Abstract
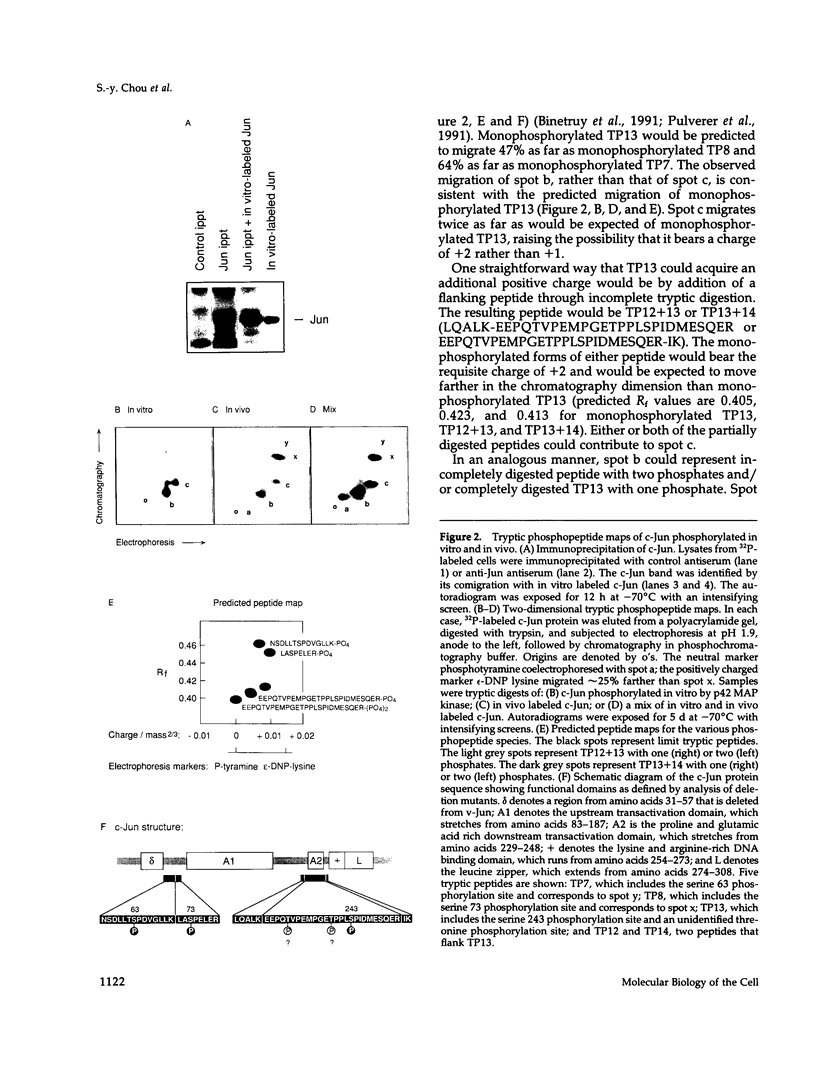
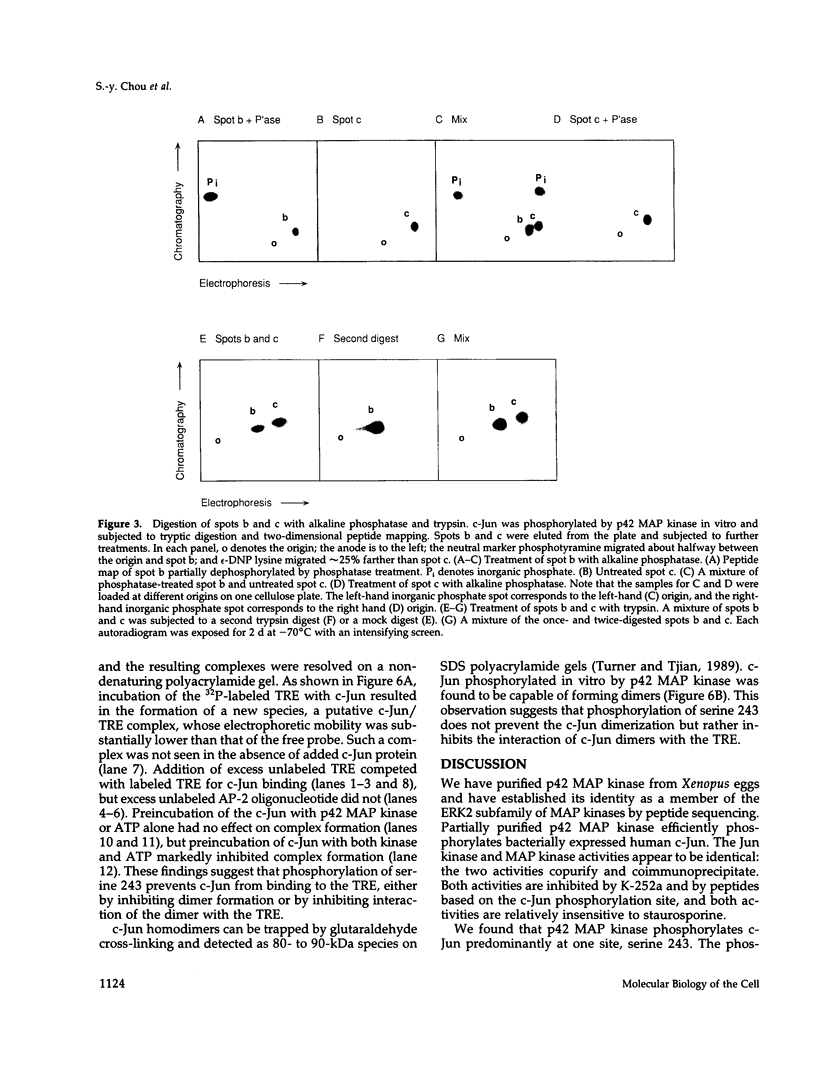
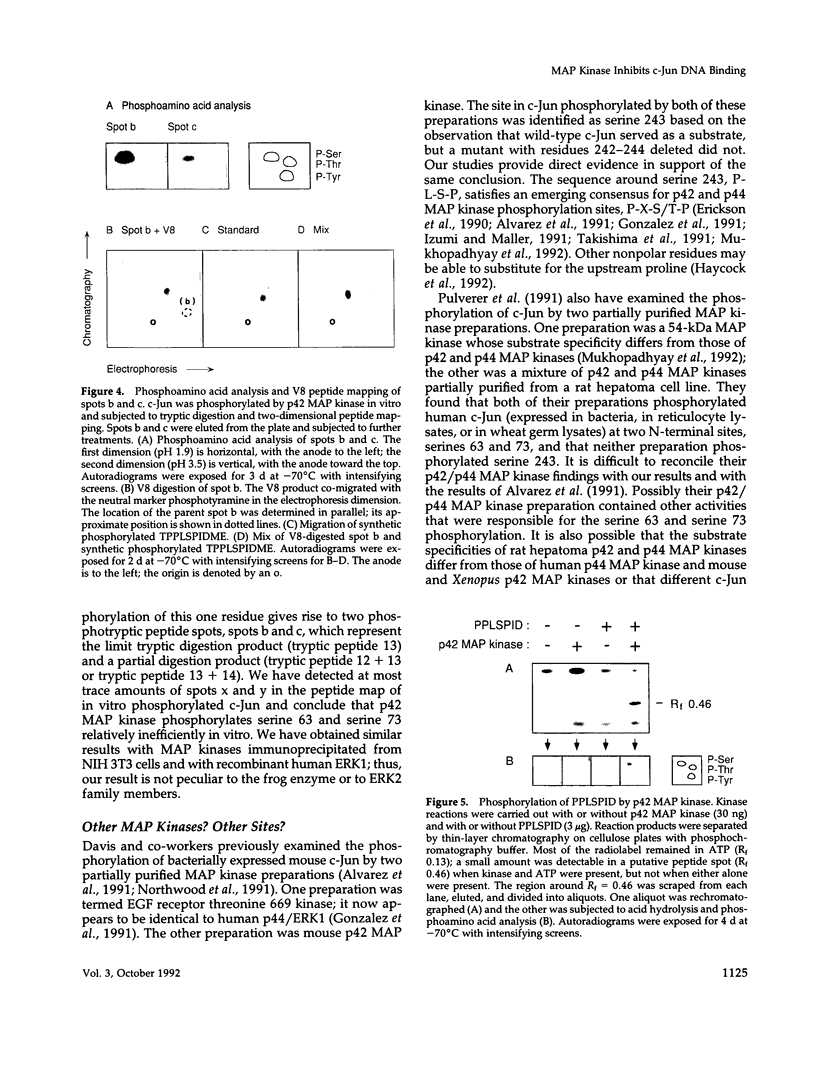
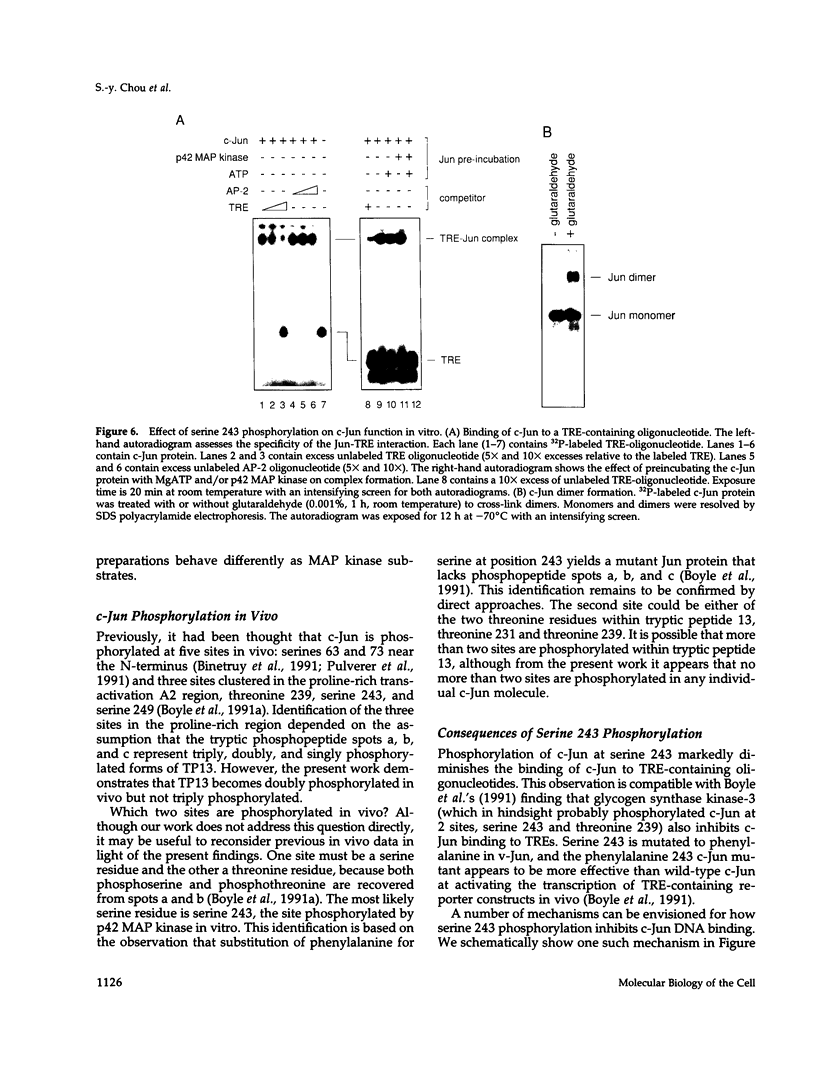
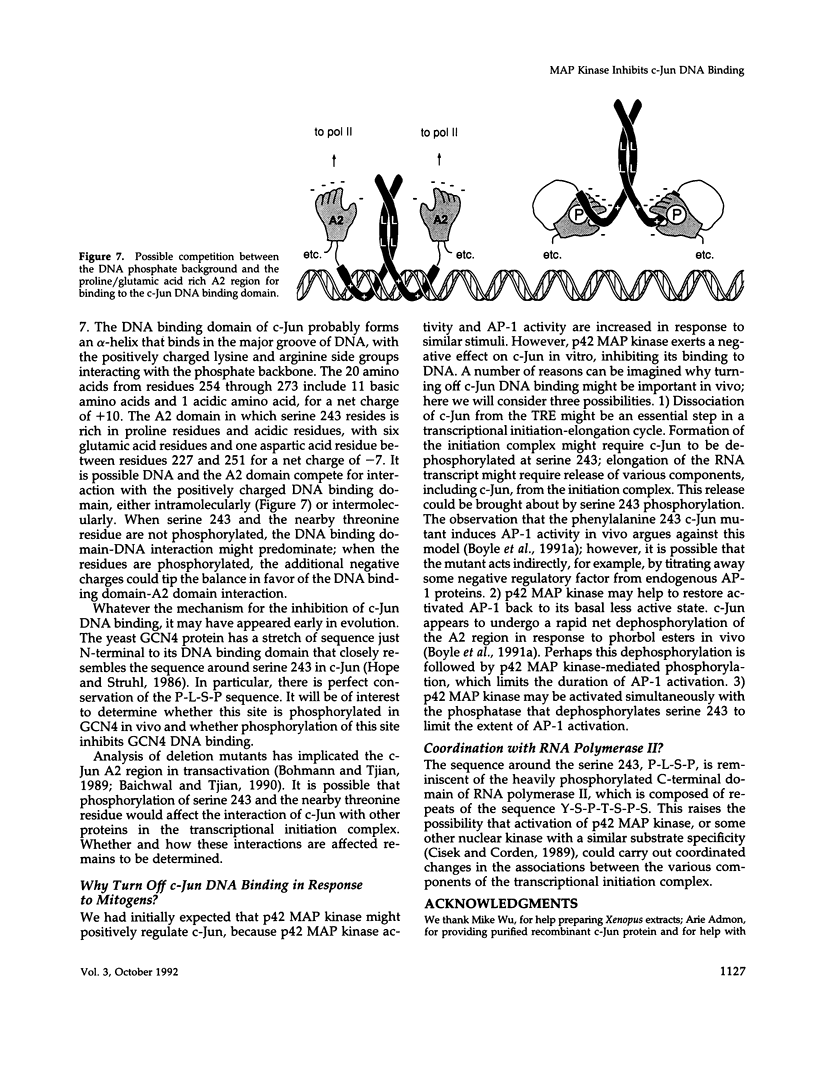
Here we demonstrate that partially purified Xenopus p42 mitogen-activated protein (MAP) kinase phosphorylates bacterially expressed human c-Jun at a single site, serine 243. Several lines of evidence argue that this phosphorylation is due to p42 MAP kinase itself rather than some contaminating species. Phosphorylation of serine 243 markedly decreases the binding of c-Jun to oligonucleotides containing the 12-O-tetradecanoylphorbol-13-acetate response element. These findings suggest that MAP kinase may play a role in the down-regulation of c-Jun or in the cycle of transcriptional initiation and elongation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Anderson N. G., Kilgour E., Sturgill T. W. Activation of mitogen-activated protein kinase in BC3H1 myocytes by fluoroaluminate. J Biol Chem. 1991 Jun 5;266(16):10131–10135. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Tjian R. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell. 1990 Nov 16;63(4):815–825. doi: 10.1016/0092-8674(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Barrett C. B., Erikson E., Maller J. L. A purified S6 kinase kinase from Xenopus eggs activates S6 kinase II and autophosphorylates on serine, threonine, and tyrosine residues. J Biol Chem. 1992 Mar 5;267(7):4408–4415. [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Tjian R. Biochemical analysis of transcriptional activation by Jun: differential activity of c- and v-Jun. Cell. 1989 Nov 17;59(4):709–717. doi: 10.1016/0092-8674(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Gregory J. S., Cobb M. H. Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry. 1991 Jan 8;30(1):278–286. doi: 10.1021/bi00215a038. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Yancopoulos G. D., Gregory J. S., Slaughter C., Moomaw C., Hsu J., Cobb M. H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990 Jul 6;249(4964):64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli M. F., Pelech S. L., Krebs E. G. Activation of multiple protein kinases during the burst in protein phosphorylation that precedes the first meiotic cell division in Xenopus oocytes. J Biol Chem. 1988 Feb 5;263(4):2009–2019. [PubMed] [Google Scholar]
- Cisek L. J., Corden J. L. Phosphorylation of RNA polymerase by the murine homologue of the cell-cycle control protein cdc2. Nature. 1989 Jun 29;339(6227):679–684. doi: 10.1038/339679a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. Related proteins are phosphorylated at tyrosine in response to mitogenic stimuli and at meiosis. Mol Cell Biol. 1989 Jul;9(7):3143–3147. doi: 10.1128/mcb.9.7.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Kunisawa R., Thorner J. A putative protein kinase overcomes pheromone-induced arrest of cell cycling in S. cerevisiae. Cell. 1989 Sep 22;58(6):1107–1119. doi: 10.1016/0092-8674(89)90509-6. [DOI] [PubMed] [Google Scholar]
- Elion E. A., Grisafi P. L., Fink G. R. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990 Feb 23;60(4):649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- Ely C. M., Oddie K. M., Litz J. S., Rossomando A. J., Kanner S. B., Sturgill T. W., Parsons S. J. A 42-kD tyrosine kinase substrate linked to chromaffin cell secretion exhibits an associated MAP kinase activity and is highly related to a 42-kD mitogen-stimulated protein in fibroblasts. J Cell Biol. 1990 Mar;110(3):731–742. doi: 10.1083/jcb.110.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. K., Payne D. M., Martino P. A., Rossomando A. J., Shabanowitz J., Weber M. J., Hunt D. F., Sturgill T. W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J Biol Chem. 1990 Nov 15;265(32):19728–19735. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Identification of a 42-kilodalton phosphotyrosyl protein as a serine(threonine) protein kinase by renaturation. Mol Cell Biol. 1990 Jun;10(6):3020–3026. doi: 10.1128/mcb.10.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funasaka Y., Boulton T., Cobb M., Yarden Y., Fan B., Lyman S. D., Williams D. E., Anderson D. M., Zakut R., Mishima Y. c-Kit-kinase induces a cascade of protein tyrosine phosphorylation in normal human melanocytes in response to mast cell growth factor and stimulates mitogen-activated protein kinase but is down-regulated in melanomas. Mol Biol Cell. 1992 Feb;3(2):197–209. doi: 10.1091/mbc.3.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Raden D. L., Davis R. J. Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem. 1991 Nov 25;266(33):22159–22163. [PubMed] [Google Scholar]
