SUMMARY
Objective
Factor XI (FXI) promotes hemostasis and thrombosis through enhancement of thrombin generation, and has been shown to play a critical role in the formation of occlusive thrombi in arterial injury models. The aim of this study was to investigate the mechanisms governing interactions between FXI and platelets.
Methods and Results
Platelet adhesion to immobilized FXI was abrogated in the presence of the low-density lipoprotein (LDL) receptor antagonist, receptor-associated protein (RAP); soluble recombinant Apolipoprotein E receptor 2 (ApoER2); or the LDL-binding domain 1 or 2 of ApoER2. FXI supported wild-type murine platelet binding; in contrast, ApoER2-deficient murine platelets did not adhere to FXI. In the presence of shear, platelet aggregates formed on FXI or activated FXI (FXIa) surfaces, while the presence of RAP, binding domain 1 of ApoER2 or an anti-GPIbα mAb blocked platelet adhesion to FXI or FXIa under shear. Soluble FXI bound to immobilized ApoER2’ with an affinity of 61 nM.
Conclusions
This study has identified apolipoprotein E receptor 2 (ApoER2, LRP8), a member of the LDL receptor family, as a platelet receptor for FXI. The interaction of FXI with other cell types that express ApoER2 remains to be explored.
Keywords: platelets, GPIb, apolipoprotein E receptor 2, LRP8, factor XI
INTRODUCTION
Factor XI (FXI) is a 160 kDa glycoprotein that participates in the intrinsic blood coagulation pathway and contributes to hemostasis. Activated FXI (FXIa) is a serine protease generated by cleavage of FXI at the Arg369-Ile370 bond by activated factor XII, thrombin or via autocatalytic activation.1 FXIa contributes to sustained thrombin generation via activation of factor IX (FIX).2 Inherited FXI deficiency causes a mild bleeding diathesis and is protective against ischemic stroke,3, 4 while an elevated FXI plasma level is an independent risk factor for thrombotic diseases such as deep vein thrombosis.5 Consistent with these observations, FXI plays a critical role in experimental thrombus growth in rabbits, mice and primates.6–10
FXI circulates as a disulfide-linked homodimer in complex with plasma high molecular weight kininogen (HK). FXI shares high sequence homology (58% amino acid identity) with the functionally-distinct plasma protein prekallikrein, which also circulates in complex with HK.11, 12 While the serine protease domain of each FXI subunit is similar to catalytic domains for other coagulation proteases, the non-catalytic portion contains four apple domains (A1 to A4), a feature shared only with prekallikrein.12, 13 The FXI A3 domain has been shown to contain binding sites for FIX and for the platelet receptor glycoprotein Ib-IX-V (GPIb).14, 15 FXI binding has been localized to the leucine-rich repeat (LRR) sequences on the NH2-terminal globular domain of GPIbα, at a site distinct from the anionic thrombin-binding domain of GPIbα.16, 17 It is unknown if FXI-platelet binding is solely mediated by GPIbα or if other platelet receptor(s) exist that can support interactions with FXI.
GPIbα has been shown to form a complex on the platelet surface with apolipoprotein E receptor 2 (ApoER2, LRP8),18–20 a member of the low density lipoprotein (LDL) family of receptors. ApoER2 initiates intracellular signaling through the adaptor protein disabled-1 (Dab-1) in platelets.21 The extracellular domain of ApoER2 consists of three regions: (i) the type A-binding repeats of LDL-binding domains displaying a negatively-charged surface that are responsible for receptor-ligand interactions; (ii) type B repeats, which are homologous to regions in the epidermal growth factor precursor; and (iii) the protein stack of O-linked sugar domains that separate the LDL-binding domains from the cellular surface. We have recently shown that platelet and leukocyte ApoER2 mediate interactions with the anticoagulant serine protease, activated protein C.22, 23 Here, we present the first evidence that identifies FXI as a ligand for ApoER2.
MATERIALS & METHODS
Reagents
Plasma-derived FXI, FXIa and FXa were purchased from Haematologic Technologies, Inc. (Essex Junction, VT, USA), fibrinogen and high molecular weight kininogen were from Enzyme Research Laboratories, Inc. (South Bend, IN, USA), fibrillar collagen was from Chrono-Log Corporation (Havertown, PA, USA), and AK2 was from GeneTex, Inc. (San Antonio, TX, USA). Soluble GPIbα, ApoER2’ (a splice variant of ApoER2 lacking LDL-binding domains 4, 5 and 6), low density lipoprotein (LDL)-binding domains 1 and 2 of ApoER2, plasma-derived β2GPI and dimeric β2GPI were cloned, expressed and purified as described.19, 24–27 HRP-labeled anti-FXI antibody was purchased from diaPharma (West Chester, OH). Mouse anti-FXI mAb has been described previously 28 and HRP-conjugated rabbit-anti-mouse IgG was from Dako (Glostrup, Denmark). All other reagents were from Sigma-Aldrich, Inc. (St. Louis, MO, USA) or previously named sources.29, 30
Preparation of Human and Murine Platelets and Red Blood Cells
Human venous blood was drawn by venipuncture from healthy volunteers into sodium citrate (final concentration 0.38% vol/vol) and acid/citrate/dextrose (ACD, 10% vol/vol). Platelet-rich plasma (PRP) was prepared by centrifugation of whole blood at 200 × g for 20 min. The platelets were then isolated from PRP by centrifugation at 1000 × g for 10 min in the presence of prostacyclin (0.1 µg/ml). Following centrifugation steps, purified human platelets were resuspended in modified Tyrode buffer (129 NaCl mM, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2; pH 7.3) as previously described.29
Following the removal of PRP, red blood cells were pelleted at 2000 × g for 10 min at 25 C. Red blood cells were subsequently washed three times with red blood cell buffer (10 mM HEPES, 140 mM NaCl, 5 mM glucose, pH 7.4) as previously described.29
Mice deficient in ApoER2 were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). Murine blood was drawn from CO2 terminally-anesthetized mice into ACD at a ratio of 1:10. PRP was obtained by centrifugation at 200 × g for 6 min. Washed platelets were prepared via centrifugation of PRP at 1000 × g in the presence of prostacyclin (0.1 µg/ml) for 6 min. The pellet was resuspended in modified Modified Tyrode buffer to the desired platelet count. All animals were maintained using housing and husbandry in accordance with institutional regulations.
