Abstract
Experimental autoimmune uveitis (EAU) serves as a model for human autoimmune uveitis and for cell-mediated autoimmunity in general. EAU induced in mice by immunization with the retinal Ag interphotoreceptor retinoid-binding protein in CFA is driven by the Th17 response. Oral calcitriol (1,25-dihydroxyvitamin D3) prevented as well as partly reversed disease and suppressed immunological responses. In vitro, calcitriol directly suppressed IL-17 induction in purified naive CD4+ T cells without inhibiting Th17 lineage commitment, as reflected by unaltered RORγt, STAT3, and FoxP3 expression. In contrast, in vivo treatment with calcitriol of mice challenged for EAU impaired commitment to the Th17 lineage, as judged by reduction of both RORγt and IL-17 in CD4+ T cells. Innate immune response parameters in draining lymph nodes of treated mice were suppressed, as was production of IL-1, IL-6, TNF-α, and IL-12/IL-23p40, but not IL-10, by explanted splenic dendritic cells (DC). Finally, supernatants of calcitriol-conditioned bone marrow-derived DC had reduced ability to support Th17 polarization of naive CD4+ T cells in vitro and in vivo. Thus, calcitriol appears to suppress autoimmunity by inhibiting the Th17 response at several levels, including the ability of DC to support priming of Th17 cells, the ability of CD4+ T cells to commit to the Th17 lineage, and the ability of committed Th17 T cells to produce IL-17.
The activated form of vitamin D (calcitriol), 1,25(OH)2D3, is a secosteroid hormone that has, in addition to its central function in calcium and bone metabolism, important effects on the growth and differentiation of many cell types, and pronounced immunoregulatory properties (1, 2). The biological effects of calcitriol are mediated by the vitamin D receptor (VDR),7 a ligand-dependent transcription factor that belongs to the superfamily of nuclear hormone receptors. VDR functions as a heterodimer with retinoid X receptor. Upon ligand binding, VDR undergoes a conformational change that promotes retinoid X receptor-VDR heterodimerization (3). The liganded heterodimer translocates to the nucleus, where VDR binds to the vitamin D response elements, induces chromatin-modifying enzymatic activities, and ultimately modulates gene transcription (4). VDR expression is found in most cell types of the immune system, including APCs such as macrophages and dendritic cells (DC), as well as both CD4+ and CD8+ T lymphocytes (1, 5).
Under appropriate stimuli, naive T cells can be driven to differentiate into distinct effectors, Th1, Th2, and Th17 cells (6, 7). VDR agonists are considered to be selective inhibitors of the Th1 pathway (8–10), whereas their effects on the Th2 pathway are controversial (11–13). It has been reported that calcitriol increases GATA3, a specific transcription factor for the Th2 response, and enhanced IgE levels, but it fails to promote development of Th2-mediated airway allergy (14). The effects of calcitriol on Th17 cells are still largely unknown.
The immunoregulatory properties of VDR agonists have been studied in different models of autoimmune diseases (8, 9, 15, 16). Furthermore, calcitriol is currently in clinical trials for treatment of secondary hyperparathyroidism, and is one of the most commonly used compounds for topical treatment of psoriasis (17, 18). Experimental autoimmune uveitis (EAU) is an animal model representing human autoimmune uveitis. Uveitis of a putative autoimmune origin accounts for ~10% of the severe visual handicap in the U.S. and is often accompanied by immune responses to retinal proteins (19 –21). The best-studied model of EAU is induced in B10.RIII mice by immunization with the retinal Ag interphotoreceptor retinoid-binding protein (IRBP) or its major uveitogenic peptide encoded by residues 161–180 in CFA. EAU in this model was recently shown to be Th17 driven (22–24). In the present study, we sought to examine the effects of calcitriol on EAU as a model of human uveitis and of cell-mediated autoimmunity in general, and to define the mechanisms involved.
We demonstrate in this study that calcitriol is able to prevent and to partially reverse EAU. This is accompanied by reduced production of IL-17. Suppression of the Th17 response by calcitriol appears to involve two independent mechanisms, as follows: direct inhibition of IL-17 production by CD4+ T cells and indirect inhibition of IL-17 lineage commitment by down-regulation of the ability of DC to support priming of T cells toward the Th17 effector pathway. Lineage commitment, as judged by induction of the Th17 lineage-specific transcription factor RORγt, is not impaired in the direct inhibitory effect on T cells, but is down-regulated in the indirect effect mediated by DC, thus permitting to distinguish between these two effects. This study indicates that calcitriol may act at several levels to suppress the proinflammatory uveitogenic Th17 response by affecting functions of both DC and CD4+ T cells.
Materials and Methods
Mice
B10.RIII female mice (H-2r) were purchased from The Jackson Laboratory or were bred in house from breeding stock purchased from The Jackson Laboratory. IFN-γ knockout (KO) mice on the C57BL/6 background (The Jackson Laboratory) were backcrossed for 10 generations onto the B10RIII background and were bred in house. FoxP3-GFP knockin mice were obtained from the University of Washington (25) and were backcrossed to B10.RIII. All mice were housed under specific pathogen-free conditions, fed standard laboratory chow ad libitum, and used between the ages of 6–8 wk. Treatment of animals was in compliance with institutional guidelines, and all animal study protocols were approved by the National Eye Institute Institutional Review Board.
Induction of EAU and treatment protocols
On day 0, EAU was induced by immunization with 8 μg/mouse of IRBP as in 0.2 cc in emulsion CFA that had been supplemented with Mycobacterium tuberculosis strain H37RA to 2.5 mg/ml. Delayed-type hypersensitivity (DTH) response was assessed by challenge on day 19 with 10 μg of IRBP in 10 μl injected into the ear pinna, and ear swelling was measured on day 21 with a spring-loaded micrometer (Mitutoya). EAU development was followed by funduscopic examination on day 13 and histological examination of eyes after H&E staining, as previously described. Briefly, the severity of EAU was evaluated in a masked fashion on a scale of 0–4 using published criteria based on the number, type, and size of lesions (19). Calcitriol powder (provided by M. Uskokvic, BioXell, Nutley, NJ) was dissolved in 100% alcohol at stock concentration of 2.4 mM and stored in −80°C until use. Treatment with calcitriol was given orally by gavage in 0.2 ml of miglyol 812 (0.5 μg/kg/day) as a prevention regimen (day −6 to day 21 postimmunization) or as a reversal regimen (days 7–21 postimmunization) 6 days per week. Vehicle controls were gavaged with Miglyol 812 only. Systemic neutralization of IL-17 and IFN-γ was performed by i.p. injection of 500 μg of clone 1D10 (provided by D. Cua, Schering-Plough/DNAX, Palo Alto, CA) and clone XMG1.2 (Harlan Bioproducts for Science), respectively, every other day, starting from day −1 through day 17.
