.
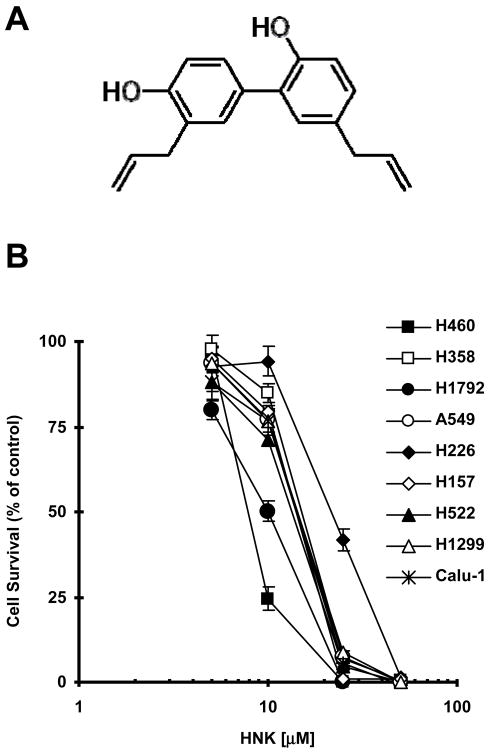
Fig. 1. Chemical structure of HNK (A) and its effects on the growth of human NSCLC cells (B).
The indicated NSCLC cell lines were seeded in 96-well cell culture plates and treated the next day with the given concentrations of HNK. After 3 days, cell number was estimated using the SRB assay. Cell survival was expressed as the percent of control (DMSO-treated) cells. Data are the means of four replicate determinations; Bars, ± SDs.
Materials and Methods
Reagents
HNK and its derivatives used in this study were synthesized in Dr. J. Arbiser’s lab (Emory University, Atlanta, GA). These compounds were dissolved in DMSO at the concentration of 30 mM, and aliquots were stored at −80°C. Stock solutions were diluted to the appropriate concentrations with growth medium immediately before use. The soluble recombinant human TRAIL and tumor necrosis factor α (TNFα) were purchased from PeproTech, Inc. (Rocky Hill, NJ). The proteasome inhibitor MG132 was purchased from Sigma Chemical Co. (St. Louis, MO). The agonistic anti-Fas antibody (EOS9.1) was purchased from BioLegend (San Diego, CA). Soluble recombinant human SuperFas ligand and mouse monoclonal anti-FLIP antibody (NF6) was purchased from Alexis Biochemicals (San Diego, CA). Rabbit polyclonal anti-DR5 antibody was purchased from ProSci Inc (Poway, CA). Mouse monoclonal anti-DR4 antibody (B-N28) was purchased from Diaclone (Stamford, CT). Mouse monoclonal anti-caspase-3 antibody was purchased from Imgenex (San Diego, CA). Rabbit polyclonal anti-XIAP, anti-caspase-8, anti-Mcl-1, anti-c-Jun, anti-phospho-c-Jun (Ser63) and anti-PARP antibodies and mouse monoclonal anti-survivin antibody were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Rabbit anti-GAPDH polyclonal antibody and mouse anti-Bax monoclonal antibody were purchased from Trevigen (Gaithersburg, MD). Mouse anti Bcl-2 and rabbit anti-Bcl-XL antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Rabbit polyclonal anti-β-actin antibody was purchased from Sigma Chemical Co.
Cell Lines and Cell Culture
Human non-small cell lung cancer (NSCLC) cell lines used in this study were purchased from the American Type Culture Collection (Manassas, VA). The stable H157-Lac Z-5, H157-FLIPL-21, H157-FLIPS-1, H460-Lac Z-9 and H460-FLIPL-15 transfectants were established as described previously (26, 27). The NF-κB reporter stable cell line A549/NFκB-luc, which maintain a chromosomal integration of a luciferase reporter construct regulated by multiple copies of the NFκB response element, was purchased from Panomics, Inc (Redwood City, CA). These cell lines were cultured in RPMI 1640 containing 5% fetal bovine serum at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
Cell Survival Assay
Cells were seeded in 96-well cell culture plates and treated the next day with the agents indicated. The viable cell number was determined using the sulforhodamine B assay, as previously described (28).
Detection of Apoptosis
Apoptosis was evaluated by Annexin V staining using Annexin V-PE apoptosis detection kit purchased from BD Biosciences (San Jose, CA) following the manufacturer’s instructions. We also detected caspase activation by Western blotting (as described below) or by fluorometric assay as described previously (29) as an additional indicator of apoptosis.
Western Blot Analysis
Whole-cell protein lysates were prepared and analyzed by Western blotting as described previously (30, 31).
Immunoprecipitation for Detection of Ubiqutinated c-FLIP
H157-FLIPL-21 cells, which stably express FLIPL, were transfected with HA-ubiquitin plasmid using the FuGENE 6 transfection reagent (Roche Diagnostics Corp., Indianapolis, IN) following the manufacturer’s instruction. After 24 h, the cells were treated with HNK or MG132 plus HNK for 4 h and then were lysed for immunoprecipitation of Flag-FLIPL using Flag M2 monoclonal antibody (Sigma Chemicals) as previously described (32) followed by the detection of ubiquitinated FLIPL with Western blotting using anti-HA antibody (Abgent; San Diego, CA).
Results
HNK Inhibits the Growth of Human NSCLC Cells
We first determined the effects of HNK as a single agent on the growth of a panel of human NSCLC cell lines. In this experiment, 9 NSCLC cell lines including H460, H358, H1792, A549, H226, H157, H522, H1299 and Calu-1 were treated with increasing concentrations of HNK. After 3 days, we found that HNK effectively inhibited the growth of the tested cell lines in a dose-dependent manner with IC50s ranging from 10 to 20 μM, except for H226 cells, the IC50 of which was approximately 30 μM (Fig. 1B).