Static adhesion assays
Glass coverslips were incubated with a 50 µg/ml solution of FXI, FXIa or fibrinogen for 1 hr at room temperature. Surfaces were then blocked with denatured fatty acid-free bovine serum albumin (BSA, 5 mg/ml) for 1 hr and washed with phosphate-buffered saline (PBS). Purified human platelets (2 × 107/ml) were incubated on protein-coated coverslips at 37°C for 45 min. Platelet spreading was imaged using Köhler illuminated Nomarski differential interference contrast (DIC) optics with a Zeiss 63× oil immersion 1.40 NA plan-apochromat lens on a Zeiss Axiovert 200M microscope (Carl Zeiss, Thornwood, NY). The degree of adhesion was computed using Image J software.31
Solid phase binding assays
Soluble ApoER2’ (sApoER2’; 5 µg/ml) was coated on Corning Costar 2595 protein assay plates as previously described.22 Coated wells were blocked with BSA for 1 hr at room temperature. Wells were washed 4 times with washing buffer (PBS with 0.1% tween-20). Prescribed dilutions of human FXI (0–200 nM) in the absence or presence of 40 µg/ml RAP were added to the wells for 1 hr at room temperature. In order to detect ApoER2’-bound FXI, plates were incubated with a mouse anti-human FXI mAb (1 µg/ml) for 1 hr prior to washing and incubation with an HRP-labeled rabbit anti-mouse antibody. The assay was developed by addition of TMB (100 µl/well) followed by acid stop with 1M H2SO4, and changes in absorbance at 450 nm were measured. The apparent Kd value was obtained from the binding data using Prism 4 software (GraphPad Software, Inc., San Diego, CA).
Flow adhesion assays
Glass capillary tubes were incubated for 1 hr at room temperature with a solution of 50 µg/ml of either FXI or FXIa. Tubes were then washed with PBS, followed by incubation with 5 mg/ml denatured BSA for 1 hr. Tubes were then incorporated into a flow system on the stage of a Zeiss Axiovert microscope (Carl Zeiss, Thornwood, NY). Reconstituted blood (autologous packed red blood cells reconstituted with washed platelets in modified Tyrode buffer at a final concentration of 3 × 108 platelets/ml, 50% vol/vol) was perfused through the chamber for 3 min at a wall shear rate of 600 s−1. Platelet interactions were imaged in real time with a Zeiss AxioCam.
Analysis of data
Data are shown as means ± SEM. Statistical significance of differences between means was determined by ANOVA. If means were shown to be significantly different, multiple comparisons were performed by the Tukey test. Probability values of P < 0.05 were selected to be statistically significant.
RESULTS
Platelets bind to and spread on factor XI
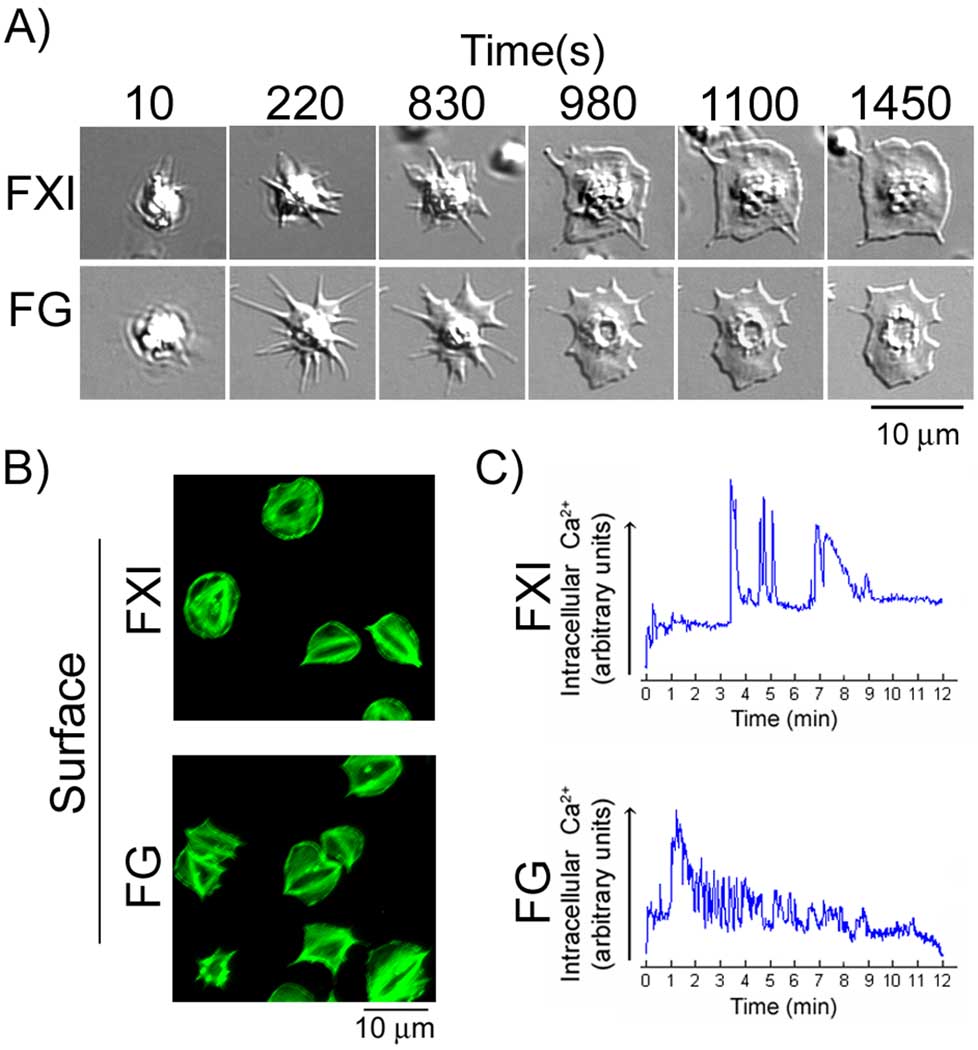
We sought to investigate the ability of FXI to support platelet adhesion and spreading. The interaction of purified platelets with surface-immobilized FXI was monitored in real-time using DIC microscopy. As shown in Fig. 1A, adhesion of platelets to immobilized FXI was followed by a series of characteristic changes in platelet morphology over a 20 minute time course resulting in the formation of large, sheet-like lamellipodia. Consistent with previous reports, surface-immobilized fibrinogen was also able to support platelet adhesion and spreading (Fig 1A). Fluorescent labeling of the actin cytoskeleton revealed that stress fibers were formed in fully spread platelets on FXI (Fig. 1B). Real-time imaging of platelets loaded with the calcium-reporter dye, Oregon Green BAPTA 1-AM, showed that platelets interacting with FXI demonstrated an initial Ca2+ release followed by a delay of up to 5 minutes, after which a further increase in intracellular calcium occurred that oscillated over 3–7 minutes before declining (Fig. 1B). On fibrinogen, platelets displayed an initial Ca2+ spike that oscillated in a descending manner over the 12 minutes shown (Fig. 1B). A similar degree of platelet spreading, actin reorganization, and intracellular calcium mobilization was recorded for platelets on immobilized FXIa (Supplemental Fig. 1).