CD4+ T cell isolation and culture
CD4+ T cells were enriched by negative selection using autoMACS (Miltenyi Biotic). The enriched CD4+ fraction was further sorted to 98–99% purity on a FACS Aria (BD Biosciences), into CD4+CD62L+CD44lowCD25− T cells (naive) or CD4+CD62L− CD44high (effector/memory phenotype). Sorted T cells were seeded into 96-well round-bottom plates at 2 ×105/well in RPMI 1640 medium supplemented with 2 mM L-glutamine, 5 ×10−5 M 2-ME, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 10% FBS. For culture under neutral conditions, the cells were stimulated with plate-bound anti-CD3 (plates were coated at 5 μg/ml) and soluble anti-CD28 (1 μg/ml) in medium supplemented as above. For culture under Th17-polarizing conditions, the cells were stimulated with anti-CD3 and anti-CD28, as above, in the presence of anti-IFN-γ (10 μg/ml), anti-IL-4 (10 μg/ml), IL-2 (20 U/ml), TGF-β1 (6 ng/ml), and IL-6 (20 ng/ml) for 72 h, as described previously (26).
Ag-specific in vitro responses
Draining lymph node (LN) cells at 2 × 105/well in 96-well plate were in vitro stimulated with 10 μg/ml IRBP in HL-1 medium for 48 h. In some experiments, 2 × 105/well of CD4+ T cells sorted from pooled LN and spleens were stimulated with 20 μg/ml IRBP in the presence of an equal amount of mytomycin C-treated APCs from naive spleen for 48 h. The cytokines in supernatant were assayed by Multiplex ELISA using SearchLight Arrays technology (27) (http://www.SearchLightOnline.com). Alternatively, production of IL-17 was assayed by sandwich ELISA (R&D Systems).
Forcing ectopic RORγt expression in CD4+ T cells by retroviral transduction
Forced RORγt expression in CD4+ T cells was performed as described by Ivanov et al. (26) with modifications. Briefly, full-length RORγt cDNA was synthesized and cloned into pMIG-W (RORγt-IRES-GFP). Phoenix cells at 95% confluence were transfected with the RORγt encoding or control plasmid (endofree) using Lipofectamine 2000 (Invitrogen). Viral supernatant was collected after 48 h and supplemented with polybrene. To generate RORγt-expressing cells, CD4+ T cells were activated for 24 h with anti-CD3 and anti-CD28 in the presence of anti-IFN-γ and anti-IL-4, and then the viral supernatant was added. The cells were spun at 2500 rpm for 1.5 h. After spin infection, the cells were recultured in the freshly prepared medium as before. A total of 10 ng/ml IL-2 was added 48 h later for 2 days. After 96 h, the GFP-positive population (expression of RORγt) cells were sorted by FACS to 98% purity.
DC conditioning with calcitriol
For splenic DC conditioning, mice were orally treated with calcitriol. On day 6, splenic DC were isolated with anti-CD11c magnetic microbeads, as previously described (24), and further sorted by FACS Aria to 95% purity. The cells were seeded at 2 × 105/well in a 96-well round-bottom plate and stimulated with 1:20 of M. tuberculosis extract overnight (24) with or without calcitriol. The cytokines in the supernatant were assayed by Multiplex ELISA.
For preparation of calcitriol-conditioned bone marrow-derived DC (BMDC), bone marrow cells from naive mice were cultured in 10 ng/ml GM-CSF with or without 10 nM calcitriol added 48 h after start of culture, as described by Griffin et al. (28). The medium was changed on days 2, 4, 6, and 8. On day 9, CD11c-positive cells were isolated with anti-CD11c magnetic microbeads. The cells were stimulated for 5 h with M. tuberculosis extract, which was prepared by sonication and repeated freeze-thawing of heat-killed M. tuberculosis H37RA (Sigma-Aldrich), as described previously (24), and the supernatants were collected for T cell culture. For adoptive transfer of DC, calcitriol-conditioned BMDC were pulsed with 100 μg/ml IRBP p161–180 and stimulated with M. tuberculosis extract for 5 h. After washing, one million cells were injected into one hind footpad. After 5 days, the draining LN were extracted and were restimulated with 20 μg/ml p161–180. After 48 h, the supernatants were collected for IL-17 assay by ELISA. Some cultures were collected after 6 h to assay RORγt mRNA expression.
Real-time PCR
To assay the RORγt expression, mRNA was extracted and reverse transcribed into cDNA. TaqMan quantitative PCR was performed per manufacturer’s instruction (assay identification: Mm01261022_m1; Applied Biosystems) using the TaqMan relative standard curve method. The expression levels of RORγt were normalized relative to the level of GAPDH expression. To assay the cytokine expression, 2−ΔΔCT method was used. GAPDH was used as endogenous control. PCR system was run by ABI 9700 following manufacturer’s protocol.
Western blotting for quantitation of STAT3 and phospho-STAT3
Purified CD4+ cells were treated with or without calcitriol under Th17-polarizing conditions. Whole-cell lysates were prepared in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, supplemented with a protease inhibitor mix). The lysates were centrifuged at 14,000 rpm for 5 min at 4°C, and the supernatants were collected and quantified for protein concentration. A total of 30 μg of protein was resolved on a 10% SDS-PAGE gel (Invitrogen Life Technologies) and then transferred to a nitrocellulose membrane (Whatman) using a semidry transfer cell apparatus (Bio-Rad). The membranes were treated with 5% nonfat milk for 2 h to block nonspecific binding, rinsed, and incubated with anti-phospho-STAT3 (Tyr705, pY-STAT3; Cell Signaling Technology) or anti-STAT3 (Signal Transduction Lab). Signals were detected with HRP-conjugated anti-rabbit or anti-mouse IgG using the ECL system (Amersham Biosciences).
Flow cytometric analysis
Purified CD4+ cells were collected from calcitriol-treated or control cultures stimulated with anti-CD3/CD28, as described above, at the indicated times. Eye-infiltrating cells were isolated on day 17 after immunization, as described (23). For surface marker analysis, cells were preincubated for 30 min at 4°C with Fc blocking Ab, before staining with fluorochrome-labeled Abs. For intracellular cytokine staining, eye-infiltrating cells were incubated with 10 ng/ml PMA/250 ng/ml ionomycin (Sigma-Aldrich) for 5 h. Cells were treated and stained using the BD Cytofix/Cytoperm kit and were analyzed on a BD FACSCalibur. At least 50,000 events per sample were collected using CellQuest software and were analyzed using FlowJo software. Fluorescent mAbs for flow cytometry analysis of T cell surface markers were CD4, CD44, CD62L, and CD25. Fluorescent mAbs for surface staining and for intracellular cytokine staining were FITC-conjugated CD4 (GK1.5), PE-conjugated anti-IL-17 (TC11-18H10), allophycocyanin-conjugated anti-IFN-γ (XMG1.2), and the appropriate isotype controls. The 7-aminoactinomycin D and annexin V-allophycocyanin staining were used to analyze cell viability. All mAbs were purchased from BD Biosciences.
