Abstract
Serotonin (5-hydroxytryptamine; 5-HT)-toxicity syndrome, an iatrogenic brain disorder induced by excessive efflux of 5-HT, has received much attention because of increasing incidents of serotonergic antidepressants. However, the neural mechanism by which extracellular 5-HT is elevated to a toxic level for the syndrome remains to be determined. The goal of the present study was to test the hypothesis that extracellular 5-HT is composed of two component effluxes responsible for distinct aspects of the syndrome. The first set of experiments was to characterize the syndrome by measuring changes in neuromuscular signs, body-core temperature and mortality rate. Our results indicate that the syndrome severity can be categorized into mild, moderate and severe levels. The second set of experiments was to determine a threshold of extracellular 5-HT for induction of each level of the syndrome. Our results demonstrate that there were an 11-fold increase in the mild syndrome and an over 55-fold increase in the severe syndrome. In the last series of experiments, the excessive increases in 5-HT were pharmacologically separated into primary and secondary component effluxes with the 5-HT2A receptor antagonists cyproheptadine and ketanserin and NMDA receptor antagonist (+)-MK-801. Our results suggest primary component efflux was caused by direct drug effects on 5-HT biosynthetic and metabolic pathways and secondary efflux ascribed to indirect drug effect on a positive feedback circuit involving 5-HT2A and NMDA receptors. In summary, the primary efflux could be an initial cause for the induction of the syndrome while the secondary efflux might involve deterioration of the syndrome.
Keywords: Serotonin-toxicity syndrome, 5-HT2A receptor, NMDA receptor, prefrontal cortex, hyperthermia
1. Introduction
Incidence of serotonin-toxicity syndrome has been on the rise in recent years due to increased prescription of antidepressant drugs, especially selective serotonin reuptake (SSRIs) and monoamine oxidase inhibitors (MAOIs) (Boyer and Shannon, 2005; Gillman, 2005; Isbister and Buckley, 2005). Although patients with a mild syndrome may feel little or no discomfort, a severe syndrome can be life-threatening. Thus insights into the neural mechanisms underlying the syndrome could be crucial for a better understanding of this iatrogenic brain disorder.
The syndrome can be reproduced experimentally in laboratory animals by administration of an MAOI combined with 5-HT precursors (Izumi et al., 2006; Speiser et al., 2008). Degrees of the syndrome are estimated by either neuromuscular responses (Izumi et al., 2006; Ma et al., 2008; Speiser et al., 2008) or autonomic changes, especially body-core temperature (Ma et al., 2008; Nisijima et al., 2001). It has been suggested that postsynaptic 5-HT1A receptors are responsible for most, if not all, of neuromuscular responses (Darmani and Zhao, 1998; Fox et al., 2007). In contrast, 5-HT2A receptors exert an essential role in autonomic dysfunction and death (Nisijima et al., 2001; Shioda et al., 2004). These two receptors have different affinities for 5-HT, a natural ligand in the CNS. The affinity of 5-HT2A receptors is 400 – 1000 times lower than that of 5-HT1A receptors (Almaula et al., 1996; Dalpiaz et al., 1995; Peroutka et al., 1981). Thus, contribution of two receptors to the toxic response, which is presumably determined by 5-HT concentration induced by drugs, is not necessarily equipotent.
In a previous study we have characterized the neuromuscular responses and changes in body-core temperature in the syndrome (Ma et al., 2008). Despite progress, neural mechanisms underlying the involvement of 5-HT in those changes were not understood. Typically there is only a 1~5-fold increase in 5-HT following therapeutic antidepressants (Fuller, 1994; Hervas et al., 2000; Rutter and Auerbach, 1993). It is widely believed that the level of 5-HT in the syndrome can be as high as 140–1100-fold over baseline (Nisijima et al., 2001; Shioda et al., 2004; also see reviews by Isbister et al., 2005 and Gillman, 2005). In the present study, we focused on the fundamental question of how extracellular 5-HT is evoked to such a toxic level, particularly the 5-HT threshold for different degrees of the syndrome. To induce the syndrome, the 5-HT immediate precursor 5-hydroxy-L-tryptophan (5-HTP; 5, 10, 15, 20, and 25 mg/kg, i.p.) was administered in rats pretreated with the MAOI clorgyline (2 mg/kg, i.p.). Degrees of the syndrome in response to drug injection were assessed in three different ways: neuromuscular responses (e.g., head shaking, myoclonus, forepaw treading and hindlimb abduction), body-core temperature and lethality. 5-HT efflux was measured using dual-probe microdialysis in the preoptic/anterior hypothalamus and prefrontal cortex. From a classic point of view, the increase in 5-HT efflux following 5-HTP and clorgyline is a direct drug effect on enzymatic activity at pre-synaptic sites (Grahame-Smith, 1971). In this study, this view was evaluated by measuring changes in 5-HT after blocking the 5-HT2A receptors with cyproheptadine and ketanserin or blocking NMDA receptors with (+)MK-801. Theoretically, pretreatments with these receptor antagonists would not have any effect on 5-HT efflux if 5-HT biosynthesis and breakdown were the exclusive mechanisms responsible for the efflux. On the contrary, we observed that these antagonists effectively reduced the increases in 5-HT efflux, suggesting that other mechanisms are involved in the course of the syndrome in addition to the direct drug effect on pre-synaptic metabolic enzymes.