Figure 1. Platelet cytoskeletal reorganization on FXI surfaces.
(A) Purified human platelets (2 × 107/ml) were exposed to surfaces coated with FXI or fibrinogen (FG) and observed in real time using differential interference contrast (DIC) microscopy. A representative time course of a single platelet spreading on each surface is shown. (B) Platelets adherent on each surface were fixed, permeabilized and stained for F-actin using FITC-conjugated phalloidin. (C) Purified human platelets loaded with the Ca2+-sensitive dye Oregon Green BAPTA 1-AM were imaged as they made contact with FXI- (top) or FG-(bottom) coated surfaces. The graphs show the basal Ca2+ level of individual platelets upon arrival to the region of interest and the subsequent Ca2+ fluctuations over a 12 min period of observation. The scale is in arbitrary units derived from the intensity of fluorescence emission.
We next aimed to determine the intracellular mechanisms that facilitate platelet spreading on FXI. Presence of an ADP scavenger (apyrase, 2 U/ml) with a cyclooxygenase inhibitor (indomethacin, 10µM) reduced platelet adhesion and spreading on FXI surfaces (Supplemental Fig. 2). Treatment of platelets with inhibitors to Src kinases (PP2, 20 µM), PI3-kinases (wortmannin, 100 nM) or an intracellular Ca2+ chelator (BAPTA-AM, 10 µM) inhibited platelet spreading on immobilized FXI (Supplemental Fig. 2). Similar results were seen on FXIa surfaces (Supplemental Fig. 2).
ApoER2 mediates platelet adhesion to FXI
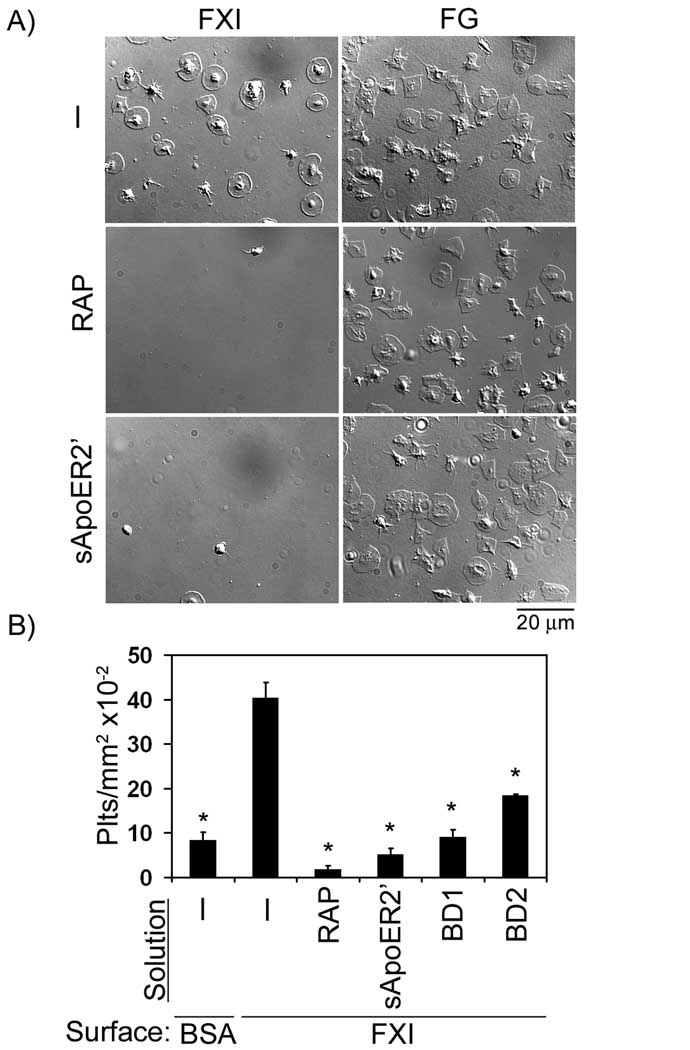
FXI has been shown to bind specifically and reversibly to high affinity sites on the platelet surface. To characterize the molecular mechanisms of platelet-FXI binding, adhesion assays were performed in the presence of receptor-associated protein (RAP), which universally inhibits ligand binding to the LDL receptor family. Our data show that RAP eliminated platelet binding to immobilized FXI, but not to fibrinogen (Fig 2). Furthermore, the presence of soluble recombinant ApoER2’ (sApoER2’) abrogated platelet binding to FXI (Fig 2). Moreover, the presence of the LDL-binding domain 1 or 2 of ApoER2 reduced platelet adhesion to FXI by 78% and 55%, respectively (Fig. 2B).
Figure 2. The role of ApoER2 in platelet adhesion to FXI.
(A) Purified human platelets (2 × 107/ml) were pipetted onto surfaces coated with FXI or fibrinogen (FG) in the absence or presence of receptor-associated protein (RAP, 40 µg/ml) or soluble ApoER2’ (sApoER2’, 40 µg/ml), followed by incubation at 37°C for 45 min. (B) Additional adhesion experiments were performed on immobilized FXI in the presence of the LDL binding domains 1 or 2 of ApoER2 (BD1 or BD2, respectively, 40 µg/ml). Adherent platelets were analyzed for each suspension treatment and reported as adherent cells/mm2 × 10−2. Values are reported as mean ± SEM of three experiments. *P < 0.05 compared to adhesion on FXI in the presence of vehicle.
