Abstract
The diagnosis and care of patients with alcohol abuse and dependence is hampered by a lack of sensitive and specific screening and monitoring tests. Proteomics is a good approach to search for biomarkers of alcohol abuse. Serum carrier protein-bound proteins have attracted significant interest because they remain a relatively un-mined region of the proteome. In the present study, a proteomic workflow including LC-MS/MS with enrichment of serum carrier protein-bound biomarkers technique was applied to profile the changes in quality and quantity of serum carrier protein-bound proteins for the discovery of novel biomarker candidates of alcohol abuse. In total, 311 proteins identified with high confidence were discovered to be bound to serum carrier proteins. Complement isoforms, Ig fragments, and apolipoprotein family proteins are the main serum carrier-bound proteins. Protein quantification analysis with and without concern as to gender revealed that gender is a critical consideration for biomarker development in alcohol abuse. Identified proteins not previously associated with alcohol abuse include gelsolin, selenoprotein P, serotransferrin, tetranectin, hemopexin, histidine-rich glycoprotein, plasma kallikrein, and vitronectin. Altered abundance of these proteins suggests that they may be potential novel biomarkers for alcohol abuse.
Keywords: Alcohol abuse, Biomarker, Proteomics, Serum carrier protein, Tandem mass spectrometry
1 Introduction
The abuse of alcohol has been recognized as a disease of addiction since the late 18th century and is defined generally by its characteristic craving for alcohol, compulsivity in alcohol-seeking behavior, impaired control over drinking and its chronic relapsing nature [1]. Alcohol is causally related to more than 60 different medical conditions, such as malignant neoplasms, neuropsychiatric disorders, cardiovascular diseases, gastrointestinal disorders, and unintentional and intentional injury [2]. Tests that can properly assess the extent of the patients’ recent and past drinking activity would be valuable in screening for alcohol use disorders, testing of potential therapy or relapse during therapy, and processing in a specific clinical setting such as in pre-liver transplant evaluation [3].
However, the diagnosis and care of patients with alcohol abuse and dependence is hampered by a lack of proper detection. Questionnaires, such as CAGE [4] and AUDIT [5], have been developed and validated to screen for or establish the diagnosis of alcoholism. However, it is well known that alcoholics often deny their drinking habit and underestimate their consumption [6]. Biomarkers, biochemical substances in the body that can indicate the presence or progress of a condition, are more objective measures of alcohol abuse. Up to now, gamma-glutamyltransferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), carbohydrate-deficient transferrin (CDT), mean corpuscular volume (MCV), whole blood-associated acetaldehyde (WBAA), N-acetyl-β-hexosaminidase (Beta-Hex), sialic acid index of apolipoprotein J (SIJ), total serum sialic acid (TSA), 5-hydroxytryptophol (5-HTOL), various fatty acid ethyl esters (FAEEs), ethyl glucuronide (EtG), acetaldehyde, salsolinol, adenylyl cyclase (AC) activity, gamma-aminobutyric acid (GABA), dopamine, beta-endorphin, and serotonin have been used or are under consideration to alert clinicians to patients’ recent drinking patterns or their inherited risk of abusing alcohol [7]. However, none of these currently available biomarkers are ideal [8, 9], and their limitations and weaknesses in sensitivity and specificity make it desirable to develop more sensitive and specific biomarkers.
In human plasma, some proteins are bound to large, high-abundance carrier proteins, which recently have been found to act as reservoirs for the accumulation and amplification of bound biomarkers - integrating, amplifying, and storing diagnostic information [10]. When they are bound to high-abundance carrier proteins, such as albumin, the half-lives of these potential biomarkers are extended in plasma by preventing excretion by the kidneys. The carrier protein-bound proteins have attracted significant interest because they remain a relatively un-mined region of the proteome.
Proteomic approaches have been used previously to search for biomarkers of alcohol consumption [11]. Two-dimensional differential gel electrophoresis (2D-DIGE) and surface enhanced laser desorption/ionization-time of flight-mass spectrometry (SELDI-TOF-MS) were used to study serum samples from alcoholics [12, 13]. 2D-DIGE suffers from several limitations, such as poor solubilization of hydrophobic proteins and basic proteins in buffers, and poor resolution of very high and low-molecular weight proteins [14]. SELDI-TOF-MS has been limited by the low sensitivity for higher molecular weight proteins [15]. Both techniques are associated with difficulties in the identification of potential biomarkers. In contrast, liquid chromatography - tandem mass spectrometry (LC-MS/MS) can be used for both identification and quantification of proteins in complex sample mixtures. Combined with enrichment of serum carrier protein-bound proteins, LC-MS/MS can provide an in-depth investigation of the serum to discover some novel biomarkers.