HNK Augments Death Receptor-mediated Apoptosis in Human NSCLC Cells
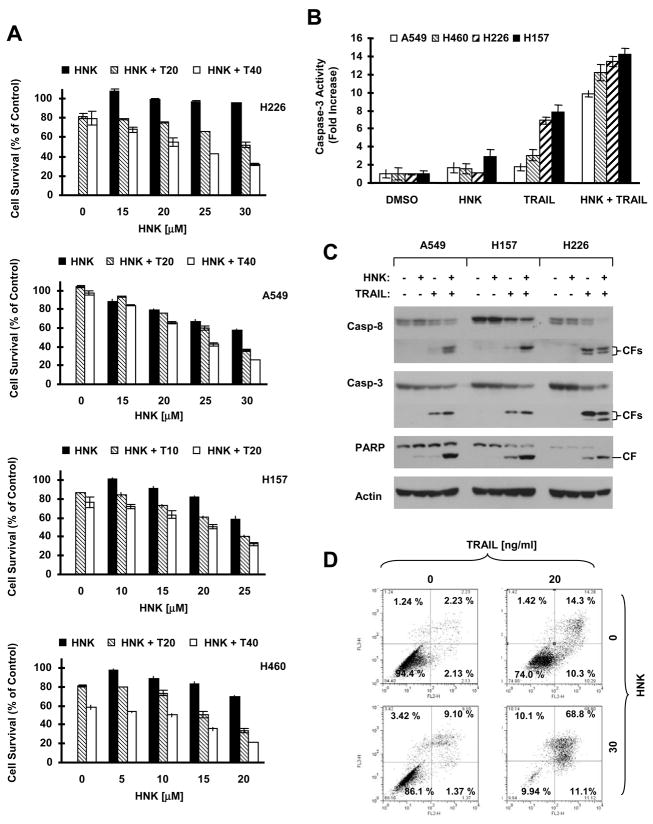
It was previously shown that HNK’s anticancer activity can be antagonized by anti-TRAIL antibody, suggesting the involvement of TRAIL in HNK-mediated anticancer activity (24). We were interested in whether HNK in combination with exogenous recombinant TRAIL augmented induction of apoptosis. To this end, we treated four NSCLC cell lines (i.e., H226, A549, H157 and H460) with TRAIL alone, HNK alone or both drugs combined, and then assessed cell survival and apoptosis. As presented in Fig. 2A, the combination of HNK at concentrations of >10 μM with either dose of TRAIL (5, 10, 20 or 40 ng/ml) was more effective in decreasing tumor cell survival than either single agent alone. For example, in H226 cells, HNK alone at 30 μM decreased cell survival by approximately 10%, and TRAIL (40 ng/ml) alone decreased cell survival by about 20%, but the combination of the two agents reduced cell survival by > 60%, which is greater than the sum of the effects of each agent alone. We detected the highest caspase-3 activity in all four tested cell lines when exposed to HNK plus TRAIL as compared to cells treated with either HNK alone or TRAIL alone, which only minimally or moderately increased caspase-3 activity (Fig. 2B). In Western blot analysis, the combination of HNK and TRAIL induced the highest levels of cleaved caspase-8, caspase-3 and PARP in comparison to HNK alone or TRAIL alone in all three tested cell lines (A549, H157 and H226) (Fig. 2C). In accordance with these findings, we detected 80% apoptotic cells when treated with HNK plus TRAIL, but only 10% and 25% apoptosis in cells exposed to HNK alone and TRAIL alone, respectively (Fig. 2D). Collectively, these results clearly show that HNK combined with TRAIL augments induction of apoptosis in human NSCLC cells.
Fig. 2. Effects of HNK combined with TRAIL on cell survival (A), caspase activation (B and C) and apoptosis induction (D).
A, The indicated cell lines were seeded in 96-well cell culture plates and treated the following day with increasing concentrations of HNK alone, TRAIL (T) alone at the two given concentrations, and their individual combinations. After 24 h, cell number was estimated using SRB assay for calculation of cell survival. Data are the means of four replicate determinations. Bars, ± SDs. B, and C, The indicated cell lines were treated with DMSO control, HNK alone at 20 μM (H460), 25 μM (H157) or 30 μM (A549 and H226), TRAIL alone at 5 ng/ml (H460), 10 ng/ml (H157) or 20 ng/ml (A549 and H226), or HNK plus TRAIL. After 24 h, the cells were subjected to preparation of whole-cell protein lysates for measuring caspase-3 activity using fluorometric assay (B) or for detecting caspase cleavage using Western blotting (C). Data in B are the means of triplicate determinations. Bars, ± SDs. Casp, caspase; CF, cleaved fragment. D, H226 cells were treated with 20 ng/ml TRAIL alone, 30 μM HNK alone or their combination for 24 h. The cells were then subjected to measurement of apoptosis using Annexin V staining. The percent positive cells in the upper right and lower right quadrants represent the total apoptotic cell population.
We also examined whether HNK enhances apoptosis when combined with Fas ligand or an agonistic anti-Fas antibody, both of which trigger Fas-mediated apoptosis. Similarly to the TRAIL findings, we found that Fas ligand or anti-Fas antibody at the tested concentrations (25–200 ng/ml) did not decrease cell survival. However, the presence of HNK substantially reduced cell survival (see supplemental Fig. S1), indicating that HNK also augments Fas-mediated apoptosis.