2. Materials and methods
2.1. Animal preparation
Adult male Sprague-Dawley rats were purchased from Charles River Laboratories (Raleigh, NC, USA) and housed in pairs under a 12-h light/dark cycle (lights on 8:00 am) in a temperature- and humidity-regulated facility. Animals were allowed to access food and water freely. Animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the local animal study committees. All efforts were made to reduce the number of animals used and their suffering.
2.2. Chemicals and drug administration
Clorgyline (N-methyl-N-propargyl-3-(2,4-dichlorophenoxy)propylamine hydrochloride; Sigma-Aldrich, St. Louis, MO, U.S.A) was intraperitoneally (i.p.) administered at a dose of 2 mg/kg 12 h before experiments. The dose used in this study was based on previous investigations (Nisijima et al., 2004; Shioda et al., 2004). Clorgyline, 5-hydroxy-L-tryptophan (5-HTP; Sigma-Aldrich, St. Louis, MO, U. S.A) and (+)MK-801 maleate (Tocris, Ellisville, MO, USA) were dissolved in isotonic saline and cyproheptadine (USP, Rockville, MD, USA) in sterilized water containing 10% ethanol. Ketanserin (Tocris, Ellisville, MO, USA) was suspended in water. Drug solutions were prepared immediately before experiments and injected in a constant volume of 1 ml/kg body weight with the exception of 5-HTP which was administered at a volume of 3 ml/kg body weight.
2.3. Assessment of the severity of a syndrome
2.3.1. Neuromuscular response
Head shaking, myoclonus, forepaw treading and hindlimb abduction were used to assess the degree of a neuromuscular response to 5-HTP in rats pretreated with clorgyline. Observers blinded to treatment were assigned to report presence or absence of these signs on a recording sheet. The defined sign was considered to be positive only after presence at least twice in each 15-min time block. The observation lasted 120 min (total eight 15-min time blocks). Dose effect on each sign was arbitrarily set at three degrees: null (no sign), partial (< 4 out of 8 blocks), and full response (> 4 time blocks) as shown in Table 1.
Table 1.
Dose-dependent effect on behavioral signs
| Presence of behavioral signs |
||||||
|---|---|---|---|---|---|---|
| 5-HTP (mg/kg, i.p.) | n | Head shakes | Myoclonus | Forepaw treading | Hindlimb abduction | Mortality |
| control | 6 | 0/6 | ||||
| 5 | 6 | X | 0/6 | |||
| 10 | 6 | √ | √ | 0/6 | ||
| 15 | 6 | √ | √ | X | X | 0/6 |
| 20 | 5 | X | X | √ | √ | 3/5 |
| 25 | 6 | X | X | √ | √ | 6/6 |
| CPH+25 | 6 | X | X | √ | √ | 0/6 |
All rats were pretreated with clorgyline (2 mg/kg, i.p.), 12 h before experiments.
Behavioral signs were recorded in 15-min intervals immediately after 5-HTP injection. Note that cyproheptadine (CPH; 10 mg/kg, i.p.) was injected 15 min before 25 mg/kg 5-HTP. The observation lasted 120 min with eight 15-min blocks. Duration of a behavioral response is indicated as following.
blank: no sign of the behavior following 5-HTP injection
X: there was a transit change lasting less than 4 blocks (<60 min)
√: there was a persistent change lasting more than 4 blocks (>60 min)
2.3.2 Lethality
Upon completion of the neuromuscular assessment, the animal underwent a lethality observation for 24 h. The syndrome was considered to be benign if animals survived in the study period and otherwise was malignant.