The intent of this study is to discover markers of abusive drinking, e.g. those proteins whose appearance in plasma or bound to abundant plasma proteins that would enable a physician or therapist to distinguish between a patient who continues to abuse alcohol from one who is complying with treatment (abstinence). To the best of our knowledge, studies of serum proteomics using LC-MS/MS to examine alcoholics before and after treatment do not appear in the scientific literature. In the present study, a proteomic workflow including LC-MS/MS with enrichment of serum carrier protein-bound biomarkers is applied to profile the changes in quantity or quality of serum carrier protein-bound proteins for discovery of novel biomarker candidates of alcohol abuse.
2 Materials and methods
2.1 Patients
The study was approved by the Indiana University Purdue University at Indianapolis and institutional review board and by the Fairbanks Addiction Treatment Center. All subjects were recruited from Fairbanks, a private, not-for profit hospital in Indianapolis, Indiana, specializing in treating individuals with alcohol abuse and drug addiction. Subjects were those seeking treatment for alcohol abuse and dependence. Upon their first visit, subjects were seen by an addiction psychiatrist at which time a full detailed interview, history, and physical examination were obtained. Inclusion criteria incorporated those subjects who met the DSM-IV Diagnostic Criteria for Alcohol Dependence/abuse and who had been actively drinking up to within 3 days of the first visit. Subjects were excluded if they had a history of any localized or systemic infectious disease within 4 weeks prior to the study and had symptoms and signs of decompensated liver disease (jaundice, ascites, or hepatic encephalopathy). Eligible subjects who met all the enrollment criteria were asked to answer questionnaires, which included the following information: a) background information and demographics, b) alcohol consumption, c) alcohol abuse and dependence, d) previous history of alcohol treatment utilization, e) family history of alcoholism, f) tobacco use and dependence, g) medicine use, h) past medical history, i) drug abuse and dependence, and j) family history of drug abuse. After acquiring written informed consent, 7 mL of blood from each subject were obtained according to the protocol described in the proteomic analysis section. This set of samples was referred to as pre-treatment samples. Alcohol treatment at Fairbanks was an intensive 6-week, daily outpatient treatment program. During this 6-week period, all patients were being followed closely by a specialist and none of the study’s subjects relapsed. At the end of the program, another set of blood samples (post-treatment samples) were drawn such that proteomic profiles could be compared between pre- and post treatment sera. During the study period, 75 subjects were recruited, and 16 out of 75 subjects met the established inclusion criteria and from whom both pre- and post-treatment samples were obtained.
2.2 Materials
Iodoethanol, triethylphosphine (TEP), and ammonium bicarbonate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile (ACN) and MS grade water were purchased from EMD Chemicals (Gibbstown, NJ, USA). Modified sequencing grade porcine trypsin was obtained from Princeton Separations (Freehold, NJ, USA). The ProXPRESSION™ Biomarker Manual Enrichment kit was obtained from PerkinElmer (Wellesley, MA, USA).
2.3 Samples
Serum samples were obtained from 75 subjects before an intensive alcohol treatment program. These included 16 subjects who met the established inclusion criteria and from whom post-treatment samples were also obtained. Seven mL of blood was obtained for clinical values’ assessment. Another 7 mL of blood was drawn and centrifuged at 1500 × g for 10 min at room temperature, within 1–3 hour(s) after acquisition. Aliquots (0.5 mL) were placed in separate 2 mL cryovials and stored at −80°C until analysis.