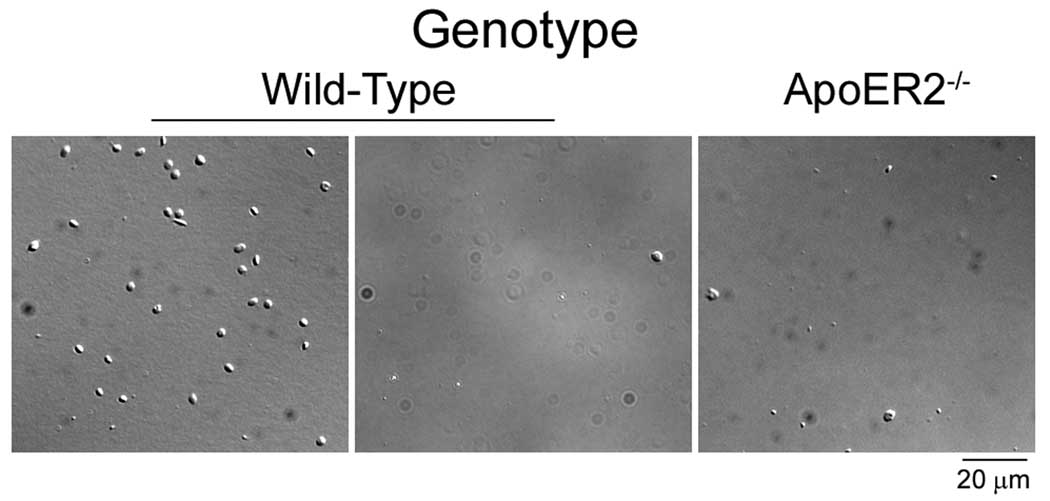
In order to verify the ability of ApoER2 to mediate platelet binding to FXI, platelets from wild-type C57Bl/6 or ApoER2-deficient mice were incubated over surfaces coated with FXI or fibrinogen. As shown in Fig. 3, a greater than 90% reduction in platelet adhesion on FXI was observed for ApoER2-deficient platelets compared to wild-type (13.6 ± 1.4 vs. 1.2 ± 0.5 × 102 platelets/mm2 on FXI for wild-type vs. ApoER2−/−, respectively). A similar level of reduction in binding was observed for wild-type platelets in the presence of binding domain 1 of ApoER2 (Fig. 3). The presence of soluble GPIbα (40 µg/ml) did not affect wild-type platelet binding to immobilized FXI (data not shown). Equivalent levels of adhesion were observed on fibrinogen for wild-type and ApoER2−/− platelets (155 ± 15 vs. 150 ± 12 × 102 platelets/mm2 on fibrinogen for wild-type vs. ApoER2−/−, respectively).
Figure 3. ApoER2-deficient platelets do not bind to FXI.
Purified platelets (2 × 107/ml) from wild-type or ApoER2-deficient mice were incubated over immobilized FXI at 37°C for 45 min in the presence of vehicle (−) or the LDL binding domain 1 of ApoER2 (BD1, 40 µg/ml). Representative images from 3–4 experiments are shown.
Mechanisms of FXI-platelet interactions under flow
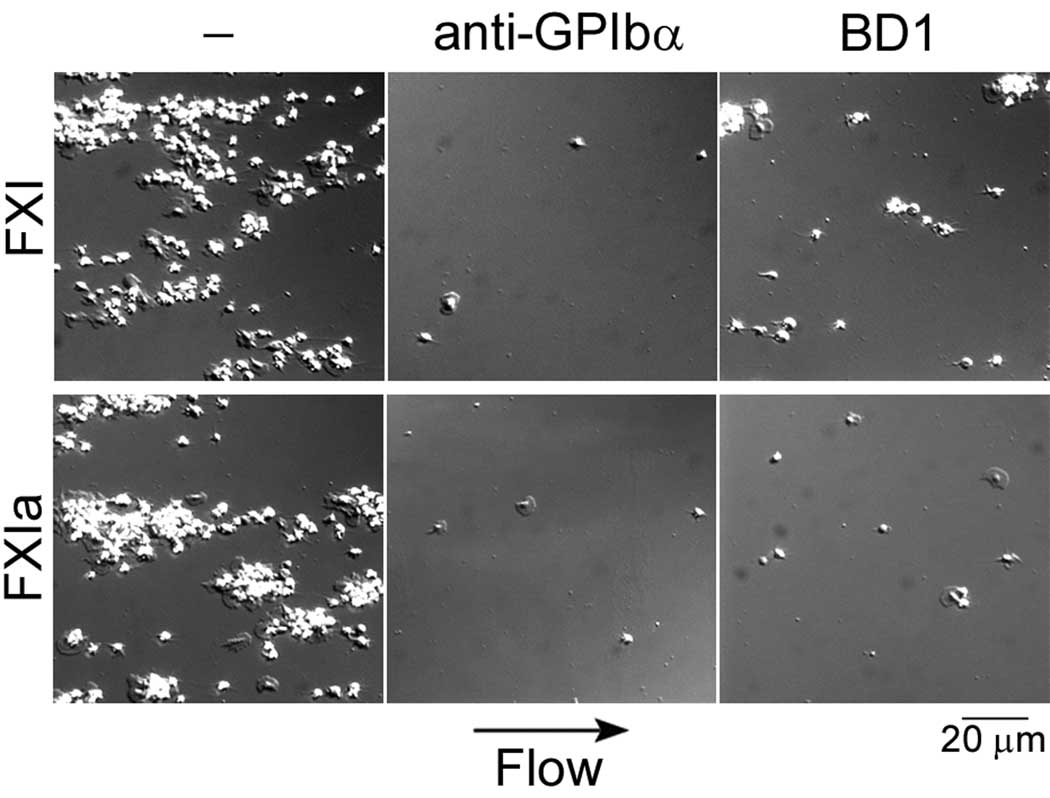
In order to evaluate the interaction between platelets and FXI in the presence of shear, washed human platelets were reconstituted with purified red blood cells (50% vol/vol, final platelet count 3 × 108 /ml) and perfused over immobilized FXI for 3 min at 600 s−1. In the presence of shear, platelets in reconstituted blood formed aggregates on immobilized FXI (Fig. 4). Addition of the anti-GPIbα mAb, AK2, or LDL-binding domain 1 of ApoER2 blocked platelet adhesion to FXI under shear conditions (Fig. 4). Similar results were observed on FXIa surfaces in the presence of shear (Fig. 4).