HNK Rapidly Reduces c-FLIP Levels in Human NSCLC Cells
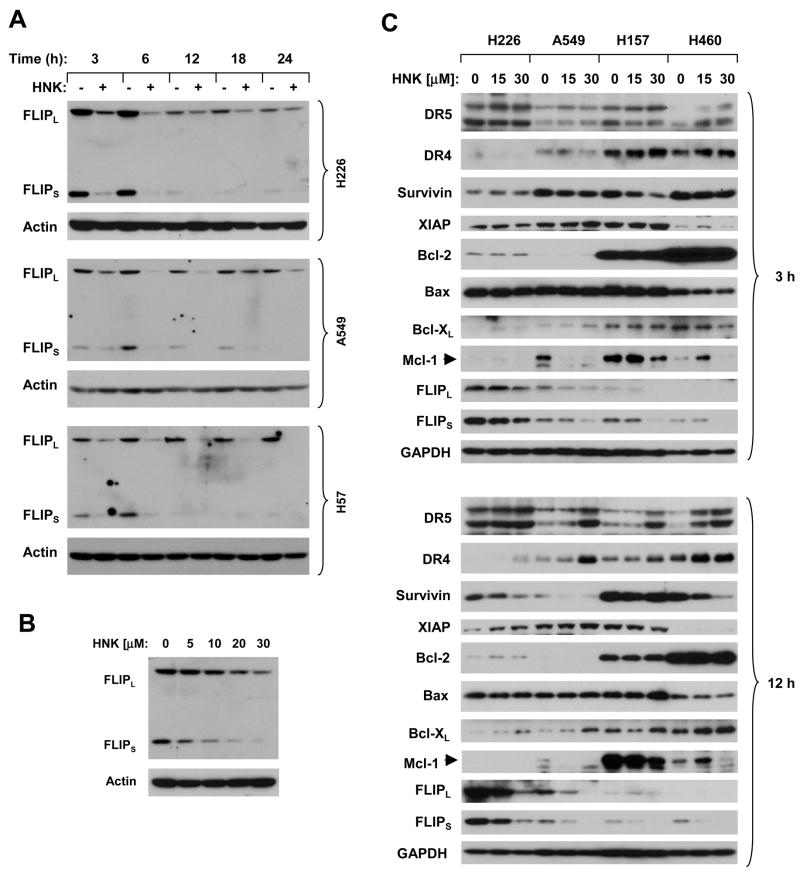
It is well known that c-FLIP is a major inhibitor of the extrinsic death receptor-mediated apoptotic pathway (6). Thus, c-FLIP downregulation is an important mechanism underlying the enhancement of death receptor-induced apoptosis by some anticancer drugs (26, 33, 34). To understand the mechanism by which HNK sensitizes cells to death receptor-mediated apoptosis, we determined whether HNK modulated c-FLIP levels. Given that c-FLIP, particularly FLIPS, are quickly turnover proteins with very short half lives (10), we had DMSO-treated cells as a control for each indicated time point when we did time-course analysis of c-FLIP modulation by Honokiol. By Western blot analysis, we detected time-dependent reduction of c-FLIP (both FLIPL and FLIPS) levels in the three tested cell lines, which occurred at 3 h post treatment and was sustained up to 24 h (Fig. 3A). Moreover, HNK’s effect on the reduction of c-FLIP was dose-dependent and decreased the levels of c-FLIP, particularly FLIPS, at concentrations as low as 5 μM (Fig. 3B). Collectively, these findings indicate that HNK exerts a potent and rapid effect on the downregulation of c-FLIP.
Fig. 3. HNK modulates c-FLIP levels (A and B) and the levels of other apoptosis-related proteins (C).
The given cell lines were treated with 25 μM (H157) or 30 μM (H226 and A549) HNK for the indicated times (A) or with the indicated concentrations of HNK for 6 h (B) or for 3 h and 12 h as indicated (C). After the treatments, the cell lines were subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis for detection of the indicated proteins.
We also determined whether HNK modulates the levels of other proteins known to be involved in the regulation of apoptosis. To this end, we made whole cell protein lysates from cells exposed to HNK for a short (3 h) or longer (12 h) time period and then analyzed the levels of proteins as presented in Fig. 3C. As expected, HNK decreased c-FLIP levels in all of the tested cell lines after both 3 h- and 12 h-treatments. Increased levels of DR5 and DR4 were detected in these cell lines exposed to HNK for 12 h; however, in cells treated with HNK for 3 h, DR5 and DR4 upregulation were observed only in one cell line (H460) and in two cell lines (H157 and H460), respectively. Similarly, survivin levels were decreased in most cell lines treated with HNK for 12 h, but only in two cell lines (A549 and H157) when exposed to HNK for 3 h. The levels of XIAP, Bcl-2 and Bax were not altered in any of these cell lines exposed to HNK for either 3 h or 12 h, whereas Bcl-XL levels were slightly increased in cells exposed to HNK for 12 h. Mcl-1 levels were effectively decreased in A549 cells exposed to both 15 μM and 30 μM for 3 h or 12 h; however, in other cell lines, Mcl-1 reduction was detected only when treated with 30 μM HNK (Fig. 3C). Thus, it appears that c-FLIP downregulation is an early event and likely plays a critical role in mediating death receptor-induced apoptosis.
HNK Donwregulates c-FLIP through Promoting Ubiqitin/proteasome-mediated Degradation Independent of JNK
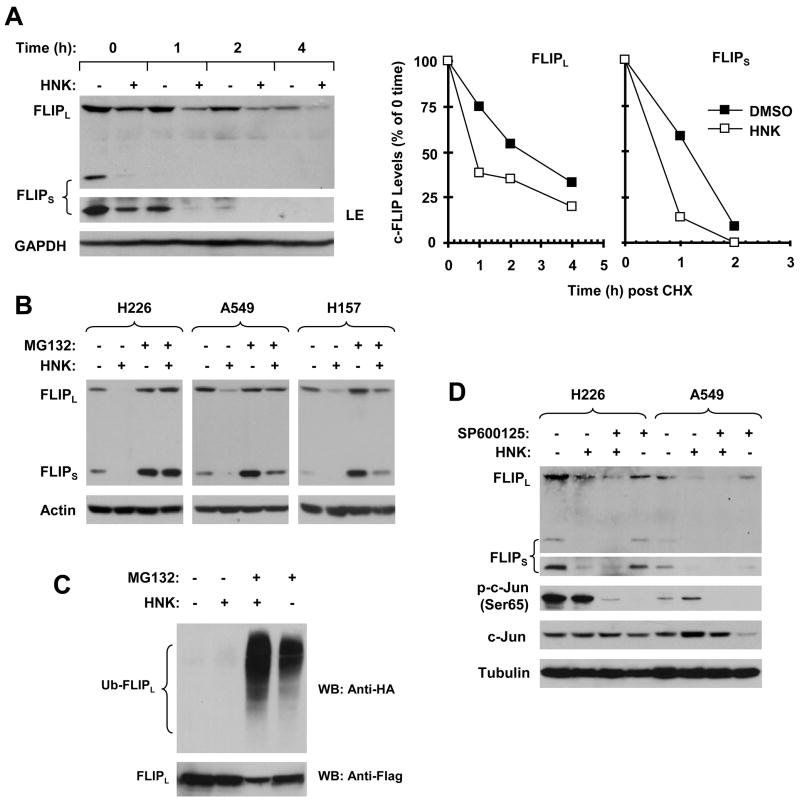
Because c-FLIP proteins are known to be regulated by ubiquitin/proteasome-mediated degradation (9, 11), we then determined whether the observed down-regulation of c-FLIP by HNK would be mediated via this process. Thus, we first examined whether HNK promotes c-FLIP degradation. To this end, we treated H226 cells with either DMSO solvent control or HNK for 5 h and then washed away the drug followed by refilling the cells with fresh medium containing the protein synthesis inhibitor cycloheximide (CHX). At the indicated times post CHX, the cells were harvested for Western blotting for analyzing c-FLIP degradation rate. As presented in Fig. 4A, the half-lives for FLIPL and FLIPS in control cells were approximately 145 min and 75 min, respectively; however, they were 50 min and 40 min, respectively, in HNK-treated cells. Thus, it is clear that HNK facilitates c-FLIP degradation. Moreover, we treated cells with HNK in the absence and presence of the proteasome inhibitor MG132 and then compared c-FLIP modulation under these conditions. We found that the HNK-induced downregulation of c-FLIP was inhibited by the presence of MG132 in all of the tested cell lines (Fig. 4B), indicating that HNK-induced c-FLIP degradation is proteasome-dependent. By immunoprecipitation/Western blotting, we detected the highest levels of ubiqutinated FLIPL in cells treated with HNK plus MG132 compared to cells exposed to HNK alone or MG132 alone (Fig. 4C), indicating that HNK increases c-FLIP ubiquitination. Taken together, we conclude that HNK initiates ubiquitin/proteasome-mediated c-FLIP degradation, leading to downregulation of c-FLIP in human NSCLC cells.