2.4 Enrichment of serum carrier protein-bound biomarkers
Enrichment of serum carrier protein-bound biomarkers was performed essentially as described in the ProXPRESSION Biomarker Manual Enrichment user guide. Briefly, 400 μL of diluted sample (2000 μg) was loaded onto the equilibrated spin column. The spin column and collection tube were then centrifuged at 250 × g for 2 min. The spin column was then washed 3 times with 400 μL of Biomarker Enrichment Binding buffer. The flow through volume was discarded. The column was eluted with 200 μL Biomarker Elution Buffer at 250 × g for 2 min, and then the elution was repeated once. Total fluid collection was 400 μL. Each sample was processed as two technical replicates. The two replicates were pooled. These protein samples were subsequently referred to as “biomarker enriched samples”. Protein concentration was determined by the Bradford assay [16]. A 100 μg aliquot of each sample was placed in a new tube and dried via SpeedVac.
2.5 Protein reduction, alkylation, and digestion for LC-MS/MS
Enriched samples were reconstituted in 200 μL of 4 M urea and then reduced and alkylated by TEP and iodoethanol as described by Lai et al. [17]. Briefly, 200 μL of the reduction/alkylation cocktail was added to the protein solution. The sample was incubated at 37°C for 120 min, dried by SpeedVac, and reconstituted with 100 μL of 100 mM NH4HCO3 at pH 8.0. A 150 μL aliquot of a 20 μg/mL trypsin solution was added to the sample and incubated at 37°C for 3 h, after which another 150 μL of trypsin was added, and the solution incubated at 37°C overnight.
2.6 LC-MS/MS
The digested samples were analyzed using a Thermo-Finnigan linear ion-trap (LTQ) mass spectrometer coupled with a Surveyor autosampler and MS HPLC system (Thermo-Finnigan). Tryptic peptides were injected onto the C18 microbore RP column (Zorbax SB-C18, 1.0 mm x 150 mm) at a flow rate of 50 μL/min. The mobile phases A, B, and C were 0.1% formic acid in water, 50% ACN with 0.1% formic acid in water, and 80% ACN with 0.1% formic acid in water, respectively. The gradient elution profile was as follows: 10% B (90% A) for 5 min, 10–95% B (90–5% A) for 120 min, 100% C for 5 min, and 10% B (90% A) for 12 min. The data were collected in the “Triple-Play” (MS scan, Zoom scan, and MS/MS scan) mode with the ESI interface using normalized collision energy of 35%. Dynamic exclusion settings were set to repeat count 1, repeat duration 30 s, exclusion duration 120 s, and exclusion mass width 0.75 m/z (low) and 2.0 m/z (high).
2.7 Data analysis
The acquired data were searched against the International Protein Index (IPI) human database (ipi.HUMAN.v3.44) using SEQUEST (v. 28 rev. 12) algorithms in Bioworks (v. 3.3). General parameters were set to: peptide tolerance 2.0 amu, fragment ion tolerance 1.0 amu, enzyme limits set as “fully enzymatic - cleaves at both ends”, and missed cleavage sites set at 2. The searched peptides and proteins were validated by PeptideProphet [18] and ProteinProphet [19] in the Trans-Proteomic Pipeline (TPP, v. 3.3.0) (http://tools.proteomecenter.org/software.php). Protein identification was reported and compared using an in-house developed platform named LASPAP (LArge-Scale Shotgun Proteomics data Analysis Platform). Gene ontology analysis was completed using the Generic GO Term Finder [20] developed by the Bioinformatics Group at the Lewis-Sigler Institute at Princeton (http://go.princeton.edu).
2.8 Relative protein quantification
Two label-free, relative quantification techniques were used to evaluate the LC-MS/MS data for potential biomarkers. 1) For all the proteins identified in both pre- and post-treatment group samples, an indirect, relative quantification was conducted by counting protein identification frequency in pre- and post-treatment groups; and 2) a semi-quantitative analysis was performed by TPP based on spectral counting of peptides [21] for proteins of specific interest.
3 Results
3.1 Patient Data
75 subjects from whom pre-treatment samples were studied, 25 females and 50 males, had an average age of 45.9 yr, height 68.9 in, weight 171.8 lbs, and BMI 26.3. All clinical data fell in the normal range with the exception of elevated AST (53.3 ± 32.6), ALT (44.4 ± 24.6), and MCV (96.5 ± 4.8). Pre-treatment clinical results for the 16 subjects who met inclusion criteria (8 males and 8 females), and on whose samples both pre- and post-treatment mass spectral analyses were conducted, had values similar to the entire group (age 48.9 yr, height 67.6 in, weight 158.8 lbs, BMI 26.2, AST 54.3 ± 37.3, ALT 37.4 ± 18.9, and MCV 98.9 ± 5.1).