Statistical analysis, reproducibility, and data presentation
Experiments were repeated at least twice, and usually three or more times. Response patterns were highly reproducible. Disease severity for each animal was calculated as an average of both eyes. Statistical analysis of EAU scores was performed by Snedecor and Cochran’s z test for linear trend in proportions. This is a nonparametric test based on frequency distribution that takes into account scores and incidence. All other statistical analyses were performed by independent two-tailed Student’s t test. Probability values of ≤ 0.05 were considered statistically significant. The data, whenever applicable, were presented as mean + SD, unless otherwise specified.
Results
Oral administration of calcitriol prevents retinal autoimmunity
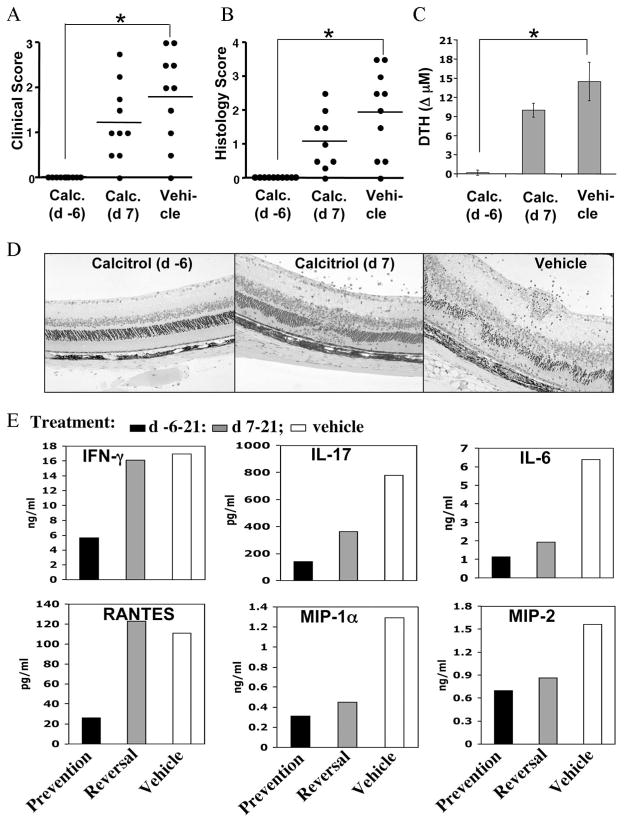
B10.RIII mice immunized for induction of EAU were treated orally with either a prevention regimen (starting on day −6), or a reversal regimen (starting on day +7) of calcitriol, as described in Materials and Methods. In mice treated with the preventive regimen, EAU pathology was completely suppressed, as determined by examination of the retina by funduscopy on day 13 (Fig. 1A). Mice remained free of disease on histological examination performed 21 days after immunization (Fig. 1, B and D). Preventive administration of calcitriol also strongly reduced DTH responses (Fig. 1C) and proinflammatory cytokine production by primed LN cells, including IFN-γ, IL-17, IL-6, RANTES, MIP-1α, and MIP-2 (Fig. 1E).
FIGURE 1.
Oral administration of calcitriol to B10.RIII mice prevents retinal autoimmune disease and decreases the uveitic response. Mice challenged for EAU were gavaged with 0.5 μg/kg calcitriol or vehicle (Miglyol 812) by the prevention or the reversal regimen. A, EAU scores by funduscopic examination on day 13. B, Final EAU readout by histology on day 21. The data reflect two combined experiments with five mice per group. Score for each mouse is an average of both eyes. C, Ag-specific DTH response in mice challenged in the ear pinna with IRBP 48 h earlier. The specific response is the difference between IRBP- and PBS-challenged ears. D, Representative histopathology of EAU in the different treatment groups. Mice treated with calcitriol from day −6 have a histological picture indistinguishable from normal. E, Ag-specific cytokine production by LN cells on day 21 after immunization measured by Multiplex ELISA in 48-h supernatants. Data are shown after background subtraction of samples without Ag stimulation. Pooled supernatants were used for the assay; therefore, although data represent a group average of five mice, no error bars could be generated. Shown is a representative experiment of two with similar results. *, p < 0.01.
In mice treated with the reversal regimen, EAU was also inhibited, although reduction of histology scores did not attain statistical significance (Fig. 1B, p = 0.13). This was accompanied by diminished production of some, but not all cytokines tested. In contrast to the prevention regimen, production of IFN-γ and the Th1-related chemokine RANTES by draining LN cells was not reduced (Fig. 1E). Oral administration of vehicle (miglyol 812) before or after immunization did not change uveitis scores, DTH responses, or Ag-specific cytokine profile, compared with untreated mice.
EAU induced by immunization depends on Th17, but not on IFN-γ
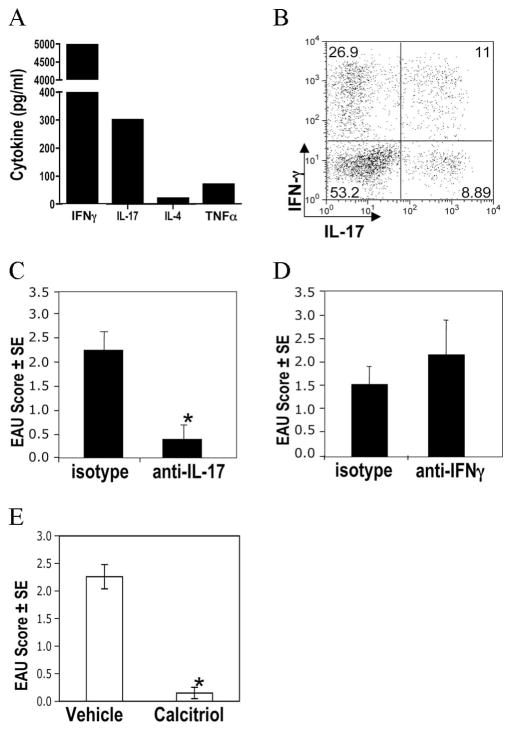
Analysis of Ag-induced cytokines showed that after immunization with IRBP in CFA, primed LN cells secrete both IFN-γ and IL-17 in response to Ag (Fig. 2A). Analysis of the infiltrating cells in uveitic eyes revealed approximately equal numbers of putative Th1 cells (CD4+ cells that were single IFN-γ producers) and putative Th17 cells (CD4+ cells that were single IL-17 producers or double IFN-γ/IL-17 producers) (Fig. 2B). However, treatment with neutralizing Abs to IFN-γ or to IL-17 revealed that only the latter was able to reduce EAU pathology (Fig. 2, C and D), in keeping with our previous observations (22, 23). This suggests that despite the mixed pattern of Th1 and Th17 responses induced, retinal pathology depends on Th17 effector rather than IFN-γ response. Furthermore, administration of calcitriol also protected IFN-γ KO mice from EAU (Fig. 2E), further supporting the notion that protection from EAU in this system is not mediated by inhibition of IFN-γ. This is not to suggest that other Th1-type cytokines, e.g., IL-6 and TNF-α, do not play an effector role in EAU. But these are not unique to Th1 cells and are produced by Th17 cells as well.