Fig. 4. HNK modulates c-FLIP levels through ubiquitin/proteasome-mediated protein degradation (A–C) independent of JNK (D).
A, H226 cells were treated with DMSO or 30 μM HNK for 5 h. The cells were then washed with PBS 3 times and refed with fresh medium containing 10 ug/ml CHX. At the indicated times, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. Protein levels were quantitated with NIH Image J software (Bethesda, MA) and were normalized to GAPGH. The results were plotted as the relative c-FLIP levels compared to those at the time 0 of CHX treatment (right panel). LE, longer exposure. B, The given cell lines were pretreated with 20 μM MG132 for 30 minutes prior to the addition of HNK (30 μM for H226 and A549; 25 μM for H157). After co-treatment for 4 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. C, H157-FLIPL-21 cells which stably express ectopic flag-FLIPL were transfected with HA-ubiquitin plasmid using FuGENE 6 transfection reagent for 24 h. The cells were then pretreated with 20 μM MG132 for 30 minutes and then co-treated with 25 μM HNK for 4 h. Whole-cell protein lysates were then prepared for immunoprecipitation using anti-Flag antibody followed by Western blotting (WB) using anti-HA antibody for detection of ubiquitinated FLIPL (Ub-FLIPL) and anti-Flag antibody for detection of ectopic FLIPL. D, The indicated cell lines were pretreated with 20 μM SP600125 for 30 min and then co-treated with 30 μM HNK for another 6 h. The cells were then subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis for detection of the indicated proteins.
Recently, JNK has been demonstrated to be responsible for tumor necrosis factor-induced, ubiquitin/proteasome-mediated FLIPL degradation (11). Therefore, we determined whether JNK activation is involved in mediating HNK-induced c-FLIP degradation. To do so, we examined the effects of HNK on c-FLIP downregulation in the presence of the JNK-specific inhibitor SP600125 in H226 and A549 cells. SP600125 at the concentration of 20 μM inhibited both the basal levels of phospho-c-Jun and increased levels of phospho-c-Jun (e.g., A549), confirming that SP600125 worked as expected in our cell systems. However, SP600125 did not block HNK-induced c-FLIP (both FLIPL and FLIPS) downregulation (Fig. 4D). Collectively, we suggest that JNK does not play a role in the HNK-induced downregulation of c-FLIP.
Enforced Expression of Ectopic c-FLIP Protects Cells from HNK/TRAIL-induced Apoptosis
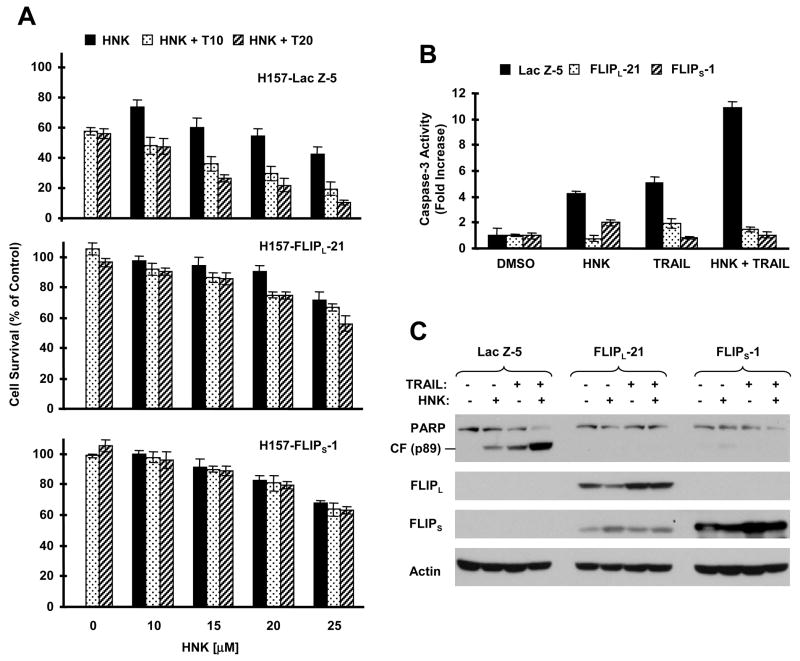
To determine the involvement of c-FLIP downregulation in HNK-mediated sensitization of death receptor-induced apoptosis, we examined the impact of enforced expression of ectopic c-FLIP on the apoptosis-inducing effects by HNK combined with TRAIL. Thus, we compared the effects of HNK plus TRAIL on the survival of three transfectants, H157-Lac Z-5, H157-FLIPL-21 and H157-FLIPS-1, which express the control protein Lac Z, FLIPL and FLIPS, respectively. As presented in Fig. 5A, the combination of HNK and TRAIL was more effective than each single agent in decreasing cell survival in H157-Lac Z-5 cells, but this enhanced effect was lost in H157-FLIPL-21 and H157-FLIPS-1 cells, indicating that enforced expression of ectopic FLIPL or FLIPS confers cell resistance to the combination of HNK and TRAIL. By measuring caspase-3 activity and PARP cleavage, we detected no increases in caspase-3 activity and PARP cleavage in both H157-FLIPL-21 and H157-FLIPS-1 cells exposed to HNK alone, TRAIL alone or in combination as compared to H157-Lac Z-5 cells, in which increased caspase-3 activity and PARP cleavage were observed upon these treatments, particularly HNK combined with TRAIL (Figs. 5B and 5C). These results further indicate that enforced expression of the ectopic c-FLIP abolishes HNK’s ability to augment TRAIL-induced apoptosis. In addition, we generated identical results in H460 transfectants that express Lac Z and FLIPL, respectively (supplemental Fig. S2). Together, we conclude that c-FLIP downregulation is a key event that mediates augmentation of the death receptor-mediated apoptosis by HNK.