Self-reported pre-treatment drinking levels for the 16 pre/post subjects averaged 16.8 ± 6.6 drinks/day and 103.1 ± 36.7 drinks/week. Values for the entire group of 75 were nearly identical. We define a “drink” as the following equivalents: 1.5 oz liquor (40% EtOH), 5 oz wine, or 12 oz beer. Many subjects self-reported drinking reflected a combination of these. Drinking onset was reported as 18.1 ± 3.3 yrs of age and 7.4 ± 6.6 drinks were consumed on the day of last use, which was reported to be from 0–144 hours prior to pretreatment sampling.
3.2 Protein identification
The peptides and proteins were identified by SEQUEST and evaluated with PeptideProphet and ProteinProphet to determine their identification probabilities. In total, 311 proteins with ≥ 90.00% confidence were identified by peptides with ≥ 90.00% confidence via TPP validation. The 160 representative proteins are listed in Table 1 (available in the supplementary data), where proteins identified by the same peptides are put into one protein group. The protein group numbers, the numbers of proteins in each group, and the number of peptides used for protein identification are provided. The complete list of identified proteins and their identification information is available in the supplementary data.
3.3 Gene ontology annotation
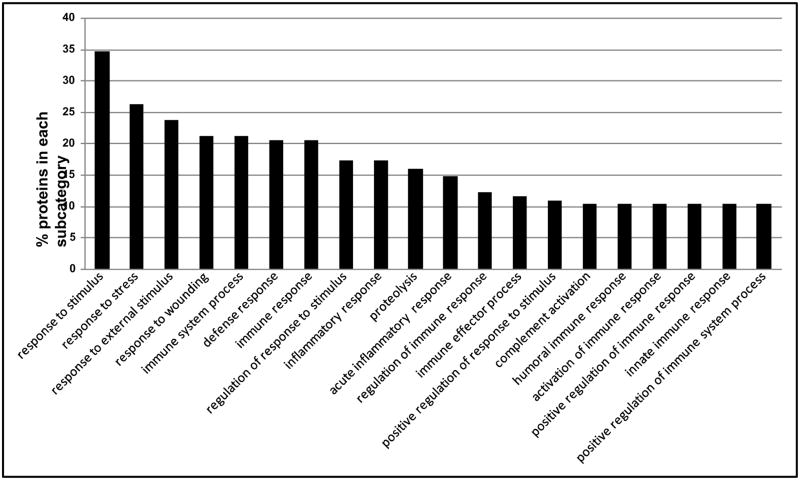
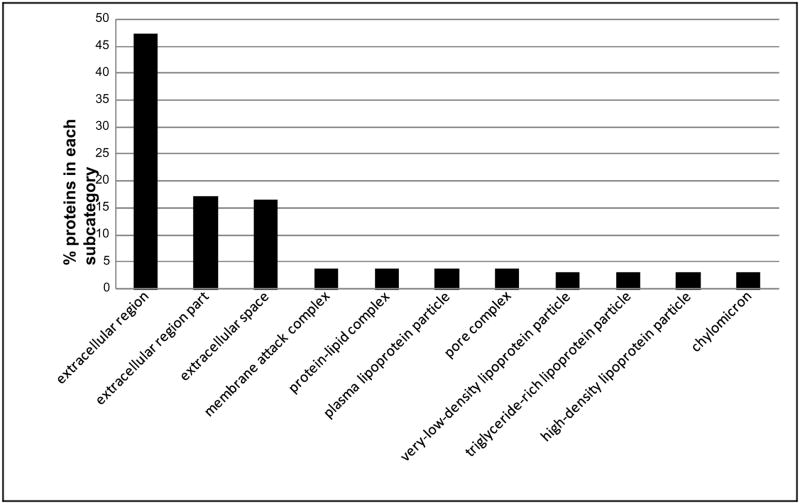
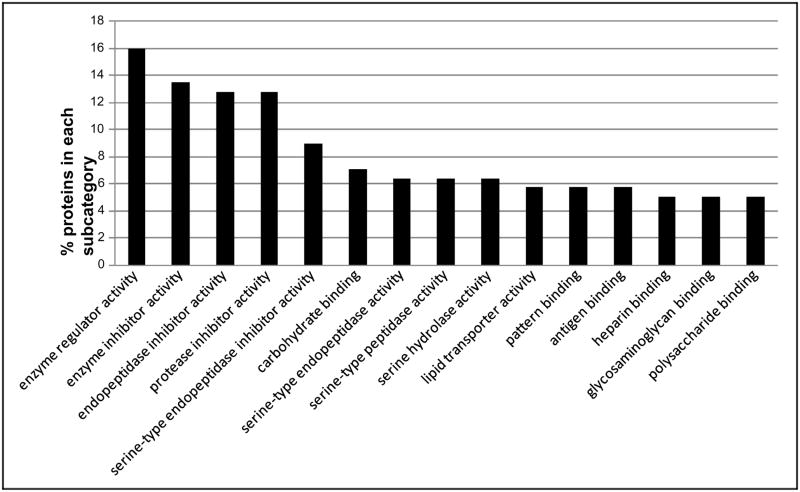
The IPI numbers of 160 identified proteins representing each protein group were submitted to the Lewis-Sigler Institute GO Term Finder, which determined that 60 identifiers were unknown and 4 were ambiguous, while the other 96 placed were categorized by ontological aspects. In addition, due to the redundancy in the reporting of subcategories by the web tool and our desire to visually simplify the histograms, some categories have been combined or nonspecific parent terms eliminated. This editing was done by consulting AmiGO (the