FIGURE 2.
Development of EAU induced with IRBP/CFA is dependent on IL-17. Mice were immunized with IRBP in CFA. A, Ag-specific cytokine secretion from LN cells as measured by Multiplex ELISA after 48-h culture. B, Cells isolated from uveitic eyes 17 days after immunization were incubated with PMA + ionomycin, and intracellular cytokine production was assayed by flow cytometry. Data shown are cells gated on CD4+ T cells. C and D, Mice were treated with neutralizing Abs to IL-17 (C) or to IFN-γ (D), or with the respective isotype. E, IFN-γ KO female mice were gavaged with calcitriol or vehicle from day −6 and immunized for EAU on day 0. Shown are scores by histology in eyes collected 17 days after immunization. Data represent one of two independent experiments with similar results. **, p < 0.01.
Calcitriol directly inhibits production of IL-17 by CD4+ T cells
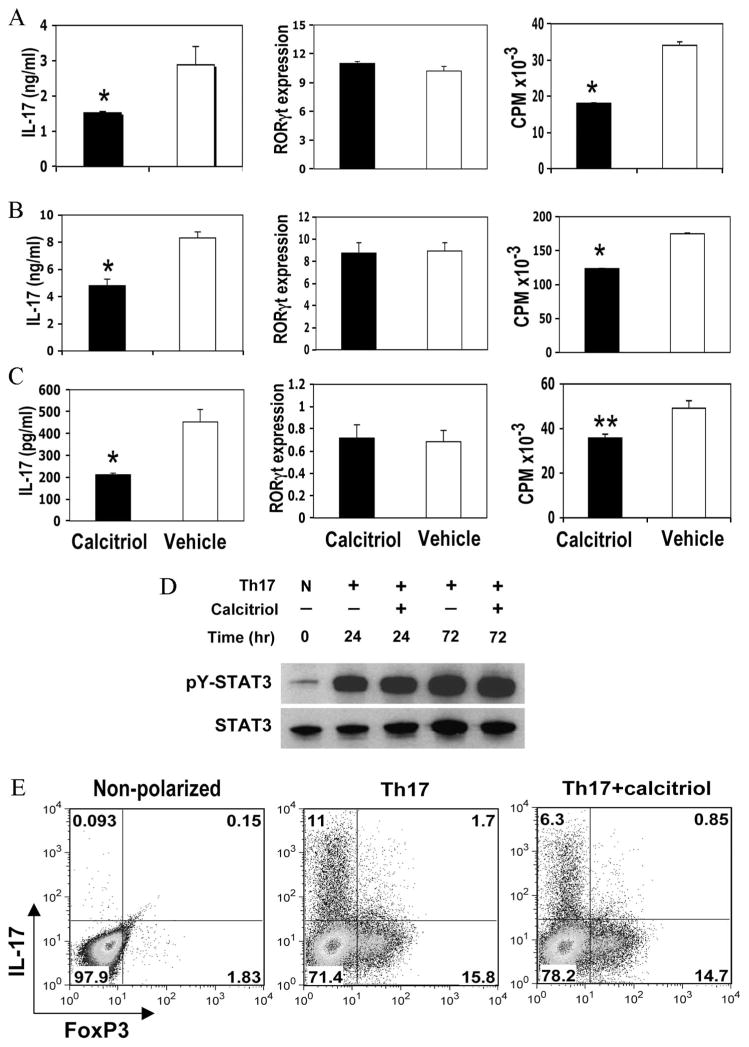
We next addressed the mechanism by which calcitriol inhibits the Th17 response and protects from EAU by examining its effects on Th17 cell generation in vitro from uncommitted precursors. T cells can be differentiated into IL-17-producing effector cells in the cytokine milieu containing IL-6, TGF-β, and IL-23 (29, 30). Highly purified naive CD4 T cells were stimulated via the TCR under Th17-polarizing conditions, as described by Ivanov et al. (26). Presence of 10 nM calcitriol in the culture significantly inhibited IL-17 production at 72 h of culture (p < 0.01; Fig. 3A). We also examined different culture conditions, including calcitriol concentrations from 5 to 100 nM and culture duration from 24 h to 5 days. All conditions consistently revealed various levels of IL-17 reduction (data not shown). Calcitriol also slightly inhibited proliferation (p < 0.05), similar to the effects on naive CD4+, Th1, and Th2 cells.
FIGURE 3.
Calcitriol treatment in vitro inhibits IL-17 production by CD4+ T cells without decreasing RORγt expression. CD4+ T cells from spleens of naive B10.RIII mice were stimulated with plate-bound anti-CD3 plus soluble anti-CD28 in the presence of 10 nM calcitriol or vehicle. After 72 h, IL-17 production was assayed by ELISA, proliferation was measured by [3H]thymidine incorporation, and intracellular staining for IL-17 and FoxP3 was performed. Relative expression levels of RORγt were measured by quantitative real-time PCR after 12 h and were normalized to GAPDH expression level. STAT3 and phospho-STAT3 expression was evaluated at the indicated times. A, Naive CD4+ T cells at 2 × 105/well cultured under Th17-polarizing conditions. B, CD4+ T cells of effector/memory phenotype cultured under Th17-polarizing conditions. C, Effector/Memory CD4+ T cells cultured under neutral conditions. *, p < 0.01; **, p < 0.05. D, STAT3 and phospho-STAT3 expression were evaluated in CD4+ cells cultured under nonpolarizing (N) or Th17-polarizing conditions in the presence or absence of calcitriol by Western blotting at the indicated times. E, CD4+ T cells from GFP-FoxP3 knockin mice were stimulated under neutral or Th17 conditions, in the presence or absence of calcitriol. PMA/Ionomycin and brefeldin A were added during the last 3 h, followed by intracellular staining for IL-17 and FoxP3. The data shown are from one of at least three independent experiments with similar results.