- Gotoh Y., Moriyama K., Matsuda S., Okumura E., Kishimoto T., Kawasaki H., Suzuki K., Yahara I., Sakai H., Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991 Sep;10(9):2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Yamashita T., Hoshi M., Kawakami M., Sakai H. Microtubule-associated-protein (MAP) kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- Gómez N., Tonks N. K., Morrison C., Harmar T., Cohen P. Evidence for communication between nerve growth factor and protein tyrosine phosphorylation. FEBS Lett. 1990 Oct 1;271(1-2):119–122. doi: 10.1016/0014-5793(90)80386-w. [DOI] [PubMed] [Google Scholar]
- Haycock J. W., Ahn N. G., Cobb M. H., Krebs E. G. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead T. A., Weiel J. E., Litchfield D. W., Tsukitani Y., Fischer E. H., Krebs E. G. Okadaic acid mimics the action of insulin in stimulating protein kinase activity in isolated adipocytes. The role of protein phosphatase 2a in attenuation of the signal. J Biol Chem. 1990 Sep 25;265(27):16571–16580. [PubMed] [Google Scholar]
- Her J. H., Wu J., Rall T. B., Sturgill T. W., Weber M. J. Sequence of pp42/MAP kinase, a serine/threonine kinase regulated by tyrosine phosphorylation. Nucleic Acids Res. 1991 Jul 11;19(13):3743–3743. doi: 10.1093/nar/19.13.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Activation of a Ca2+-inhibitable protein kinase that phosphorylates microtubule-associated protein 2 in vitro by growth factors, phorbol esters, and serum in quiescent cultured human fibroblasts. J Biol Chem. 1988 Apr 15;263(11):5396–5401. [PubMed] [Google Scholar]