Fig. 5. Enforced expression of ectopic c-FLIP confers resistance to induction of apoptosis by the combination of HNK and TRAIL.
A, The indicated transfectants were seeded in 96-well plates and treated with the indicated concentrations of HNK alone, 10 ng/ml TRAIL (T10) or 20 ng/ml TRAIL (T20) alone, or individual combination of HNK with TRAIL. After 24 h, the cells were subjected to the SRB assay for measurement of cell survival. Data are the means of four replicate determinations. Bars; ± SDs. B and C, The indicated transfectants were treated with DMSO, 25 μM HNK alone, 10 ng/ml TRAIL alone, or HNK plus TRAIL for 24 h and then subjected to preparation of whole-cell protein lysates for measuring caspase-3 activity using fluorometric assay (B) or for detecting PARP cleavage and c-FLIP using Western blotting (C).
We noted that HNK alone was less effective in decreasing the survival of H157-FLIPL-21 and H157-FLIPS-1 cells in comparison to that of H157-Lac Z-5 cells (Fig. 5A). In agreement, we found that HNK increased caspase-3 activity (Fig. 5B) and PARP cleavage (Fig. 5C) in H157-Lac Z-5 cells, but not in H157-FLIPL-21 and H157-FLIPS-1 cells. These results show that overexpression of ectopic c-FLIP also protects cells from HNK-induced apoptosis, suggesting that c-FLIP downregulation also plays a role in mediating HNK-induced apoptosis.
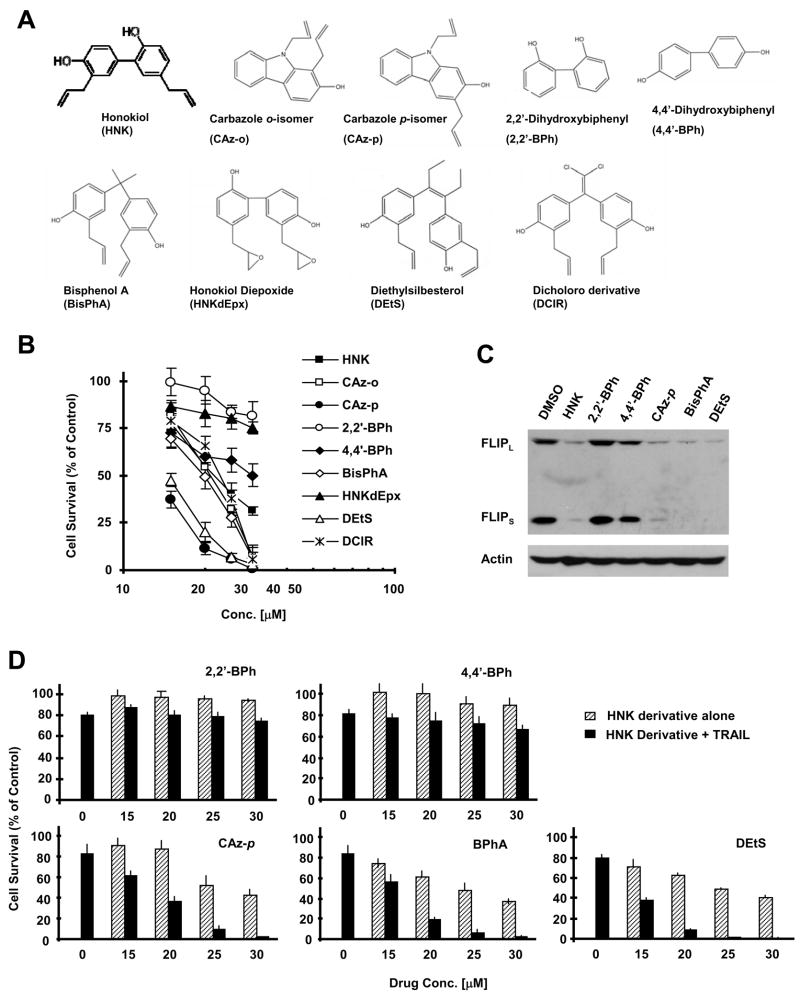
The Potencies of HNK Derivatives in Decreasing Cell Survival and Enhancing Death Receptor-mediated Apoptosis Are Associated with Their Abilities to Downregulate c-FLIP
To improve HNK’s anticancer efficacy, we synthesized some derivatives of HNK as presented in Fig. 6A. These derivatives had different activities in decreasing the survival of H226 cells. Among these compounds, 2,2′-BPh, 4,4′-BPh and HNKdEpx had the weakest activity, whereas DEtS and CAz-p had the most potent activity in decreasing cell survival. BisPhA, CAz-o and DCIR had activity in between, but slightly more potent than HNK in decreasing cell survival (Fig. 6B). By comparing their effects on modulation of c-FLIP levels, we found that both 2,2′-BPh and 4,4′-BPh had minimal effects on reducing c-FLIP levels, whereas CAz-p, particularly BisPhA and DEtS, were even more potent than HNK in decreasing c-FLIP levels (Fig. 6C). Accordingly, we found that 2,2′-BPh or 4,4′-BPh, when combined with TRAIL, did not exhibit augmented effects on decreasing cell survival; however, BisPhA, DEtS or CAz-p in combination with TRAIL showed more potent activity than HNK in decreasing cell survival (Fig. 6D). Collectively, these results demonstrate that there is a tight association between downregulation of c-FLIP and sensitization of death receptor-mediated apoptosis by HNK and its derivatives.