RORγt was recently identified as the transcription factor controlling T cell lineage commitment to IL-17 production (7). We, therefore, examined the effect of calcitriol on RORγt expression by real-time PCR in naive T cells cultured under Th17-polarizing conditions, as described in Materials and Methods. Surprisingly, calcitriol did not reduce RORγt mRNA expression in CD4+ T cells at 12 h of culture (Fig. 3A). We further examined RORγt expression from up to 36 h of culture, but even at later time points no reduction of RORγt expression was apparent (data not shown). To investigate whether inhibition could vary with T cell differentiation status, we sorted effector/memory phenotype (CD62L−CD44low) CD4+ T cells and cocultured them with calcitriol under Th17-favoring or neutral conditions. Again, no apparent inhibition of RORγt expression was seen (Fig. 3, B and C). In line with this, phosphorylation of STAT3, which is upstream of RORγt and regulates its expression (31), was also not measurably affected for up to 72 h in culture, as determined by Western blotting (Fig. 3D).
Recently, IL-2 has been shown to inhibit IL-17 commitment through activation of STAT5 (32). To assess whether IL-2 in the culture could contribute to inhibition of Th17 polarization, we examined effects of calcitriol on naive CD4+ T cells under polarization conditions, as in Fig. 3A, but without IL-2 and instead adding IL-23. This change in culture conditions did not alter the effects on T cells, as examined at the 72-h time point, in terms of IL-17 production, RORγt expression, or proliferation (data not shown).
Because of the reciprocal relationship that exists between Th17 and T regulatory (Treg) cells, it was important to examine whether inhibition of IL-17 might be due to induction of FoxP3 and conversion to a Treg phenotype. To address this question, we used CD4+ T cells purified from FoxP3-GFP knockin mice (25) back-crossed at least five generations onto the B10.RIII background. Moderate levels of FoxP3 expression were induced under Th17-polarizing conditions in parallel to intracellular IL-17. Addition of calcitriol reduced the number of cells staining for intracellular IL-17, but did not enhance FoxP3 expression during the 72-h time frame of the assay (Fig. 3E).
Calcitriol inhibits IL-17 production by T cells with forced expression of RORγt
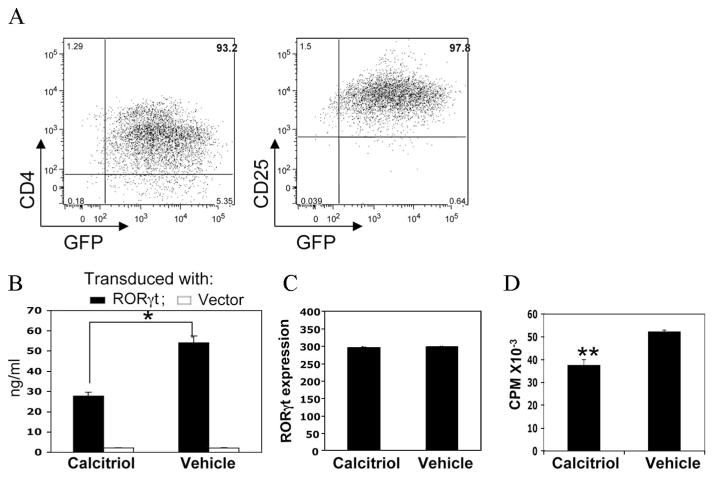
To investigate whether calcitriol can inhibit IL-17 production in T cells with forced ectopic expression of RORγt, CD4+ T cells were activated by CD3 ligation and were transduced with a retroviral construct of RORγt-GFP prepared as described Ivanov et al. (26), as described in Materials and Methods. On day 5, GFP-expressing cells from the expanding cultures and from control GFP vector-transduced cells were sorted and characterized phenotypically and functionally. Most CD4+ GFP+ cells expressed CD25 (Fig. 4A). GFP-RORγt-expressing cells were then stimulated with anti-CD3/IL-2 for 48 h in the presence or absence of calcitriol. Large amounts of IL-17 were produced by cells transduced with the RORγt plasmid, but not with the control plasmid. Similarly to cells expressing RORγt endogenously, we found significant reduction by calcitriol of IL-17 production by cells expressing RORγt ectopically (Fig. 4B), in the face of unchanged RORγt expression (Fig. 4C). Consistent with data in endogenously RORγt-expressing T cells shown above, calcitriol only marginally reduced proliferation (Fig. 4D). These results indicate that calcitriol can directly inhibit production of IL-17 from T cells without affecting induction and levels of RORγt. The effect may involve events downstream of RORγt that are related to IL-17 production, but do not regulate RORγt expression.
FIGURE 4.
Calcitriol inhibits IL-17 production by CD4+ T cells expressing ectopic RORγt. Naive CD4+ T cells were activated with anti-CD3/anti-CD28 in presence of anti-IFN-γ and anti-IL-4 mAbs, and retrovirally transduced with GFP-RORγt or GFP control plasmid. After 96 h, cells were sorted for GFP and were restimulated with anti-CD3 plus IL-2 with or without calcitriol. A, Phenotype of FACS-sorted, RORγt-expressing T cells before restimulation with anti-CD3 mAb. B, IL-17 production by sorted cells after 48 h of activation. C, RORγt expression by sorted cells after 12 h of activation. The relative expression levels of RORγt were normalized to the level of GAPDH expression. D, Proliferation by [3H]thymidine uptake after 48 h of activation. *, p < 0.01; **, p < 0.05. The data represent one of two independent experiments with similar results.