official tool for searching and browsing the Gene Ontology database) to insure correct attribution of subcategories and to respect the gene ontology dendogram [22]. These annotated proteins grouped by subcategories are included in a graphical display in Figures 1–3, which illustrate proteins categorized by the gene ontology biological process, molecular function, and cellular component aspects.
Fig. 1.
Distribution of the Gene Ontology (GO) Biological Process aspect for 160 identified proteins. The ordinate value is the percent of proteins that match a specific subcategory of this aspect.
Fig. 3.
Distribution of the Gene Ontology (GO) Cellular Component aspect for 160 identified proteins. The ordinate value is the percent of proteins that match a specific subcategory of this aspect.
3.4 Protein quantification
If protein abundance in the analyzed sample is too low to be detected by the mass spectrometer, this protein will not be identified. As a result, comparison of protein identification frequency in pre- vs. post-treatment groups can reveal differential protein expression. This is an easy, useful, and label-free relative quantification method. Using this analysis, the identification frequency of 84 proteins was found to differ in at least 3 out of 16 samples, pre- vs. post-treatment. The comparison of 7 potential biomarkers by identification frequency counting is shown in Figure 4, which indicates gelsolin, apolipoprotein A-II, apolipoprotein C-I, and selenoprotein P were decreased, while serotransferrin and tetranectin were increased by abstinence in post-treatment group.
Fig. 4.
Pre- and post-treatment group comparison of 6 proteins by identification frequency counting. Gelsolin (IPI00646773), apolipoprotein A-II (IPI00021854), apolipoprotein C-I (IPI00021855), and selenoprotein P (IPI00029061) are decreased by treatment; serotransferrin (IPI00022463) and tetranectin (IPI00009028) are increased during abstinence in post-treatment group.