2.3.3. Body-core temperature measurement
Animals were individually kept in transparent Plexiglas cages (40 cm × 20 cm × 20 cm) in a test room (ambient temperature 22.0 ± 1.0 °C, humidity controlled) for at least 2 h before experiments. A flexible, 4.6-cm thermoprobe connected to a digital thermometer (Traceable®, Fisher Science) was inserted into rat colon. 5-HTP injection was made after two baseline measurements. The experiments lasted 2 h at 15-min intervals for each measurement. Changes in body-core temperature in response to drug injection were categorized into three levels: hypothermia, normothermia and hyperthermia. If there was a persistent increase by +2 °C and higher above pre-drug measurements, the response was considered to be hyperthermia. Conversely, it was defined as hypothermia if the body-core temperature in response to drug injection was persistently reduced by −1 °C and further below pre-drug levels. Changes between +2 °C and −1 °C were considered to be normothermia.
2.4. 5-HT microdialysis
Guide cannulae, 10 mm in length prepared from 22 gauge stainless steel tubing, were implanted in anesthetized rats mounted in a stereotaxic frame (Stoelting Co., Wood Dal, Illinois, USA). Stereotaxic coordinates were AP −1.1 mm relative to bregma, ML ± 1.0 mm and DV −2.0 mm targeting toward the preoptic/anterior hypothalamus and coordinates AP +3.3, ML ± 0.7 mm and DV −2.0 mm toward the prefrontal cortex. Rats were then allowed a recovery period of at least one week.
I-shaped microdialysis probes (cut-off 18 kD) were used in this study. The length of the exchange surface was 1.0 mm for the hypothalamus and 2.5 mm for the cortex. One day before experiments, rats were briefly anaesthetized with isoflurane, and dialysis probes were inserted through the guide cannulae to the hypothalamus (coordinates: AP −1.1 mm relative to bregma, ML ± 1.0 mm, DV −9.2 mm) and to the cortex (AP +3.3 mm, ML ± 0.7 mm, DV −4.5 mm). The probes were secured in place with dental cement. In this study, we employed a dual-probe microdialysis technique to measure 5-HT changes simultaneously in these two regions. It was found that neurochemical and behavioral responses to drugs were not covariant with numbers of probes implanted in the brain. Also, there was no obvious difference in neurochemical changes between the left and right probes, and thus all probes from both hemispheric regions were pooled for the data analysis. Rats attached to a Raturn™ system (Bioanalytical System Inc., W. Lafayette, IN, USA) were allowed unrestricted behavior in test chambers with food and water available. The hypothalamus and cortex via the respective microdialysis probes were perfused overnight with artificial cerebrospinal fluid (aCSF; containing 140 mM NaCl, 3 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 0.25 mM NaH2PO4, and 1.0 mM Na2HPO4; pH: 7.4) at a flow rate of 1 μl/min. Animals were injected with clorgyline (2 mg/kg, i.p.) 12 h before 5-HTP injection.
Samples were manually collected at intervals of 15 min starting from 11:00 the next morning and analyzed by high performance liquid chromatography (HPLC). 5-HT was separated via a reversed phase column packed with a TSK gel ODS-80 TM and measured using a voltammetric detector (HTEC-500; EICOM, Japan). The potential on the graphite electrode was set at + 400 mV (relative to Ag/AgCl reference electrode). The mobile phase consisted of 0.1 M phosphate buffer (pH 6.0), 1% methanol, 500 mg/L sodium-1-octanesulfonate and 50 mg/L ethylenediamine-tetracetic acid. The detection limit was 0.05 pg/sample.
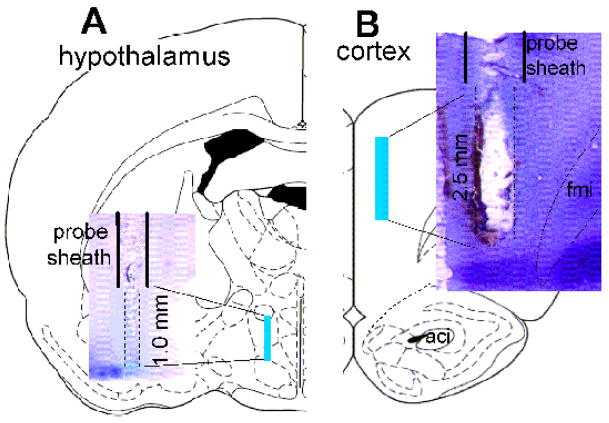
Upon completion of an experiment, the rats were detached from the Raturn™ system and deeply anesthetized with pentobarbital (100 mg/kg, i.p.). A histological dye, 2% Fast Green solution, was infused via the probes to the target areas for 10 min. The animals were then decapitated, and brains were removed and frozen. In most cases, brains were sliced freehand with a razor blade. The probe location was determined by comparison to the rat brain atlas (Paxinos and Watson, 1998). Data were excluded from analysis if probes were determined to be outside the target coordinates. For the photomicrographs, coronal sections (40 μm) were cut on a freezing microtome. A series of one-in-three sections were subsequently processed for cresyl violet staining. Fig. 1 shows a representative photomicrograph of a dual-probe location in the preoptic/anterior hypothalamus (fig. 1A) and prefrontal cortex (fig. 1B).