Calcitriol treatment in vivo suppresses both the Th17 response and lineage commitment
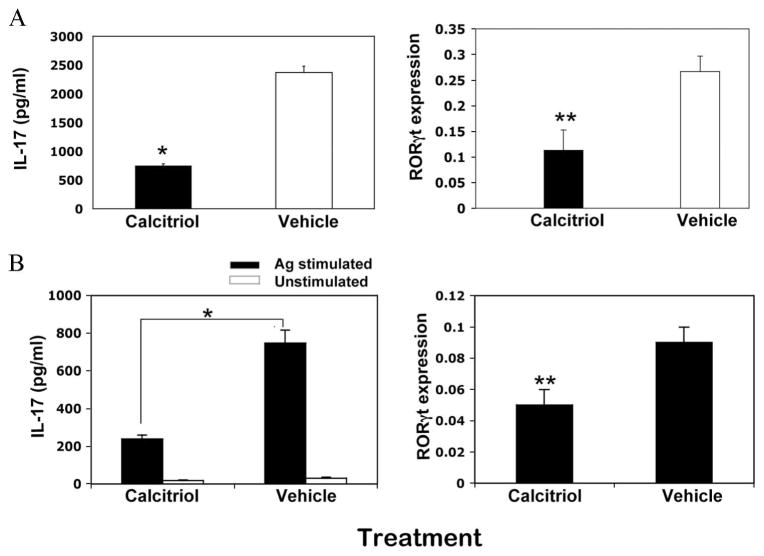
We next examined the effects of calcitriol treatment on the development of the Th17 response in vivo. Mice were gavaged with calcitriol daily from day −6 and were immunized on day 0, and lymphoid organs were collected on day 9. Isolated CD4+ T cells from primed LN and spleens were stimulated under nonpolarizing conditions with soluble anti-CD3 or IRBP in the presence of mytomycin C-treated APCs from naive spleen, such that any IL-17 production would derive from in vivo polarization. After 72 h in culture, IL-17 production was significantly reduced in mice that had been treated with calcitriol in vivo (Fig. 5). Unlike the Th17 cells induced in vitro, RORγt expression in Th17 cells induced in vivo under the cover of calcitriol treatment was reduced, suggesting not only inhibition of IL-17 synthesis, but also inhibition of Th17 lineage commitment (Fig. 5). The results indicate that calcitriol treatment in vivo suppresses both the Th17 response and lineage commitment, and suggest the involvement of factors additional to direct suppressive effects on IL-17 production by CD4+ T cells.
FIGURE 5.
Calcitriol treatment in vivo suppresses the Th17 response. B10.RIII mice were treated orally with calcitriol in the prevention regimen and were immunized with a uveitogenic regimen of IRBP on day 0. Draining LN and spleen cells were pooled, and CD4+ T cells were purified by negative immunomagnetic selection and FACS sorting, and were stimulated with anti-CD3 (A) or 20 μg/ml IRBP in the presence of mytomycin-treated splenic APCs (B). IL-17 production in the supernatants after 72 h was assayed by ELISA. RORγt expression after 12 h was assayed by real-time PCR. The relative expression levels of RORγt are normalized to GAPDH. The data represent one of three independent experiments with similar results. *, p < 0.01; **, p < 0.05.
Calcitriol-conditioned DC have a reduced ability to prime for a Th17 response
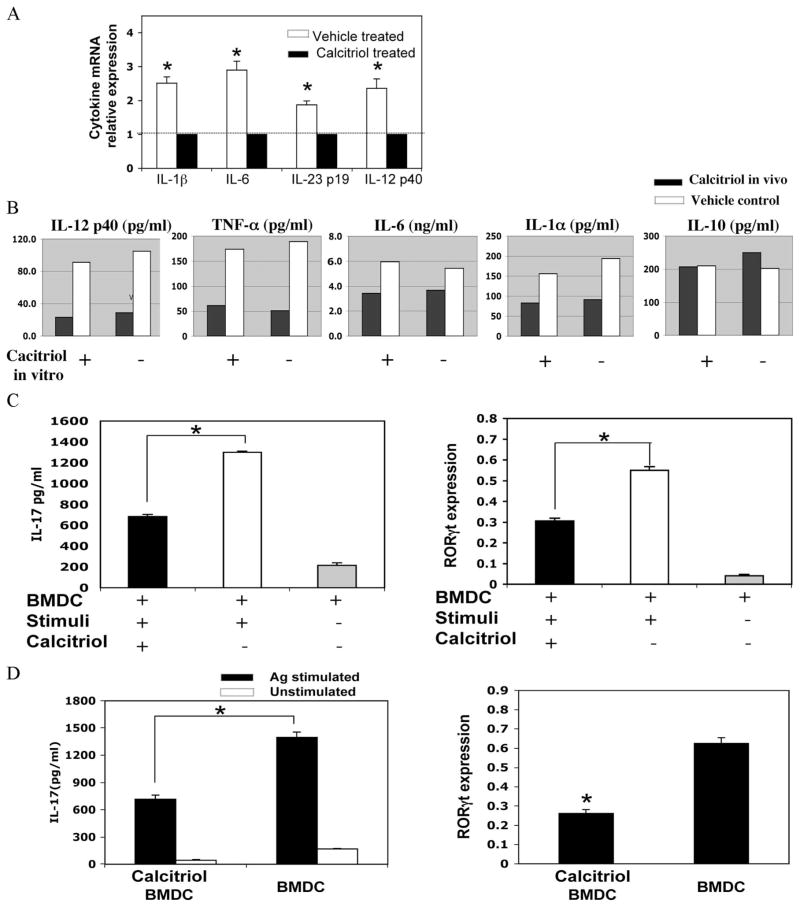
In vitro induction of Th17 cells can be achieved directly with anti-CD3/CD28 and cytokines. However, priming of Th17 cells in vivo requires DC that present Ag, provide costimulatory signals, and help create the cytokine milieu of IL-1, IL-6, TGF-β, TNF-α, and IL-23 (26, 29, 30). We therefore examined the cytokine response in mice treated with calcitriol from day −6 and immunized on day 0 for EAU. Draining LN cells were collected 48 h after immunization, and cytokine expression was assayed ex vivo without further stimulation using real-time PCR. LN cells of mice treated with calcitriol had reduced expression of message for IL-12p40, IL-23p19, IL-6, and IL-1β compared with control (Fig. 6A). In another type of experiment, we examined the ability of DC from calcitriol-treated mice to respond to M. tuberculosis (the microbial stimulant incorporated in CFA) in vitro. Naive mice were orally treated with calcitriol for 6 days, and their splenic CD11c+ DC were isolated and stimulated with M. tuberculosis extract overnight. Multiplex ELISA of the supernatants revealed that DC exposed in vivo to calcitriol had impaired ability to respond to M. tuberculosis stimulation by production of IL-12p40, TNF-α, IL-6, and IL-1α, but IL-10 production was not reduced.
FIGURE 6.