Fig. 4. Platelet binding to FXI under physiological shear.
Human reconstituted blood (autologous packed red blood cells and washed human platelets combined to 50% vol/vol, yielding a final platelet concentration of 3 × 108/ml) was perfused over a surface of FXI or FXIa at a shear rate of 600 s−1 for 3 min in the presence of 25 µM Zn2+. Separate experiments were performed in the presence of either an anti-GPIbα antibody (AK2, 20 µg/ml) or LDL-binding domain 1 of ApoER2 (BD1, 50 µg/ml). Images are representative of at least 3 separate experiments.
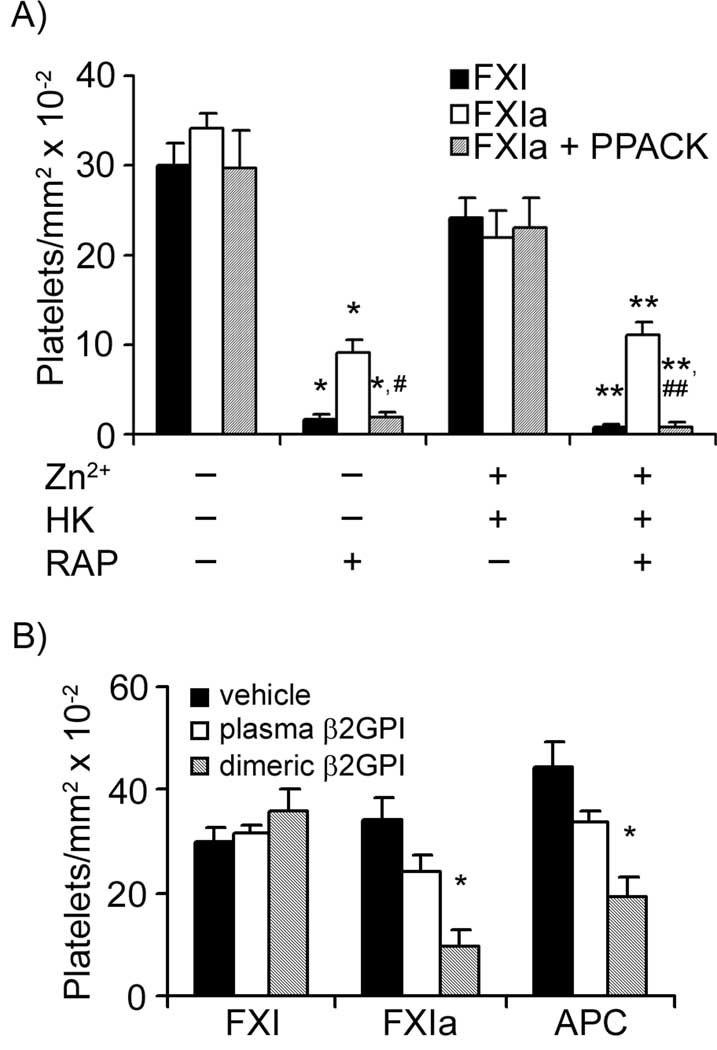
Effects of Zn2+ and HK on platelet adhesion to FXI surfaces
FXI circulates in the plasma in complex with HK, and earlier studies have shown that FXI-platelet interactions in solution require both HK and the divalent cation, Zn2+.32, 33 As shown in Fig. 5A, FXI surfaces supported platelet adhesion in the presence of either vehicle or the combination of Zn2+ and HK. The presence of RAP abrogated platelet adhesion to FXI in the absence or presence of Zn2+ and HK (Fig. 5A). The degree of platelet adhesion to surfaces of the active site mutant FXI-Ala557, which lacks proteolytic activity, in the presence of vehicle was similar to adhesion to surfaces of native FXI (31.4 ± 4.2 vs. 33.9 ± 3.6× 102 platelets/mm2 on FXI-Ala557 vs. native FXI, respectively).
Figure 5. Platelet-FXI interactions in the presence of Zn2+, high molecular weight kininogen or β2 glycoprotein I.
Washed human platelets (2 × 107/ml) were incubated over surfaces of FXI or FXIa in the absence or presence of (A) Zn2+ (25µM) and high molecular weight kininogen (HK, 42 nM). Selected experiments were performed in the presence of PPACK (40 µM) or RAP (40 g/ml). P < 0.05 compared to adhesion on each respective surface in the presence of vehicle (*) or Zn2+ and HK (**). P < 0.05 compared to adhesion on FXIa with RAP in the presence of vehicle (#) or Zn2+ and HK (##). (B) Additional adhesion experiments were performed over FXI, FXIa or activated protein C (APC) in the presence or absence of plasma-derived beta2-glycoprotein I (β2GPI, 350 nM) or dimeric β2GPI (350 nM). Adherent platelets were calculated for each treatment and are reported as adherent cells/mm2 × 10−2. Adhesion numbers are reported as mean ± SEM of at least three experiments. *P < 0.05 compared to adhesion on each respective surface in the presence of vehicle.
FXIa surfaces supported platelet adhesion either in the presence of vehicle or the combination of Zn2+ and HK. Addition of RAP significantly, but incompletely, inhibited platelet adhesion to FXIa in the absence or presence of Zn2+ and HK (Fig. 5A). The remaining platelet adhesion on FXIa in each case was eliminated with the combination of RAP in combination with the serine protease inhibitor, D-phenylalanyl- L-prolyl-L-arginine chloromethyl ketone (PPACK, 40 µM).
Effects of known ApoER2 ligands on platelet adhesion to FXI
ApoER2 has been shown to bind to plasma components such as antiphospholipid antibody-dimerized beta 2 glycoprotein I (β2GPI) and activated protein C (APC).19, 22 As shown in Fig. 5B, platelet adhesion to FXI was unaffected by the presence of either plasma-derived or dimerized β2GPI. Conversely, platelet adhesion to immobilized FXIa or APC was significantly reduced in the presence of dimeric β2GPI (Fig. 5B). Platelet adhesion to FXI or FXIa was not inhibited by the presence of APC in solution (data not shown).