- Izumi T., Maller J. L. Phosphorylation of Xenopus cyclins B1 and B2 is not required for cell cycle transitions. Mol Cell Biol. 1991 Aug;11(8):3860–3867. doi: 10.1128/mcb.11.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- Kyriakis J. M., Avruch J. pp54 microtubule-associated protein 2 kinase. A novel serine/threonine protein kinase regulated by phosphorylation and stimulated by poly-L-lysine. J Biol Chem. 1990 Oct 5;265(28):17355–17363. [PubMed] [Google Scholar]
- Miyasaka T., Sternberg D. W., Miyasaka J., Sherline P., Saltiel A. R. Nerve growth factor stimulates protein tyrosine phosphorylation in PC-12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2653–2657. doi: 10.1073/pnas.88.7.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay N. K., Price D. J., Kyriakis J. M., Pelech S., Sanghera J., Avruch J. An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J Biol Chem. 1992 Feb 15;267(5):3325–3335. [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Rheeder A., Williams K., Pollack S., Katz R., Landreth G. E. Stimulation of MAP-2 kinase activity in T lymphocytes by anti-CD3 or anti-Ti monoclonal antibody is partially dependent on protein kinase C. J Immunol. 1990 Apr 1;144(7):2683–2689. [PubMed] [Google Scholar]
- Northwood I. C., Gonzalez F. A., Wartmann M., Raden D. L., Davis R. J. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991 Aug 15;266(23):15266–15276. [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Posada J., Sanghera J., Pelech S., Aebersold R., Cooper J. A. Tyrosine phosphorylation and activation of homologous protein kinases during oocyte maturation and mitogenic activation of fibroblasts. Mol Cell Biol. 1991 May;11(5):2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Sanghera J. S., Marsden L. A., Weber M. J., Pelech S. L., Sturgill T. W. Biochemical characterization of a family