Calcitriol-conditioned DC have a reduced ability to prime the Th17 response. A, Naive B10.RIII mice were gavaged with calcitriol or vehicle for 6 days and were immunized with a uveitogenic regimen of IRBP in CFA. After 48 h, the draining LN were extracted and the cytokine expression was quantitated by real-time PCR using the 2−ΔΔCT method. Data were combined from two independent experiments. B, Naive B10.RIII were gavaged with calcitriol or vehicle for 6 days, but were not immunized. Splenic CD11c+ DC were stimulated with M. tuberculosis extract in the presence or absence of calcitriol overnight. Cytokines in the supernatant were assayed by Multiplex ELISA. C, BMDC conditioned or not with calcitriol were stimulated with M. tuberculosis extract for 5 h. The supernatant at 1/10 dilution was added to naive CD4+ T cells stimulated with anti-CD3/anti-CD28 mAbs. IL-17 in the supernatants was assayed after 48 h. RORγt expression was quantitated after 12 h. The relative expression levels of RORγt are normalized to GAPDH. D, BMDC conditioned or not with calcitriol were pulsed with IRBP p161–180 and M. tuberculosis extract for 5 h. One million cells were injected into footpads of B10.RIII mice. After 5 days, draining LN were extracted and recalled with p161–180. RORγt was quantitated after 6 h, and IL-17 production was measured after 48 h. *, p < 0.05. Data represent one of two independent experiments with similar results.
We then asked whether impaired cytokine production from DC in calcitriol-treated mice might underlie their reduced ability to mount a Th17 effector response. Because larger amounts of supernatant were required than could be produced from splenic DC obtained from in vivo treated mice, we used BMDC. BMDC cultured as described in Materials and Methods were conditioned, or not, with calcitriol, washed, and stimulated with M. tuberculosis extract to induce cytokine production. Their culture supernatant was collected and assayed for ability to support Th17 cell development. The results confirmed that naive CD4 T cells activated with anti-CD3/anti-CD28 in presence of M. tuberculosis-elicited supernatant from calcitriol-conditioned BMDC expressed less RORγt and produced less IL-17 than parallel T cells activated in presence of M. tuberculosis-elicited supernatants from nonconditioned BMDC (Fig. 6C). Supplementation of the cultures with exogenous IL-6 (to reach 4 ng/ml) restored about one-half of the deficit in IL-17 secretion by the T cells, suggesting that inhibition of IL-6 was partly responsible for the effect.
For an in vivo parallel, the calcitriol-conditioned or control BMDC after M. tuberculosis stimulation and pulsing with IRBP p161–180 were injected into the footpads of B10.RIII mice. The draining LN cells were collected after 5 days, and responses were recalled in vitro with the IRBP p161–180 peptide. Similar to T cells from IRBP-immunized mice that were treated in vivo with calcitriol (Fig. 5), draining LN cells of mice injected with calcitriol-conditioned DC produced less IL-17 and expressed reduced RORγt levels (Fig. 6D). These data suggested that calcitriol reduced the ability of DC to prime for a Th17 response, separately from and in addition to its direct effect on IL-17 production by CD4+ T cells.
Discussion
The data in the present study demonstrate that oral administration of calcitriol prevents EAU and reduces the associated immunological responses, including DTH responses and Ag-specific production of proinflammatory cytokines by primed LN cells. Calcitriol administration started 7 days after disease induction reduces disease scores by one-half, inhibiting DTH and most cytokine responses.
Although EAU induced by immunization of IRBP in CFA manifests as a mixed Th1/Th17 response, neutralization studies indicate that IL-17 is necessary for pathogenesis of the disease. Furthermore, recent publications indicate that IL-17 may be involved in human uveitis and scleritis, as well as in uveitis accompanying Vogt-Koyanagi-Harada disease (33, 34). Our interest was, therefore, to dissect the effects of calcitriol on induction of the Th17 response. To examine direct inhibitory effects on T cells, we triggered Th17 polarization in purified CD4+ T cells in vitro, in the absence of APCs, by TCR and CD28 ligation in presence of Th17-polarizing Abs and cytokines (anti-IFN-γ, anti-IL-4, TGF-β1, IL-6, and IL-2) (26). Irrespective of whether sorted naive or effector/memory phenotype T cells were used, calcitriol diminished IL-17 production. This effect was independent of culture conditions and occurred even in the absence of IL-2 and in presence of IL-23. The inhibitory effect on proliferation under Th17-polarizing conditions is consistent with the capacity of calcitriol to inhibit naive T, Th1, and Th2 cell proliferation (11). It is interesting to note that cytokine inhibition does not always parallel inhibition of proliferation. In our hands, inhibition of proliferation under Th17-polarizing conditions was accompanied by reduced IL-17 production. Other investigators reported suppression of IFN-γ and IL-2 from Th1; however, production of IL-4 from Th2 was enhanced in the presence of calcitriol (9, 11, 13, 35). Thus, effects on cytokine production vs proliferation can be dissociated.
Murine RORγt is a splice variant of RORγ encoded by the RORc gene located on chromosome 3. It was identified exclusively in cells of lymphoid lineage (36). Naive spleen T cells do not express RORγt (37). As a lineage-specific transcription factor, RORγt is necessary for differentiation of Th17 cells and is also sufficient to direct the expression of IL-17, the hallmark cytokine of this lineage. Under conditions of genetic absence of RORγt, IL-17+ cells in the lamina propria were reduced at least 10-fold, and Th17 polarization in vitro was reduced at least 50-fold (7, 26). Our study reveals that calcitriol can inhibit IL-17 production without suppressing either endogenous or ectopically expressed RORγt levels, irrespective of the stage of differentiation of the affected T cells, and does not involve induction of FoxP3 or inhibition of STAT3 phosphorylation. Thus, calcitriol inhibits IL-17 production separately and independently of processes affecting specific lineage commitment. Recently, other studies revealed that some widely distributed genes are also required for the full Th17 cell differentiation, including NF-AT, STAT3, and IFN regulatory factor 4, as reviewed previously (7, 38). NF-AT is a transcription factor involved in T cell activation via TCR ligation, and a molecular target for calcitriol (39). Although the details of how calcitriol impairs Th17 lineage differentiation at the transcriptional level remain to be studied further, our current data raise two non-mutually exclusive possibilities, as follows: 1) the inhibitory effect occurs downstream of STAT3 and RORγt induction, or 2) full Th17 development requires cooperation of genes regulated by RORγt with other genes, such as NF-AT or NF-κB. The ability to inhibit IL-17 production by lineage-committed T effectors may explain the ability of calcitriol to reduce disease scores and immunological responses in mice treated with the reversal regimen started 7 days after uveitogenic immunization, a time when effector cells had already been generated.