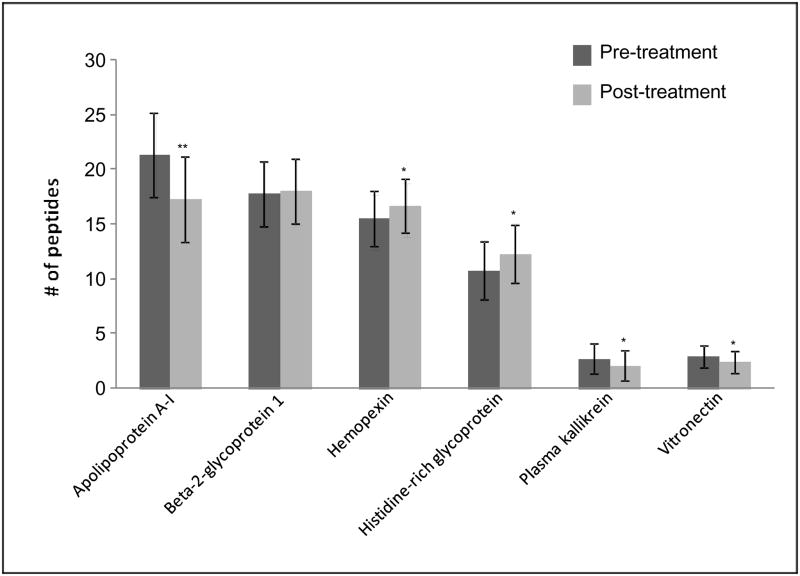
A semi-quantitative analysis also was performed based on spectral counting of peptides using TPP. The comparison of 6 potential biomarkers was carried out by peptide counting (Figure 5). Beta-2-glycoprotein 1 had no significant difference between pre- and post-treatment groups. Apolipoprotein A-I, plasma kallikrein, and vitronectin significantly decreased levels, while hemopexin and histidine-rich glycoprotein increased levels during abstinence in post-treatment group. When the group of 16 subjects was separated by gender (8 females, 8 males), the comparisons illustrated in Figure 6 revealed that most of the group differences observed in Fig. 5 were the result of the significant changes observed in the female cohort. Plasma kallikrein and vitronectin significantly decreased only in female, while hemopexin and histidine-rich glycoprotein increased levels only in the females during abstinence in the post-treatment group. Beta-2-glycoprotein 1, which had no significant differences without considering gender, significantly decreased in female, but increased in male between pre- and post-treatment groups.
Fig. 5.
Pre- and post-treatment group comparison of 6 proteins by peptide counting. Mean ± S.D. are presented, with significant differences indicated by asterisks: *P<0.05, **P<0.01.
Fig. 6.
Pre- and post-treatment group comparison of 6 proteins identified by peptide counting in female and male samples. Mean ± S.D. are presented, with significant differences indicated by asterisks: * p < 0.05, ** p < 0.01.
4 Discussion
4.1 Enrichment of serum carrier protein-bound biomarkers
The serum carrier-bound proteome is one of the LC-MS/MS-based proteomics strategies in the analysis of plasma/serum to discover the potential blood-based biomarker candidates [23]. The ProXPRESSION™ Protein Enrichment Kit enables capture of carrier proteins and carrier-bound proteins, protein fragments and peptides. The carrier-bound proteins, protein fragments and peptides are eluted, and then only the low molecular weight proteins, protein fragments and peptides are targeted according to the standard sample preparation procedure. This strategy has been used to investigate biomarkers of ovarian cancer [24], uterine endometrial cancer [25], prostate cancer [10], tumor-stage mycosis fungoides [26], and Alzheimer’s disease [27]. However, in the present study, we applied a sample preparation procedure that was a little different from the standard and targeted all the carrier-bound proteins, protein fragments and peptides, known as the serum carrier-bound proteome. We believe that the research of carrier-bound proteins remains a relatively un-mined region of the plasma/serum proteome, which are depleted with the most abundant proteins in the general high-abundance protein depletion approach to serum/plasma-based biomarker development, and also ignored in the high-throughput MALDI-TOF approach.
4.2 Gene ontology annotation
In the individual ontological categories or “aspects”, some IPI numbers were recognized but were not annotated relative to any aspect in the database. A review of unannotated IPI numbers yielded mainly complement isoforms, Ig fragments, and uncharacterized proteins. These annotated proteins grouped by subcategories are included in a graphical display in Figures 1–3.
Figure 1 illustrates the percent of proteins categorized according to the biological process aspect. This category includes a high proportion of proteins that are involved in responses to external stimulus and wounding. Defense, inflammatory, and acute inflammatory response proteins present high percentage of the proteins as well. Alcohol consumption leads to inflammation of the liver [28]. It is reasonable that many proteins are included in inflammatory response in the present study. Figure 2 illustrates proteins categorized by the gene ontology molecular function aspect. Alcohol metabolism is completed mainly by oxidative pathways involving alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP2E1), and catalase enzymes [29]. A large number of proteins’ functions are identified as enzyme inhibitors, specifically serine-type endopeptidase inhibitors, in this study. Lipid and carbohydrate binding are also the molecular functions of many of the proteins identified in the samples. Figure 3 illustrates the percent of proteins categorized according to the cellular component aspect. In the subcategories comprising the cellular component aspect, the annotated proteins fell into the extracellular region. This would be expected of serum proteins which are secreted into the extracellular space.