Fig. 1. Representative histological sections of dual-probe microdialysis.
Microdialysis probes were inserted via guide cannulae into the preoptic hypothalamus (A) and prefrontal cortex (B) indicated by shaded areas in the drawing adopted from the rat brain atlas (Paxinos and Watson, 1998). Insets: crystal violet staining of probe placements.
2.5. Data analysis
In fig. 2, body-core temperature (on the y-axis) was plotted against sampling time (on the x-axis). 5-HT efflux expressed as absolute values (pg/sample) was uncorrected from probe recovery. In some cases, only a hypothalamic or cortical datum was included in the analysis because of technical problems (for instance, obstruction in the infusion lines, damaged probe or missed target areas). For comparison, microdialysis data were normalized to relative changes expressed as fold increases over their respective baselines. Note that the baseline was calculated from the mean of four sequential samples before 5-HTP injection. To understand an accumulative effect of antagonistic drugs, Area under the Curve (AUC) was used to compare overall changes in 5-HT efflux between vehicle-treatment and antagonist-treatment. AUC data were obtained via computing accumulative changes in 5-HT over 90 min following 5-HTP injection.
Fig. 2. Changes in body-core temperature.
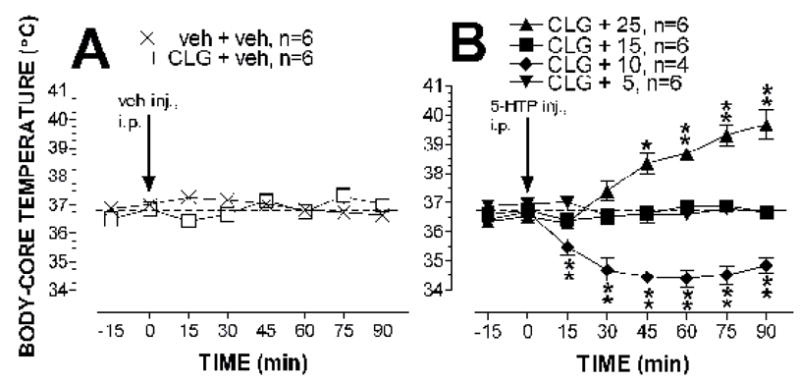
Data are expressed as mean ± S.E.M. 5-HTP or vehicle was administered at time zero, as indicated by the arrows. Panel A shows the body-core temperature response to vehicle injection (veh; 3 ml/kg, i.p.) in animals pretreated with vehicle (veh; 1 ml/kg, i.p.) or clorgyline (CLG; 2 mg/kg, i. p.) 12 h before the experiment. In panel B, all animals were pretreated with clorgyline (CLG; 2 mg/kg, i.p.) 12 h before experiments. 5-HTP injection produced a dose-dependent change in body-core temperature. The 5-HTP doses were 5 mg/kg, 10 mg/kg, 15 mg/kg and 25 mg/kg. * P< 0.05 and ** P < 0.01 vs. the control (CLG + veh) group examined by the two-way factorial ANOVA followed by the post-hoc Scheffe test.
Statistical analysis was performed by a two-way (drug treatment × time course) factorial ANOVA. If there was a significant difference between drug and vehicle treatment, further statistical analysis was carried out using the post-hoc Scheffe test in determining the significance of the respective time points. If appropriate, the unpaired t-test was employed for the data analysis. The level of P-value was set at 0.05 for a statistically significant effect.
3. Results
3.1. Dose-dependent effect of 5-HTP on the severity of serotonin syndrome in clorgyline-pretreated rats
Neuromuscular responses including head shaking, myoclonus, forepaw treading and hindlimb abduction were recorded every 15 min for a total of 120 min as described in the Methods section. Administration of 5-HTP had no effect on naïve animals but elicited a series of neuromuscular responses in rats pretreated with clorgyline (2 mg/kg, i.p.). As shown in table 1, 5 mg/kg 5-HTP did not elicit any response except for head shaking which lasted less than 45 min. At the dose of 10 mg/kg, 5-HTP produced persistent head shaking behavior and myoclonus throughout the 120-min experiment, but had no effect on other responses. In addition to the increases in number of head shakes and myoclonus, which were soon overcome by other symptoms, 5-HTP at 20 mg/kg and 25 mg/kg produced persistent forepaw treading and hindlimb abduction, lasting 90 min in the 120-min observation. The dose of 15 mg/kg elicited persistent head shakes and myoclonus, but the effect on the limbs was brief, ~30 min. Based on the profiles of intensity and duration, neuromuscular responses were fully induced by 5-HTP at doses over 20 mg/kg but partially evoked by 10 mg/kg or 15 mg/kg. Since there was no other symptom except for head shakes, 5 mg/kg 5-HTP had a negligible effect on inducing the syndrome.