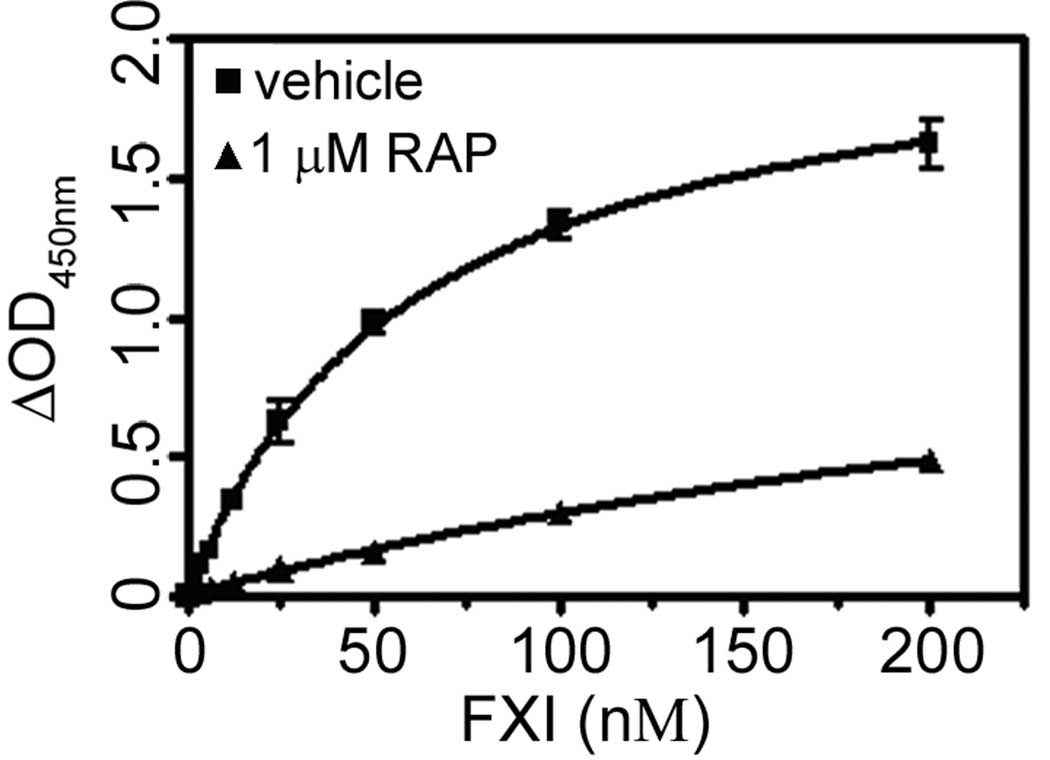
Evaluation of soluble FXI binding to purified sApoER2’
To quantify direct binding of FXI to ApoER2, prescribed dilutions of FXI were incubated with immobilized sApoER2’, and the level of bound FXI was determined using anti-FXI antibodies as described in “Materials and Methods”. As shown in Fig. 6, soluble FXI bound to immobilized sApoER2’ with an apparent affinity of 61 nM. The presence of RAP significantly reduced FXI binding to sApoER2’ (Fig. 6). Taken together, our data identify ApoER2 as a novel receptor for FXI.
Fig. 6. Soluble FXI binding to purified ApoER2’.
FXI at the indicated concentrations was incubated over a surface of recombinant ApoER2’. Designated experiments were performed in the presence of either vehicle (squares) or 1 µM RAP (triangles). Bound FXI was detected by anti-FXI antibodies as described in ‘Materials & Methods’. Values are reported as mean ± SEM of 3 experiments.
DISCUSSION
The aim of this study was to investigate the molecular mechanisms of FXI-platelet interactions. We identified FXI as a novel ligand for the receptor ApoER2. Our initial data demonstrated that purified, recombinant forms of ApoER2 abrogated platelet binding to FXI, and that platelets from ApoER2-deficient mice did not adhere to immobilized FXI. Immobilized ApoER2’ specifically bound FXI in a cell-free assay with an affinity of 61 nM. Our data identify FXI as a ligand for the platelet receptor ApoER2.
FXI binding to platelets was first described over 30 years ago.34 GPIbα was identified as the major receptor for FXI on activated platelets,16, 17, 35 but studies have failed to show that GPIbα mediates FXIa binding to activated platelets under static conditions.36, 37 The data reported here support a role for GPIbα in mediating shear-dependent interactions between resting platelets and both FXI and FXIa. The reason for the discrepancy between our results and earlier stucies is not clear, but it is noteworthy that ApoER2’ has been shown to co-localize with the GPIb/IX/V complex on the platelet surface.19 As FXI and FXIa exist as homodimers, perhaps one FXI/FXIa molecule can simultaneously bind both GPIbα and ApoER2’ on the platelet surface. Since ApoER2 is expressed on the platelet surface at far lower levels than the GPIb complex,19 this may explain why a total of only 1500 FXI binding sites per platelet have been reported,33 even though GPIb is present at 25,000 copies per platelet.38
Since the expression of ApoER2 on platelets was discovered in 1999, platelet ApoER2 has been shown to bind a handful of plasma components.19, 22, 39, 40 Data reported here suggest that FXI has a similar affinity for ApoER2 as recently-described ligands such as dimeric β2GPI and activated protein C (APC).22, 41 Autoantibodies to β2GPI correlate highly with recurrent arterial thrombosis and fetal loss associated with antiphospholipid antibody syndrome (APS).21 The in vitro properties of APS patient β2GPI-anti-β2GPI antibody complexes are mimicked by dimeric β2GPI.27 While neither dimeric β2GPI nor APC in our experiments inhibited platelet adhesion to FXI, the presence of dimeric β2GPI significantly reduced platelet adhesion to immobilized FXIa. β2GPI has been shown to inhibit the activation of FXI by FXIIa and thrombin, whereas cleavage of β2GPI at Lys317-Lys318 by FXIa eliminates this inhibitory effect.42 Because β2GPI is able to interact directly with FXI and FXIa, and FXIa is able to cleave β2GPI, further studies are required to determine the exact mechanisms leading to the differential effects of dimeric β2GPI on platelet binding to FXI and FXIa.42, 43 Perhaps the platelet receptor ApoER2 plays a role in mediating the interaction of β2GPI with FXI on the surface of activated platelets and subsequent FXIa-mediated proteolytic cleavage of β2GPI. It remains to be determined whether deregulation of this process by autoantibodies to β2GPI may be an important mechanism for thrombosis in patients with APS.