of serine/threonine protein kinases regulated by tyrosine and serine/threonine phosphorylations. J Biol Chem. 1991 Oct 25;266(30):20270–20275. [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Bader S. A., Pelech S. L. Purification and characterization of a maturation-activated myelin basic protein kinase from sea star oocytes. J Biol Chem. 1990 Jan 5;265(1):52–57. [PubMed] [Google Scholar]
- Schanen-King C., Nel A., Williams L. K., Landreth G. Nerve growth factor stimulates the tyrosine phosphorylation of MAP2 kinase in PC12 cells. Neuron. 1991 Jun;6(6):915–922. doi: 10.1016/0896-6273(91)90232-o. [DOI] [PubMed] [Google Scholar]
- Schieven G., Martin G. S. Nonenzymatic phosphorylation of tyrosine and serine by ATP is catalyzed by manganese but not magnesium. J Biol Chem. 1988 Oct 25;263(30):15590–15593. [PubMed] [Google Scholar]
- Takishima K., Griswold-Prenner I., Ingebritsen T., Rosner M. R. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated "MAP" kinase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H., Aletta J. M., Greene L. A. Nerve growth factor and fibroblast growth factor selectively activate a protein kinase that phosphorylates high molecular weight microtubule-associated proteins. Detection, partial purification, and characterization in PC12 cells. J Biol Chem. 1990 Sep 15;265(26):15471–15480. [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- White M. F., Backer J. M. Preparation and use of anti-phosphotyrosine antibodies to study structure and function of insulin receptor. Methods Enzymol. 1991;201:65–79. doi: 10.1016/0076-6879(91)01009-q. [DOI] [PubMed] [Google Scholar]