Fig. 2.
Distribution of the Gene Ontology (GO) Molecular Function aspect for 160 identified proteins. The ordinate value is the percent of proteins that match a specific subcategory of this aspect.
4.3 Notable proteins
Thousands of protein components are present in serum with more than 10 orders of magnitude of dynamic concentration range [30]. The large and abundant carrier proteins, such as albumin, within the blood bind various physiologically important molecules, such as hormones, cytokines, and lipoproteins [31]. The abnormal physiological states are expected to alter presence or abundance of the proteins in serum [31] and also the interaction of different proteins, such as the biomarker with the larger carrier protein. Therefore, it is also important to present the binding state change of bound biomarker in serum.
In the present research, 311 proteins were discovered to be bound with the carrier proteins, such as albumin. Among the bound proteins, it is not surprising that 38.9% of proteins are complement isoforms and Ig fragments, which constitute more than 26% of the protein content of plasma [31]. Apolipoproteins are another common type of proteins bound to the carrier proteins. However, apolipoproteins have different bound states with the carrier proteins. Apolipoprotein C-II, D, F, M, and L were not detected in any enriched sample. Apolipoprotein A-IV and E appeared in no more than 3 enriched samples, which means they are not likely to be bound to the carrier proteins. Apolipoprotein B-100, C-III, and J (Clusterin) were found almost in every sample, but their quantification revealed there was no significant difference between the pre- and post-treatment groups, even when the group of 16 subjects was separated by gender (8 females, 8 males). Apolipoprotein A-II and C-I were found to decrease after alcohol withdrawal in the abusive drinking subjects. Apolipoprotein A-I level significantly decreased with and without considering gender, after six weeks treatment in our subjects, which makes it a potential biomarker for both female and male. The change in concentration of apolipoprotein H (beta-2-glycoprotein 1) in the bound state to carrier proteins is very interesting. Without considering gender, its level has no significant difference after 6 weeks of abstinence. However, with considering gender, its level significantly decreased in female, while significantly increased in male. This discovery reveals that apolipoprotein H alters between female and male differently, and gender is a critical consideration for development of some biomarkers in alcohol abuse.
Gelsolin, known for its involvement in dynamic changes in the actin cytoskeleton, has unexpected roles in neurite filopodial retraction, neuronal ion channel modulation, and as a regulator and mediator of apoptosis and phosphoinositide signaling [32]. Its level decreased after six weeks treatment in our subjects, which indicates that its binding to the carrier proteins was affected by the alcohol abuse. In the current study, selenoprotein P showed the same trend as gelsolin, while the levels of serotransferrin and tetranectin increased after six weeks treatment in our subjects.
Without considering gender, hemopexin and histidine-rich glycoprotein were found to significantly increase, while plasma kallikrein and vitronectin significantly decrease after 6 weeks of abstinence. With considering gender, hemopexin and histidine-rich glycoprotein were noticed to significantly increase only in female, while plasma kallikrein and vitronectin significantly decrease only in female. These results further confirm gender is a critical consideration for biomarker development in alcohol abuse.
Identified proteins not previously associated with alcohol abuse include gelsolin, selenoprotein P, serotransferrin, tetranectin, hemopexin, histidine-rich glycoprotein, plasma kallikrein, and vitronectin. Our findings of these proteins’ alterations indicate that they may be potential novel biomarkers for alcohol abuse.
In summary, using a mass spectrometric approach with the carrier proteins bound biomarker enrichment technique, we have detected novel potential biomarkers of excessive alcohol consumption in heavy drinkers seeking treatment and found gender is a critical consideration for biomarker development in alcohol abuse. A follow-up study including significantly more subjects will confirm these findings and enable the design and completion of biomarker validation studies for potential application to the general population.
Supplementary Material
Acknowledgments
This work was supported by NIH-NIAAA R21 AA016217-01.