Fig. 6. Comparing the effects of HNK derivatives (A) on cell growth (B), downregulation of c-FLIP (C) and augmentation of TRAIL-induced apoptosis (D).
A, Chemical structures of HNK derivatives. B, H226 cells were seeded in 96-well plates and treated with the indicated concentrations of HNK. After 3 days, the cells were subjected to the SRB assay for measurement of cell survival. C, H226 cells were treated with 30 μM of the indicated HNK derivatives for 4 h and then subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis. D, H226 cells were seeded in 96-well plates and treated with the indicated concentrations of HNK derivative alone, 20 ng/ml TRAIL alone, or their combination. After 24 h, the cells were subjected to the SRB assay for measurement of cell survival. Data in B and D are the means of four replicate determinations. Bars; ± SDs.
Discussion
In this study, we have demonstrated that the natural product HNK and its derivatives inhibit the growth of human NSCLC cells and, more importantly, augment TRAIL-induced apoptosis in human NSCLC cells. To our knowledge, this is the first study demonstrating that HNK and its derivatives can function as sensitizers of death receptor-mediated apoptosis. Given that the tumor-selective TRAIL is a potential cancer therapeutic protein and is being tested in phase I clinical trials, our findings suggest a potential strategy for the utilization of HNK in combination with TRAIL in the treatment of lung cancer, and possibly other types of cancer. Thus, the current finding is of clinical significance and also warrants further evaluation on the efficacy of HNK combined with TRAIL in animal models.
By comparing the modulatory effects of HNK on the expression levels of several proteins involved in the regulation of apoptosis including c-FLIP, DR4, DR5, survivin, XIAP, Mcl-1, Bcl-2, Bcl-XL and Bax, c-FLIP, a major inhibitor of the death receptor-induced apoptosis, was rapidly downregulated in a dose-dependent manner in all of the tested NSCLC cell lines at 3 h post HNK treatment (Fig. 3C). Thus, it appears that HNK preferentially decreases the levels of c-FLIP (both FLIPL and FLIPS). Moreover, we have shown that HNK downregulates c-FLIP levels by facilitating ubiquitin/proteasome-mediated degradation of c-FLIP. This is evidenced by enhancement of c-FLIP turnover rates in HNK-treated cells, by prevention of the HNK-induced c-FLIP reduction using the proteasome inhibitor MG132, and by the increased levels of ubiquitinated c-FLIP, which are detected in cells co-treated with MG132 and HNK using immunoprecipitation-Western blotting (Fig. 4). Although JNK activation is suggested in regulating ubiquitin/proteasome-dependent degradation of FLIPL (11), we failed to demonstrate a role of JNK in mediating HNK-induced c-FLIP degradation based on the following facts: First, HNK decreases both forms of c-FLIP (i.e., FLIPL and FLIPS), whereas JNK regulates the degradation of only the long form of c-FLIP (i.e., FLIPL) (11). Second, HNK only slightly increases JNK activation in one cell line (i.e., A549), but not in another cell line (i.e., H226), in which c-FLIP was still downregulated. Third, the JNK inhibitor SP600125 does not prevent HNK-induced downregulation of c-FLIP.
It has been documented that modulation of c-FLIP levels alters cell sensitivity to death receptor-mediated apoptosis (7, 9, 35–38). In this study, enforced expression of ectopic FLIPL or FLIPS abrogated induction of apoptosis by HNK combined with TRAIL (Fig. 5), suggesting a critical role of c-FLIP downregulation in mediating the augmentation of TRAIL-induced apoptosis by HNK. Through studies on the HNK derivatives, we found that the potencies of these derivatives on enhancing TRAIL-induced apoptosis were tightly associated with their abilities to decrease c-FLIP levels (Fig. 6), further supporting the notion that c-FLIP downregulation is a key mechanism by which HNK and its derivatives sensitize TRAIL-induced apoptosis. Given that c-FLIP is a major inhibitor of death receptor-mediated apoptosis, we believe that HNK can function as a general sensitizer of the pathway. Indeed, HNK combined with Fas ligand or the agonistic Fas antibody also exhibits augmented effects on decreasing cell survival (supplemental Fig. S1). Thus, we speculate that HNK as well as its derivatives can also sensitize cancer cells to agonistic anti-DR4 or anti-DR5 antibody-triggered apoptosis, implying the potential utilization of HNK in combination with an agonistic anti-TRAIL death receptor antibody for cancer therapy.
It is known that TRAIL/TRAIL death receptor- and/or Fas ligand/Fas-mediated apoptosis are vital components of immunosurveillance of cancer cell by both T cells and NK cells (39, 40). Accordingly, upregulation of c-FLIP in tumor cells will protect tumor cells from being eradiated by this immunosurveillace mechanism and hence favors tumor immune escape (40). HNK downregulates c-FLIP in cancer cells, resulting in sensitization of cancer cells to both TRAIL- and Fas ligand-mediated apoptosis. These results imply that HNK may be able to sensitize cancer cell to T cell- and/or NK cell-mediated immunosurveillace or immunotherapy through such a mechanism, which needs further investigation in the future.