Next, lethality was used to distinguish between a benign and malignant syndrome following varied doses of 5-HTP. There was no death till the dose reached 20 mg/kg and higher. The lethal dose at 50% death rate (LD50) was 20 mg/kg and LD100 was 25 mg/kg. Thus, the syndrome was malignant when doses were over 20 mg/kg and benign at 15 mg/kg and lower. To test whether 5-HT2A receptors were involved in the syndrome, cyproheptadine (10 mg/kg, i.p.) was administered 30 min before 25 mg/kg 5-HTP in the clorgyline-pretreated rats. As elucidated in table 1, cyproheptadine had no effect on the severe neuromuscular response, however, it blocked deaths.
Changes in body-core temperature were used to estimate autonomic dysfunctions in the serotonin syndrome. Clorgyline injected (2 mg/kg, i.p.) 12 h before experiments did not have any effect on basal body-core temperature (Fig. 2A). This conclusion was confirmed by the two-way factorial ANOVA showing the insignificant main effect of clorgyline pretreatment (F(1,10) = 0.081, P = 0.782) or time course (F(5,50) = 1.716, P = 0.1481). Fig. 2B shows the dose-dependent effect of 5-HTP on body-core temperature in rats pretreated with clorgyline. Two-way factorial ANOVA indicated a significant main effect of 5-HTP doses (F(2,13) = 68.51, P < 0.0001) and time course (F(5,65) = 12.091, P < 0.0001). In addition, there was a significant interaction between 5-HTP treatment × sampling time (F(10,65) = 14.22, P < 0.0001). Specifically, administration of 5 mg/kg 5-HTP had no significant effect on core temperature. At the dose of 10 mg/kg, 5-HTP produced hypothermia, and the maximum reduction was −2.2 °C (± 0.3) below the pre-drug level. The reduction lasted at least 60 min during the observation. At the dose of 25 mg/kg, 5-HTP evoked hyperthermia. Maximum elevation was +3.2 °C (± 0.4) above the pre-drug level, and the effect sustained at least 60 min. Interestingly, 15 mg/kg 5-HTP had no significant effect on body-core temperature.
Up to this point, the degrees of the syndrome in relationship with 5-HTP doses can be pictured as follows. The low doses of 5-HTP (≤10 mg/kg) produced a mild syndrome, indicated by head shakes, myoclonus and hypothermia, while the high doses (≥20 mg/kg) evoked a severe syndrome, showing persistent forepaw treading, hindlimb abduction, hyperthermia and death. The syndrome following 15 mg/kg 5-HTP was considerably moderate as indicated by head shaking, myoclonus and normothermia along with minor forepaw treading or hindlimb abduction. Therefore, the doses of 10, 15 and 20 mg/kg 5-HTP were used in the following experiments to evoke the mild, moderate and severe syndrome, respectively.
3.2. Changes in 5-HT efflux in the serotonin syndrome
Dual-probe microdialysis was used to measure 5-HT efflux in the preoptic/anterior hypothalamic and prefrontal cortex. Fig. 3A shows the dose-dependent effect of 5-HTP on 5-HT efflux in the hypothalamus of clorgyline-pretreated animals. The baseline 5-HT was 0.39 ± 0.03 (pg in 15 min sample collection; n = 42). Two-way factorial ANOVA revealed overall significant differences in 5-HTP doses (F(5, 50) = 15.731, P< 0.0001) and sampling time (F(5, 150) = 46.448, P< 0.0001). A dose × sampling time interaction indicated that 5-HTP produced a higher increase across time than did the vehicle control (F(25, 150) = 13.191, P< 0.0001).
Fig. 3. 5-HT efflux in the hypothalamus and cortex.