While the universal LDL-receptor ligand-binding inhibitor RAP abolished platelet adhesion to FXI, its presence significantly, but incompletely inhibited platelet adhesion to FXIa. Previous studies have shown that the catalytic domain of FXIa is involved in platelet interactions.44 In this study, the remaining platelet adhesion to FXIa was eliminated with the addition of the serine protease inhibitor, PPACK, in combination with RAP. A parallel set of observations was made in the presence of HK/Zn2+. Taken together, these data point to a possible role for the catalytic domain in platelet-FXIa interactions, suggesting that a distinct, RAP-insensitive, site exists on the platelet surface that is capable of interacting with FXIa. One such possibility is that FXIa is capable of cleaving PAR receptors. Perhaps FXIa, once bound to the platelet surface via ApoER2, contributes to platelet activation via proteolytic cleavage of platelet PAR receptors. It remains to be determined if FXI binding to monocytes and endothelial cells, which express ApoER2,22, 23 is mediated by ApoER2, and whether FXIa ligation of ApoER2 promotes a Reelin-like signaling pathway in these cells.
Recent findings by Tucker, et al. support the hypothesis that FXI activity is necessary for thrombus stability, as inhibition of FXI led to instability and dissolution of platelet-rich thrombi in a nonhuman primate thrombosis model.45 FXIa bound to activated platelets has been shown to be resistant to inhibition by exogenous protease nexin 2.36 It could be that ApoER2’, in concert with GPIbα, plays a critical role in localizing FXI/FXIa to the growing platelet plug, subsequently facilitating FXIa activation of FIX, accelerating thrombin formation and thus promoting thrombus stability. Future work will be focused on identifying the significance of ApoER2 in FXI-dependent thrombus formation.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by American Heart Association Grants 09GRNT2150003 (O.J.T.M.), 0850056Z (A.G.), 0910025G (T.C.W) and 09PRE2230117 (M.A.B.), a Dutch Heart Foundation Grant 2003B074, and National Institutes of Health (NIH) Grant HL58837 (D.G., A.G.). J.M.G. was supported by the UR Reach Program. T.C.W. is a Vertex Scholar and T.C.W. and M.A.B. are ARCS Scholars.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 2.Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 3.Asakai R, Chung DW, Davie EW, Seligsohn U. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325:153–158. doi: 10.1056/NEJM199107183250303. [DOI] [PubMed] [Google Scholar]
- 4.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 5.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 6.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 7.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–776. [PubMed] [Google Scholar]
- 9.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Smith PL, Hsu MY, Ogletree ML, Schumacher WA. Murine model of ferric chloride-induced vena cava thrombosis: evidence for effect of potato carboxypeptidase inhibitor. J Thromb Haemost. 2006;4:403–410. doi: 10.1111/j.1538-7836.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Q, Sun MF, Kravtsov DV, Aktimur A, Gailani D. Factor XI apple domains and protein dimerization. J Thromb Haemost. 2003;1:2340–2347. doi: 10.1046/j.1538-7836.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 12.Fujikawa K, Chung DW, Hendrickson LE, Davie EW. Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochemistry. 1986;25:2417–2424. doi: 10.1021/bi00357a018. [DOI] [PubMed] [Google Scholar]
- 13.Seligsohn U. Factor XI in haemostasis and thrombosis: past, present and future. Thromb Haemost. 2007;98:84–89. [PubMed] [Google Scholar]
- 14.Baglia FA, Walsh PN. A binding site for thrombin in the apple 1 domain of factor XI. J Biol Chem. 1996;271:3652–3658. doi: 10.1074/jbc.271.7.3652. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Gailani D. Identification of a factor IX binding site on the third apple domain of activated factor XI. J Biol Chem. 1996;271:29023–29028. doi: 10.1074/jbc.271.46.29023. [DOI] [PubMed] [Google Scholar]
- 16.Baglia FA, Gailani D, Lopez JA, Walsh PN. Identification of a binding site for glycoprotein Ibalpha in the Apple 3 domain of factor XI. J Biol Chem. 2004;279:45470–45476. doi: 10.1074/jbc.M406727200. [DOI] [PubMed] [Google Scholar]
- 17.Baglia FA, Shrimpton CN, Emsley J, Kitagawa K, Ruggeri ZM, Lopez JA, Walsh PN. Factor XI interacts with the leucine-rich repeats of glycoprotein Ibalpha on the activated platelet. J Biol Chem. 2004;279:49323–49329. doi: 10.1074/jbc.M407889200. [DOI] [PubMed] [Google Scholar]
- 18.May P, Herz J, Bock HH. Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci. 2005;62:2325–2338. doi: 10.1007/s00018-005-5231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennings MT, Derksen RH, van Lummel M, Adelmeijer J, VanHoorelbeke K, Urbanus RT, Lisman T, de Groot PG. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2'. J Thromb Haemost. 2007;5:369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 20.Stolt PC, Bock HH. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal. 2006;18:1560–1571. doi: 10.1016/j.cellsig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta(2)-glycoprotein I requires signaling via both glycoprotein Ibalpha and Apolipoprotein E Receptor 2'. J Thromb Haemost. 2008 doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 22.White TC, Berny MA, Tucker EI, Urbanus RT, De Groot PG, Fernandez JA, Griffin JH, Gruber A, McCarty OJ. Protein C supports platelet binding and activation under flow: role of glycoprotein Ib and apolipoprotein E receptor 2. J Thromb Haemost. 2008;6:995–1002. doi: 10.1111/j.1538-7836.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang XV, Banerjee Y, Fernandez JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC, McCarty OJ, Griffin JH. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci U S A. 2009;106:274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 25.Pennings MT, Derksen RH, Urbanus RT, Tekelenburg WL, Hemrika W, de Groot PG. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J Thromb Haemost. 2007;5:1538–1544. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 26.Horbach A, van Oort E, Donders RC, Derksen RH, de Groot PG. Lupus anticoagulant is the strongest risk factor for both venous and arterial thrombosis in patients with systemic lupus erythematosus. Comparison between different assays for the detection of antiphospholipid antibodies. Thromb Haemost. 1996;76:916–924. [PubMed] [Google Scholar]