The enhanced effectiveness of calcitriol in a prevention regimen compared with a reversal regimen could be due, at least in part, to its dual effects on DC and T cells, combining mechanisms acting before as well as after Th17 lineage commitment. The VDR-mediated suppression of RelB in APCs is considered to play an essential role in its regulatory function at the level of the innate response (40). DC conditioned by calcitriol inhibit Th1 responses by increasing IL-10 and decreasing IL-12 production (15, 35). Our present data provide direct evidence that DC are a target of calcitriol also for inhibition of Th17 response in vivo and in vitro by decreasing the IL-17-polarizing cytokines IL-1, IL-6, IL-12/IL-23p40, and IL-23p19. Previous publications reported induction of Treg cells in vivo by functional criteria (41) and confers on myeloid DC (although not plasmacytoid DC) tolerogenic properties that enhance induction of CD4+CD25+FoxP3+ Treg cells (42). Although we did not see a clear up-regulation of FoxP3+ T cells in the peripheral lymphoid organs of calcitriol-treated mice (data not shown), Ag-specific Treg cells are only a tiny fraction of total FoxP3+ cells, so that a change in their number, even if functionally meaningful, might easily be missed. Calcitriol was also reported to prompt a response biased toward Th2 (14, 16), although how calcitriol-educated DC contribute to a Th2 response is not well understood. In the present study, we did not observe evidence supporting Th2 skewing of the response in vivo in either wild-type or IFN-γ KO mice protected by calcitriol treatment, as judged by unaltered IL-4 production (data not shown).
In conclusion, our data suggest that in vivo calcitriol acts not only on T cells to inhibit IL-17 production, but also on DC, with consequent impairment of the induction of Th17 response at the level of priming and lineage commitment. The ability of calcitriol not only to prevent, but also to partially reverse the disease process suggests that VDR agonists could potentially be useful in the clinical setting as an approach to therapy of uveitis. Although procalcemic effects of calcitriol limit its use as a therapeutic agent, synthetic VDR agonists have been developed that have lower toxicity and improved efficacy (43). It would be of interest to examine whether the direct in vitro inhibitory effects on Th17 cells may be applicable to screening synthetic VDR agonists to identify candidates with the desired therapeutic effect.
Acknowledgments
We thank the staff of the National Eye Institute Histology Core Facility for expert processing of the eyes for histopathological evaluation. We thank Drs. Dan Cua and Edward Bowman for their gift of anti-IL-17 Abs.
Footnotes
Abbreviations used in this paper: VDR, vitamin D receptor; BMDC, bone marrow-derived dendritic cell; CT, cycle threshold; DC, dendritic cell; DTH, delayed-type hypersensitivity; EAU, experimental autoimmune uveitis; IRBP, interphotoreceptor retinoid-binding protein; KO, knockout; LN, lymph node; Treg, T regulatory.
Disclosures
L. Adorini is a past employee of BioXell and has been involved with development of calcitriol and its derivatives as therapeutic agents.
This work was supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health.
References
- 1.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 2.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 3.Lemon BD, Freedman LP. Selective effects of ligands on vitamin D3 receptor- and retinoid X receptor-mediated gene activation in vivo. Mol Cell Biol. 1996;16:1006–1016. doi: 10.1128/mcb.16.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D3 receptor: a network of coactivator interactions. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 5.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 6.Glimcher LH. Lineage commitment in lymphocytes: controlling the immune response. J Clin Invest. 2001;108:s25–s30. [PubMed] [Google Scholar]
- 7.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Sanvito F, Doglioni C, Adorini L. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504–8511. doi: 10.4049/jimmunol.177.12.8504. [DOI] [PubMed] [Google Scholar]
- 9.Van Halteren AG, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C. Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D3. Diabetes. 2002;51:2119–2125. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Khalifa B, Yee YK, Lu J, Memezawa A, Savkur RS, Yamamoto Y, Chintalacharuvu SR, Yamaoka K, Stayrook KR, et al. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J Clin Invest. 2006;116:892–904. doi: 10.1172/JCI25901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 12.Staeva-Vieira TP, Freedman LP. 1,25-Dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168:1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 13.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 14.Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J Immunol. 2004;173:3432–3436. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 15.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 17.Mathieu C, Jafari M. Immunomodulation by 1,25-dihydroxyvitamin D3: therapeutic implications in hemodialysis and renal transplantation. Clin Nephrol. 2006;66:275–283. doi: 10.5414/cnp66275. [DOI] [PubMed] [Google Scholar]
- 18.Van de Kerkhof PC, Wasel N, Kragballe K, Cambazard F, Murray S. A two-compound product containing calcipotriol and β methasone dipropionate provides rapid, effective treatment of psoriasis vulgaris regardless of baseline disease severity. Dermatology. 2005;210:294–299. doi: 10.1159/000084753. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med. 2004;102:395–419. doi: 10.1385/1-59259-805-6:395. [DOI] [PubMed] [Google Scholar]
- 20.Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB. A new model of autoimmune disease: experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495. [PubMed] [Google Scholar]
- 21.Caspi RR, Chan CC, Wiggert B, Chader GJ. The mouse as a model of experimental autoimmune uveoretinitis (EAU) Curr Eye Res. 1990;9(Suppl):169–174. doi: 10.3109/02713689008999438. [DOI] [PubMed] [Google Scholar]
- 22.Caspi RR, Chan CC, Grubbs BG, Silver PB, Wiggert B, Parsa CF, Bahmanyar S, Billiau A, Heremans H. Endogenous systemic IFN-γ has a protective role against ocular autoimmunity in mice. J Immunol. 1994;152:890–899. [PubMed] [Google Scholar]
- 23.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang J, Zhu W, Silver PB, Su SB, Chan CC, Caspi RR. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol. 2007;178:5578–5587. doi: 10.4049/jimmunol.178.9.5578. [DOI] [PubMed] [Google Scholar]
- 25.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 26.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Moody MD, Van Arsdell SW, Murphy KP, Orencole SF, Burns C. Array-based ELISAs for high-throughput analysis of human cytokines. BioTechniques 31. 2001;186–190:192–194. doi: 10.2144/01311dd03. [DOI] [PubMed] [Google Scholar]
- 28.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701–708. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 29.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 32.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 34.Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, Chen L, Zhou H, Huang X, Kijlstra A. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol. 2007;119:1218–1224. doi: 10.1016/j.jaci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 36.Eberl G, Littman DR. The role of the nuclear hormone receptor RORγt in the development of lymph nodes and Peyer’s patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 37.He YW, Deftos ML, Ojala EW, Bevan MJ. RORγt, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1α,25-dihydroxyvitamin D3-mediated effects. J Immunol. 1998;160:209–218. [PubMed] [Google Scholar]
- 40.Griffin MD, Dong X, Kumar R. Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation. Arch Biochem Biophys. 2007;460:218–226. doi: 10.1016/j.abb.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 42.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 43.Amuchastegui S, Daniel KC, Adorini L. Inhibition of acute and chronic allograft rejection in mouse models by BXL-628, a nonhypercalcemic vitamin D receptor agonist. Transplantation. 2005;80:81–87. doi: 10.1097/01.tp.0000164619.49828.7a. [DOI] [PubMed] [Google Scholar]