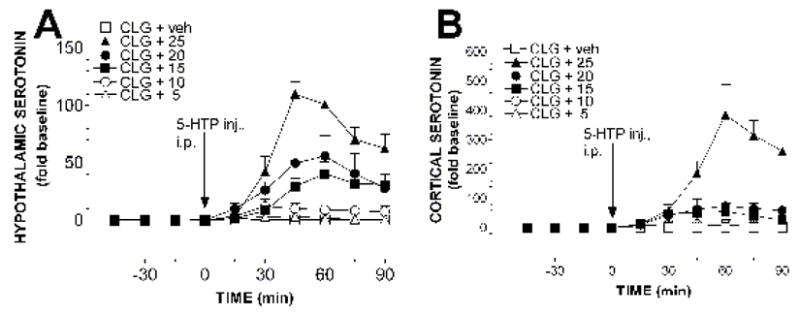
Rats were pretreated with clorgyline (CLG; 2 mg/kg, i.p.) 12 h before dual-probe microdialysis. Data for changes in 5-HT efflux in the hypothalamus (panel A) and cortex (panel B) were obtained from the same animals. 5-HTP (mg/kg; 5, 10, 15, 20 and 25) was intraperitoneally administered at time zero, as indicated by the solid arrows. Data are expressed as mean (fold increase above baseline, sample size n = 5–6 rats) ± S.E.M. Asterisks (*) indicating significance (P< 0.05) are omitted from the graphs for the sake of clarity.
Fig. 3B shows changes in 5-HT in the cortex of the same rats described in fig. 3A. The mean basal level was 0.26 ± 0.03 (pg in 15 min sample collection; n = 43). Similarly, 5-HTP produced a dose-dependent increase in the cortex. This conclusion was confirmed by the two-way factorial ANOVA, which revealed the significant effect of doses (F(5, 30) = 23.206, P < 0.0001), sampling time (F(5, 150) = 11.364, P < 0.0001) and dose × sampling time interaction (F(25, 150) = 7.783, P < 0.0001). The post-hoc Scheffe test revealed that 5-HTP at the dose as low as 5 mg/kg was able to cause a significant increase (F(1, 12) = 6.061, P = 0.0299).
Table 2 shows the relationship between doses of 5-HTP (column 1), behavioral syndrome (column 2), body-core temperature (column 3) and 5-HT efflux in the hypothalamus (column 4) and cortex (column 5). Criteria for hypothermia, normothermia and hyperthermia are described in the Methods section. Note that, except for 1 mg/kg 5-HTP, the efflux (column 4 and 5) was a replot of the mean maximum increase between 45 – 75 min of the data presented in fig. 3. The dose of 1 mg/kg 5-HTP, which did not elicit any behavioral or body-core temperature change in the clorgyline-pretreated animals, induced a 0- to 2-fold increase in 5-HT in the brain. The dose of 5 mg/kg, which produced a low frequency (<5 times per 15 min) of head shaking behavior but not other neuromuscular symptoms or body-core temperature change, evoked an approximate 5-fold increase in 5-HT efflux. Following 10 mg/kg 5-HTP, there was an 11-fold increase in 5-HT efflux associated with a mild and benign syndrome. Remarkably, animals could well tolerate the excessive efflux up to a 33- to 53-fold increase and survive, as examined by 15 mg/kg 5-HTP. However, following 20 mg/kg 5-HTP (LD50), hyperthermia and death occurred as the efflux reached a 55- to 69-fold increase to the threshold level for a severe and malignant syndrome. All animals were killed following 25 mg/kg 5-HTP as the efflux was over 109-fold (LD100). In general, increases in 5-HT efflux in the hypothalamus in response to 5-HTP injection were relatively smaller than those in the prefrontal cortex. Statistical analysis revealed a significant difference between two regions in response to 25 mg/kg 5-HTP(F(1, 10) = 20.346, P = 0.0011).
Table 2.
Dose-response relationship between 5-HTP and behavioral responses, body-core temperature or 5-HT in the CNS in rats pretreated with clorgyline
| 5-HT efflux (fold increase above baseline) |
||||
|---|---|---|---|---|
| 5-HTP doses | behavioral responses | body-core temperature | hypothalamus | cortex |
| 1 | no sign | no | 0 | 2 ± 0.3 |
| 5 | negligible | no | 3 ± 2 | 11 ± 5 |
| 10 | mild | hypothermia | 11 ± 6 | 11 ± 3 |
| 15 | moderate | normothermia | 33 ± 8 | 53 ± 23 |
| 20 | severe | hyperthermia | 55 ± 15 | 69 ± 23 |
| 25 | severe | hyperthermia | 109 ± 18 | 350 ± 69 |
Clorgyline (2 mg/kg, i.p.) was administered 12 h before experiments. Observations were based on 5 – 6 rats. Changes in 5-HT efflux from pre-drug level were expressed as the mean maximum increase (± S.E.M) which took place between 45 – 75 min as illustrated in fig. 3.