- 27.Lutters BC, Meijers JC, Derksen RH, Arnout J, de Groot PG. Dimers of beta 2-glycoprotein I mimic the in vitro effects of beta 2-glycoprotein I-anti-beta 2-glycoprotein I antibody complexes. J Biol Chem. 2001;276:3060–3067. doi: 10.1074/jbc.M008224200. [DOI] [PubMed] [Google Scholar]
- 28.Von dem Borne PA, Bajzar L, Meijers JC, Nesheim ME, Bouma BN. Thrombin-mediated activation of factor XI results in a thrombin-activatable fibrinolysis inhibitor-dependent inhibition of fibrinolysis. J Clin Invest. 1997;99:2323–2327. doi: 10.1172/JCI119412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berny MA, White TC, Tucker EI, Bush-Pelc LA, Di Cera E, Gruber A, McCarty OJ. Thrombin mutant W215A/E217A acts as a platelet GPIb antagonist. Arterioscler Thromb Vasc Biol. 2008;28:329–334. doi: 10.1161/ATVBAHA.107.156273. [DOI] [PubMed] [Google Scholar]
- 30.White TC, Berny MA, Robinson DK, Yin H, DeGrado WF, Hanson SR, McCarty OJ. The leech product saratin is a potent inhibitor of platelet integrin alpha2beta1 and von Willebrand factor binding to collagen. Febs J. 2007;274:1481–1491. doi: 10.1111/j.1742-4658.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- 31.McCarty OJ, Zhao Y, Andrew N, Machesky LM, Staunton D, Frampton J, Watson SP. Evaluation of the role of platelet integrins in fibronectin-dependent spreading and adhesion. J Thromb Haemost. 2004;2:1823–1833. doi: 10.1111/j.1538-7836.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 32.Baird TR, Walsh PN. Activated platelets but not endothelial cells participate in the initiation of the consolidation phase of blood coagulation. J Biol Chem. 2002;277:28498–28503. doi: 10.1074/jbc.M203427200. [DOI] [PubMed] [Google Scholar]
- 33.Greengard JS, Heeb MJ, Ersdal E, Walsh PN, Griffin JH. Binding of coagulation factor XI to washed human platelets. Biochemistry. 1986;25:3884–3890. doi: 10.1021/bi00361a022. [DOI] [PubMed] [Google Scholar]
- 34.Walsh PN, Mills DC, Pareti FI, Stewart GJ, Macfarlane DE, Johnson MM, Egan JJ. Hereditary giant platelet syndrome. Absence of collagen-induced coagulant activity and deficiency of factor-XI binding to platelets. Br J Haematol. 1975;29:639–655. doi: 10.1111/j.1365-2141.1975.tb02750.x. [DOI] [PubMed] [Google Scholar]
- 35.Ho DH, Badellino K, Baglia FA, Sun MF, Zhao MM, Gailani D, Walsh PN. The role of high molecular weight kininogen and prothrombin as cofactors in the binding of factor XI A3 domain to the platelet surface. J Biol Chem. 2000;275:25139–25145. doi: 10.1074/jbc.M001890200. [DOI] [PubMed] [Google Scholar]
- 36.Baird TR, Walsh PN. The interaction of factor XIa with activated platelets but not endothelial cells promotes the activation of factor IX in the consolidation phase of blood coagulation. J Biol Chem. 2002;277:38462–38467. doi: 10.1074/jbc.M205902200. [DOI] [PubMed] [Google Scholar]
- 37.Sinha D, Seaman FS, Koshy A, Knight LC, Walsh PN. Blood coagulation factor XIa binds specifically to a site on activated human platelets distinct from that for factor XI. J Clin Invest. 1984;73:1550–1556. doi: 10.1172/JCI111361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergmeier W, Chauhan AK, Wagner DD. Glycoprotein Ibalpha and von Willebrand factor in primary platelet adhesion and thrombus formation: lessons from mutant mice. Thromb Haemost. 2008;99:264–270. doi: 10.1160/TH07-10-0638. [DOI] [PubMed] [Google Scholar]
- 39.Korporaal SJ, Koekman CA, Verhoef S, van der Wal DE, Bezemer M, Van Eck M, Akkerman JW. Downregulation of Platelet Responsiveness Upon Contact With LDL by the Protein-Tyrosine Phosphatases SHP-1 and SHP-2. Arterioscler Thromb Vasc Biol. 2009;29:372–379. doi: 10.1161/ATVBAHA.108.173278. [DOI] [PubMed] [Google Scholar]
- 40.Korporaal SJ, Relou IA, van Eck M, Strasser V, Bezemer M, Gorter G, van Berkel TJ, Nimpf J, Akkerman JW, Lenting PJ. Binding of low density lipoprotein to platelet apolipoprotein E receptor 2' results in phosphorylation of p38MAPK. J Biol Chem. 2004;279:52526–52534. doi: 10.1074/jbc.M407407200. [DOI] [PubMed] [Google Scholar]
- 41.van Lummel M, Pennings MT, Derksen RH, Urbanus RT, Lutters BC, Kaldenhoven N, de Groot PG. The binding site in {beta}2-glycoprotein I for ApoER2' on platelets is located in domain V. J Biol Chem. 2005;280:36729–36736. doi: 10.1074/jbc.M504172200. [DOI] [PubMed] [Google Scholar]
- 42.Shi T, Giannakopoulos B, Iverson GM, Cockerill KA, Linnik MD, Krilis SA. Domain V of beta2-glycoprotein I binds factor XI/XIa and is cleaved at Lys317-Thr318. J Biol Chem. 2005;280:907–912. doi: 10.1074/jbc.M410291200. [DOI] [PubMed] [Google Scholar]
- 43.Shi T, Iverson GM, Qi JC, Cockerill KA, Linnik MD, Konecny P, Krilis SA. Beta 2-Glycoprotein I binds factor XI and inhibits its activation by thrombin and factor XIIa: loss of inhibition by clipped beta 2-glycoprotein I. Proc Natl Acad Sci U S A. 2004;101:3939–3944. doi: 10.1073/pnas.0400281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller TN, Sinha D, Baird TR, Walsh PN. A catalytic domain exosite (Cys527–Cys542) in factor XIa mediates binding to a site on activated platelets. Biochemistry. 2007;46:14450–14460. doi: 10.1021/bi701310x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–944. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.