Abbreviations
- SELDI-TOF-MS
surface enhanced laser desorption/ionization-time of flight-mass spectrometry
- TPP
trans-proteomic pipeline
- GO
gene ontology
References
- 1.Li TK, Hewitt BG, Grant BF. Addiction. 2007;102:1522–1530. doi: 10.1111/j.1360-0443.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- 2.Room R, Babor T, Rehm J. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 3.Mathurin P. Liver Transpl. 2005;11:S21–24. doi: 10.1002/lt.20601. [DOI] [PubMed] [Google Scholar]
- 4.Ewing JA. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 5.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson L, Ries R, Russo J. J Addict Dis. 2003;22:79–90. doi: 10.1300/J069v22n02_07. [DOI] [PubMed] [Google Scholar]
- 7.Peterson K. Alcohol Res Health. 2004;28:30–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn WS, Park SP, Bae SM, Lee JM, et al. Cancer Sci. 2005;96:197–201. [Google Scholar]
- 9.Conigrave KM, Degenhardt LJ, Whitfield JB, Saunders JB, et al. Alcohol Clin Exp Res. 2002;26:332–339. [PubMed] [Google Scholar]
- 10.Petricoin EF, Ornstein DK, Liotta LA. Urol Oncol. 2004;22:322–328. doi: 10.1016/j.urolonc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Kasinathan C, Vrana K, Beretta L, Thomas P, et al. Alcohol Clin Exp Res. 2004;28:228–232. doi: 10.1097/01.alc.0000113779.35260.a8. [DOI] [PubMed] [Google Scholar]
- 12.Nomura F, Tomonaga T, Sogawa K, Ohashi T, et al. Proteomics. 2004;4:1187–1194. doi: 10.1002/pmic.200300674. [DOI] [PubMed] [Google Scholar]
- 13.Wu D, Tomonaga T, Sogawa K, Satoh M, et al. Alcohol Clin Exp Res. 2007;31:S67–71. doi: 10.1111/j.1530-0277.2006.00289.x. [DOI] [PubMed] [Google Scholar]
- 14.Faber MJ, Agnetti G, Bezstarosti K, Lankhuizen IM, et al. Cell Biochem Biophys. 2006;44:11–29. doi: 10.1385/CBB:44:1:011. [DOI] [PubMed] [Google Scholar]
- 15.Seibert V, Wiesner A, Buschmann T, Meuer J. Pathol Res Pract. 2004;200:83–94. doi: 10.1016/j.prp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Lai XY, Bacalla RL, Blazer-Yost BL, Hong D, et al. Proteomics Clin Appl. 2008;2:1140–1152. doi: 10.1002/prca.200780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 19.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 20.Boyle EI, Weng S, Gollub J, Jin H, et al. Bioinformatics. 2004;20:3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Sadygov RG, Yates JR., 3rd Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner M, Ball CA, Blake JA, Botstein D, et al. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez CR, Piersma S, Pham TV. Biomark Med. 2007;1:541–565. doi: 10.2217/17520363.1.4.541. [DOI] [PubMed] [Google Scholar]
- 24.Lopez MF, Mikulskis A, Kuzdzal S, Golenko E, et al. Clin Chem. 2007;53:1067–1074. doi: 10.1373/clinchem.2006.080721. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi S, Honda K, Handa Y, Kato H, et al. Cancer Sci. 2007;98:822–829. doi: 10.1111/j.1349-7006.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowen EW, Liu CW, Steinberg SM, Kang S, et al. Br J Dermatol. 2007;157:946–953. doi: 10.1111/j.1365-2133.2007.08185.x. [DOI] [PubMed] [Google Scholar]
- 27.Lopez MF, Mikulskis A, Kuzdzal S, Bennett DA, et al. Clin Chem. 2005;51:1946–1954. doi: 10.1373/clinchem.2005.053090. [DOI] [PubMed] [Google Scholar]
- 28.McClain CJ, Shedlofsky S, Barve S, Hill DB. Alcohol Health Res World. 1997;21:317–320. [PMC free article] [PubMed] [Google Scholar]
- 29.Zakhari S, Li TK. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 30.Anderson NL, Anderson NG. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 31.Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, et al. Mol Cell Proteomics. 2003;2:1096–1103. doi: 10.1074/mcp.M300031-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Kwiatkowski DJ. Curr Opin Cell Biol. 1999;11:103–108. doi: 10.1016/s0955-0674(99)80012-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.