We noted that HNK alone had much weaker activity on decreasing cell survival and on increasing caspase-3 activity and PARP cleavage in cells expressing an ectopic FLIPL or FLIPS as compared to control cells expressing Lac Z (Fig. 5). These data also suggest that c-FLIP downregulation contributes to HNK-induced apoptosis. Several recent studies have documented that c-FLIP downregulation participates in the induction of apoptosis by certain types of anticancer agents including chemotherapeutic agents. Thus, the relationship between c-FLIP downregulation and HNK-induced apoptosis warrants further investigation.
It has been suggested that c-FLIP plays a critical role in anoikis resistance and distant tumor formation (41). Thus, c-FLIP may be a potential therapeutic target against advanced cancer or metastatic cancer. Accordingly, agents that specifically inhibit c-FLIP may have therapeutic potential for these types of cancers, particularly when combined with TRAIL or agonistic anti-DR4 or anti-DR5 antibody that triggers the death receptor-mediated apoptosis. HNK is a natural product purified from magnolia, a plant used in traditional Chinese and Japanese medicine and more recently used as a component of dietary supplements and cosmetic products (42). It is a systemically available and well-tolerated compound in mice with potent antitumor activity (21, 24, 25, 43). Importantly, the maximal plasma concentrations of HNK in mice can safely reach higher than 1 mg/ml (43). Moreover, HNK has a simple chemical structure and should be easily modified. Therefore, HNK is an ideal lead compound for synthesizing c-FLIP inhibitors with more potent activity than HNK in sensitizing the death receptor-mediated apoptosis and better cancer therapeutic efficacy.
We noted that HNK did modulate the expression of a few other apoptotic proteins such as DR4, DR5, Mcl-1 and survivin at either a high concentration (e.g., 30 uM) and/or relatively late times (e.g., 12 h). In some cell lines (e.g., H460 or H157), the modulation of these proteins by HNK also occurred at 3 h post treatment. In A549 cells, Mcl-1 levels were effectively reduced even by 15 μM HNK for a 3 h exposure (Fig. 3C). Given that these proteins are also involved in modulating death receptor-induced apoptosis (44), it is possible that the modulation of these proteins can also contribute to HNK-mediated sensitization of the death receptor-induced apoptosis to a certain extent, at least in some cell lines. It is likely that the initial HNK-mediated removal of c-FLIP by induction of its degradation followed by upregulation of DR4 and/or DR5 and decreases in the levels of the downstream inhibitors survivin and Mcl-1 make cancer cells sensitive to death receptor-induced apoptosis. Nonetheless, the roles of these proteins in modulating death receptor-induced apoptosis need further investigation.
It has been shown that HNK enhances TNFα-induced apoptosis by inhibiting TNF-induced NF-κB activation and the expression of certain antiapoptotic genes regulated by NF-κB including Mcl-1, survivin and c-FLIP (22). Our study aimed at modulation of basal levels of protein involved in regulation of apoptosis. Indeed, HNK effectively inhibited NF-κB activation induced by TNFα in our assay (see supplemental Fig. S3). Although TRAIL activates NF-κB in some cell systems or under certain conditions (45, 46), we failed to demonstrate that TRAIL increased NF-κB activity under the condition in our assay. Moreover, we found that HNK only minimally inhibited the basal levels of NF-κB activity (Fig. S3). Given that HNK promotes c-FLIP degradation as demonstrated in our study, we collectively suggest that it is unlikely that HNK sensitizes NSCLC cells to TRAIL-induced apoptosis through inhibition of NF-κB.
In summary, the present study for the first time demonstrates that the natural product HNK sensitizes death receptor-mediated apoptosis in human NSCLC cells by facilitating the ubiquitin/proteasome-mediated degradation of c-FLIP, thus warranting further in vivo evaluation of HNK combined with TRAIL or an agonistic TRAIL death receptor antibody as a potential cancer therapeutic regimen.
Supplementary Material
Acknowledgments
Grant support: Georgia Cancer Coalition Distinguished Cancer Scholar award (to S-Y. S.), Department of Defense grant W81XWH-04-1-0142-VITAL (to S-Y. Sun for Project 4), NIH/NCI SPORE P50 grant CA128613-01 (to S-Y. Sun for Project 2), National Institute of Health grant 5R01AR050727 (to J.L. Arbiser), Jamie Rabinowitch-Davis Foundation and the Minsk Foundation (to J. L. Arbiser) and a VA Merit award (to J. L. Arbiser).
We are grateful to Dr. X. Liu in our lab for establishing c-FLIP-expressing cell lines.