3.3. Involvement of 5-HT2A receptors
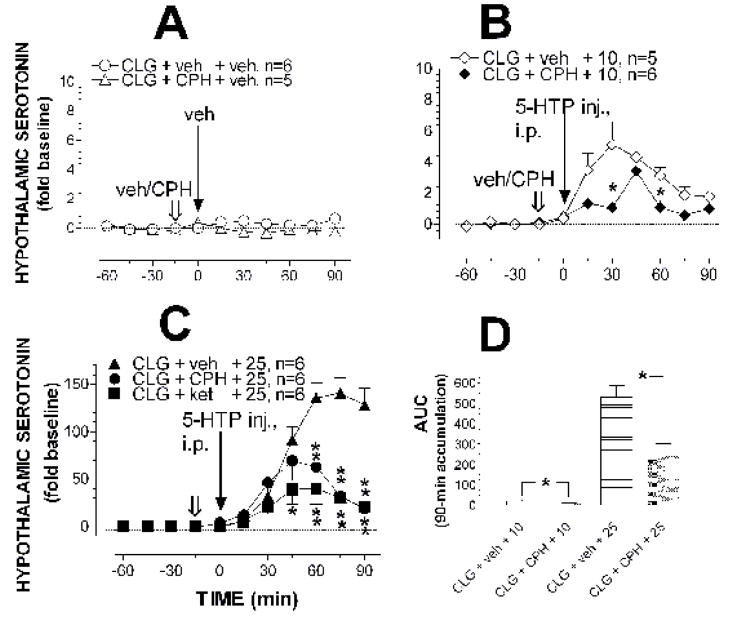
Fig. 4 shows 5-HT efflux in the hypothalamus of rats treated with cyproheptadine (10 mg/kg, i.p.) 15 min before 5-HTP injection. Cyproheptadine alone as illustrated in fig. 4A significantly reduced basal 5-HT efflux (F(1,9) = 12.185, P = 0.0068) without significant effects on sampling time (F(5,9) = 0.689, P = 0.6346) or interactions of drug treatment × sampling time (F(5,45) = 1.307, P = 0.2781). Fig. 4B shows that cyproheptadine pretreatment reduced the increase in 5-HT efflux induced by 10 mg/kg 5-HTP. The two-way factorial ANOVA indicated a significant main effect of cyproheptadine treatment (F(1, 9) = 5.329, P = 0.0464) and sampling time (F(5,45) = 11.714, P > 0.0001 ). Significant interactions between treatment and sampling time were also found (F(5,45) = 4.127, P = 0.0034). The post-hoc Scheffe test indicated the difference in 5-HT efflux between treatments at 30 min and 60 min but not at any other time points. Cyproheptadine pretreatment also reduced the effect of 25 mg/kg 5-HTP (fig. 4C). The two-way factorial ANOVA showed that there were significant differences in the main effect of the pretreatment (F(1, 10) = 13.92, P = 0.0039), the sampling time (F(5,50) = 10.435, P < 0.0001) and pretreatment × sampling time interaction (F(5,50) = 24.829, P < 0.0001). As evidenced by the post-hoc Scheffe test, a significant difference in antagonizing the excessive increase was observed at the later time points (e.g., 60 min after 5-HTP injection). To uncover an accumulative effect of the treatment, the cyproheptadine data were further analyzed by comparison of the Area under the Curve (AUC) in a summation of efflux between 0–90 min. As shown in fig. 4D, pretreatment with cyproheptadine also reduced the accumulative efflux of 5-HT in 90 min (10 mg/kg 5-HTP, unpaired t = 2.307, P < 0.05; 25 mg/kg, unpaired t = 7.959, P < 0.05).
Fig. 4. Effect of cyproheptadine or ketanserin on 5-HT efflux in the hypothalamus.
Rats were pretreated with clorgyline (CLG; 2 mg/kg, i.p.) 12 h before in vivo microdialysis. Open arrows indicate the time for injection of vehicle (veh), cyproheptadine (CPH; 10 mg/kg, i.p.) or ketanserin (ket; 5 mg/kg, i.p.), and solid arrows show the time for 5-HTP administration. Data are expressed as mean ± S.E.M. There was a small but significant decrease in basal 5-HT efflux in rats pretreated with cyproheptadine compared to vehicle control (A). The effect of 10 mg/kg (B) and 25 mg/kg 5-HTP (C), which produced a dose-dependent increase in 5-HT efflux in the hypothalamus, was significantly reduced in rats pretreated with cyproheptadine or ketanserin. *P < 0.05 and **P < 0.01 indicate significant differences between vehicle and antagonist pretreatment, examined by the two-way factorial ANOVA followed by the post-hoc Scheffe test. Accumulative efflux over 90 min in B and C was replotted as Area under the Curve (AUC) shown in D. * P < 0.05 indicates significant differences between two pretreatments, examined by unpaired t-test.