References
- 1.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 2.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–9. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Rowinsky EK. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol. 2005;23:9394–407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ. Targeting death-inducing receptors in cancer therapy. Oncogene. 2007;26:3745–57. doi: 10.1038/sj.onc.1210374. [DOI] [PubMed] [Google Scholar]
- 5.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–54. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6:196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- 7.Wajant H. Targeting the FLICE Inhibitory Protein (FLIP) in cancer therapy. Mol Interv. 2003;3:124–7. doi: 10.1124/mi.3.3.124. [DOI] [PubMed] [Google Scholar]
- 8.Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/critrevimmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Suh N, Sporn M, Reed JC. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem. 2002;277:22320–9. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- 10.Poukkula M, Kaunisto A, Hietakangas V, et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem. 2005;280:27345–55. doi: 10.1074/jbc.M504019200. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Kamata H, Solinas G, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–13. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Kamarajan P, Sun NK, Chao CC. Up-regulation of FLIP in cisplatin-selected HeLa cells causes cross-resistance to CD95/Fas death signalling. Biochem J. 2003;376:253–60. doi: 10.1042/BJ20030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longley DB, Wilson TR, McEwan M, et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–48. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 14.Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23:6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- 15.Wilson TR, McLaughlin KM, McEwan M, et al. c-FLIP: a key regulator of colorectal cancer cell death. Cancer Res. 2007;67:5754–62. doi: 10.1158/0008-5472.CAN-06-3585. [DOI] [PubMed] [Google Scholar]
- 16.Rogers KM, Thomas M, Galligan L, et al. Cellular FLICE-inhibitory protein regulates chemotherapy-induced apoptosis in breast cancer cells. Mol Cancer Ther. 2007;6:1544–51. doi: 10.1158/1535-7163.MCT-06-0673. [DOI] [PubMed] [Google Scholar]
- 17.Hibasami H, Achiwa Y, Katsuzaki H, et al. Honokiol induces apoptosis in human lymphoid leukemia Molt 4B cells. Int J Mol Med. 1998;2:671–3. doi: 10.3892/ijmm.2.6.671. [DOI] [PubMed] [Google Scholar]
- 18.Yang SE, Hsieh MT, Tsai TH, Hsu SL. Down-modulation of Bcl-XL, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem Pharmacol. 2002;63:1641–51. doi: 10.1016/s0006-2952(02)00894-8. [DOI] [PubMed] [Google Scholar]
- 19.Battle TE, Arbiser J, Frank DA. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood. 2005;106:690–7. doi: 10.1182/blood-2004-11-4273. [DOI] [PubMed] [Google Scholar]
- 20.Ishitsuka K, Hideshima T, Hamasaki M, et al. Honokiol overcomes conventional drug resistance in human multiple myeloma by induction of caspase-dependent and -independent apoptosis. Blood. 2005;106:1794–800. doi: 10.1182/blood-2005-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf I, O'Kelly J, Wakimoto N, et al. Honokiol, a natural biphenyl, inhibits in vitro and in vivo growth of breast cancer through induction of apoptosis and cell cycle arrest. Int J Oncol. 2007;30:1529–37. [PubMed] [Google Scholar]
- 22.Ahn KS, Sethi G, Shishodia S, Sung B, Arbiser JL, Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol Cancer Res. 2006;4:621–33. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 23.Konoshima T, Kozuka M, Tokuda H, et al. Studies on inhibitors of skin tumor promotion, IX. Neolignans from Magnolia officinalis. Journal of natural products. 1991;54:816–22. doi: 10.1021/np50075a010. [DOI] [PubMed] [Google Scholar]
- 24.Bai X, Cerimele F, Ushio-Fukai M, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–7. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 25.Shigemura K, Arbiser JL, Sun SY, et al. Honokiol, a natural plant product, inhibits the bone metastatic growth of human prostate cancer cells. Cancer. 2007;109:1279–89. doi: 10.1002/cncr.22551. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. Cellular FLICE-inhibitory protein down-regulation contributes to celecoxib-induced apoptosis in human lung cancer cells. Cancer Res. 2006;66:11115–9. doi: 10.1158/0008-5472.CAN-06-2471. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. CHOP-dependent DR5 induction and ubiquitin/proteasome-mediated c-FLIP downregulation contribute to enhancement of TRAIL-induced apoptosis by dimethyl-celecoxib in human non-small cell lung cancer cells. Mol Pharmacol. 2007;72:1269–79. doi: 10.1124/mol.107.037465. [DOI] [PubMed] [Google Scholar]
- 28.Sun SY, Yue P, Dawson MI, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–9. [PubMed] [Google Scholar]
- 29.Sun SY, Yue P, Hong WK, Lotan R. Augmentation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by the synthetic retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) through up-regulation of TRAIL receptors in human lung cancer cells. Cancer Res. 2000;60:7149–55. [PubMed] [Google Scholar]
- 30.Sun SY, Yue P, Wu GS, et al. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene. 1999;18:2357–65. doi: 10.1038/sj.onc.1202543. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–80. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Sun X, Ran Q, et al. Ubiquitin-proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene. 2005;24:3319–27. doi: 10.1038/sj.onc.1208497. [DOI] [PubMed] [Google Scholar]
- 33.Zou W, Liu X, Yue P, Khuri FR, Sun SY. PPARgamma Ligands Enhance TRAIL-induced Apoptosis through DR5 Upregulation and c-FLIP Downregulation in Human Lung Cancer Cells. Cancer Biol Ther. 2007;6:99–106. doi: 10.4161/cbt.6.1.3555. [DOI] [PubMed] [Google Scholar]
- 34.Roth W, Reed JC. FLIP protein and TRAIL-induced apoptosis. Vitam Horm. 2004;67:189–206. doi: 10.1016/S0083-6729(04)67011-7. [DOI] [PubMed] [Google Scholar]
- 35.Sharp DA, Lawrence DA, Ashkenazi A. Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem. 2005;280:19401–9. doi: 10.1074/jbc.M413962200. [DOI] [PubMed] [Google Scholar]
- 36.Xiao C, Yang BF, Song JH, Schulman H, Li L, Hao C. Inhibition of CaMKII-mediated c-FLIP expression sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Exp Cell Res. 2005;304:244–55. doi: 10.1016/j.yexcr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Rippo MR, Moretti S, Vescovi S, et al. FLIP overexpression inhibits death receptor-induced apoptosis in malignant mesothelial cells. Oncogene. 2004;23:7753–60. doi: 10.1038/sj.onc.1208051. [DOI] [PubMed] [Google Scholar]
- 38.Mathas S, Lietz A, Anagnostopoulos I, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med. 2004;199:1041–52. doi: 10.1084/jem.20031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature's TRAIL--on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- 40.French LE, Tschopp J. Defective death receptor signaling as a cause of tumor immune escape. Semin Cancer Biol. 2002;12:51–5. doi: 10.1006/scbi.2001.0405. [DOI] [PubMed] [Google Scholar]
- 41.Mawji IA, Simpson CD, Hurren R, et al. Critical role for Fas-associated death domain-like interleukin-1-converting enzyme-like inhibitory protein in anoikis resistance and distant tumor formation. J Natl Cancer Inst. 2007;99:811–22. doi: 10.1093/jnci/djk182. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Song Y, Zhang W, et al. Evaluation of the in vitro and in vivo genotoxicity of magnolia bark extract. Regul Toxicol Pharmacol. 2007 doi: 10.1016/j.yrtph.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Chen F, Wang T, Wu YF, et al. Honokiol: a potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol. 2004;10:3459–63. doi: 10.3748/wjg.v10.i23.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 45.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–48. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 46.MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicology letters. 2003;139:89–97. doi: 10.1016/s0378-4274(02)00422-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.