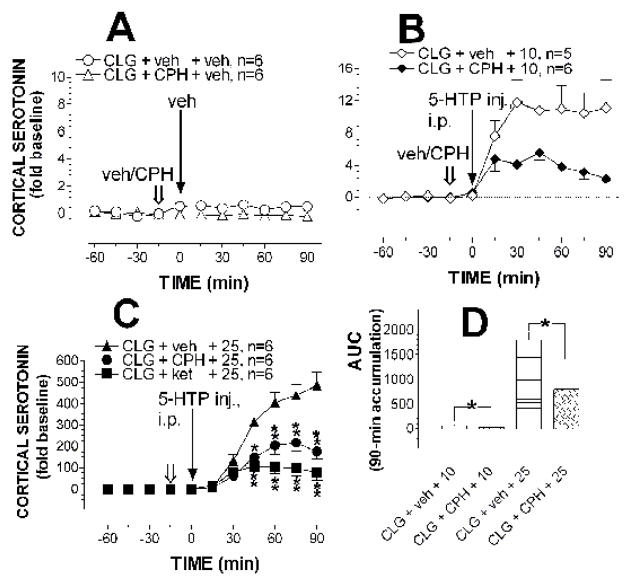
Similar to that in the hypothalamus, cyproheptadine alone caused a small reduction in 5-HT in the cortex (Fig. 5A). Compared to control, the main effect of cyproheptadine injection was significant (F(1,10) = 13.382, P = 0.0044) although there was no statistical significance between sampling times (F(1, 10) = 0.862, P = 0.5135) or of interactions of drug × sampling times (F(5,50) = 0.336, P = 0.889). Fig. 5B shows that cyproheptadine pretreatment reduced the increase evoked by 10 mg/kg 5-HTP. The two-way factorial ANOVA revealed a significant main effect of cyproheptadine pretreatment (F(1,9) = 7.913, P = 0.0203) without a significant change in sampling times (F(5,9) = 1.109, P = 0.3691) or interactions of drug × sampling times (F(5,45) = 2.099, P = 0.083). Fig. 5C shows the effect of cyproheptadine on 25 mg/kg 5-HTP-induced increases in 5-HT efflux. Apparently the magnitude of the increase was reduced following cyproheptadine pretreatment. The two-way factorial ANOVA revealed significant main effects of pretreatment (F(1,10) = 17.016, P = 0.0021), sampling time (F(5,50) = 37.457, P < 0.0001) and pretreatment × time interaction (F(5, 50) = 6.318, P = 0.0001). Post-hoc Scheffe comparisons showed that the significant reduction began 60 min after cyproheptadine injection (or 45 min after 5-HTP). Fig. 5D depicts an overall effect of cyproheptadine on 5-HT by comparison of AUC between vehicle and cyproheptadine pretreatment. The unpaired t-test showed a significant reduction in 5-HT efflux in rats pretreated with cyproheptadine (for 10 mg/kg, t = 2.287, P = 0.048; for 25 mg/kg, t = 4.125, P = 0.0021).
Fig. 5. Effect of cyproheptadine or ketanserin on 5-HT efflux in the cortex.
Rats were pretreated with clorgyline (CLG; 2 mg/kg, i.p.) 12 h before in vivo microdialysis. Open arrows indicate the time for injection of vehicle (veh), cyproheptadine (CPH; 10 mg/kg, i.p.) or ketanserin (ket; 5 mg/kg, i.p.), and solid arrows show the time for 5-HTP administration. Values are expressed as means ± S.E.M. There was a significant reduction in basal 5-HT in rats injected with cyproheptadine compared to the control (A). Injection of 10 mg/kg (B) or 25 mg/kg (C) 5-HTP produced a dose-dependent increase in 5-HT efflux in the cortex in the control groups. This effect was significantly reduced in rats pretreated with cyproheptadine or ketanserin. * P < 0.05 and ** P < 0.01 show significant differences between vehicle and cyproheptadine pretreatment, examined by the two-way factorial ANOVA followed by a post-hoc Scheffe test. The total accumulative increase in 5-HT efflux over 90 min in B and C was replotted in D as Area under the Curve (AUC). * P < 0.05 indicates significant differences between vehicle and cyproheptadine pretreatment, examined by unpaired t-test.
The antagonistic effect of cyproheptadine was confirmed with another 5-HT2A receptor antagonist ketanserin at a dose shown to block hyperthermia and death in the previous study (5 mg/kg, i.p.; Ma et al., 2008). Likewise, ketanserin pretreatment reduced the increases in 5-HT efflux induced by 25 mg/kg in the hypothalamus (fig. 4C) and cortex (fig. 5C). Data analysis revealed that there was a significant main effect of the ketanserin treatment (hypothalamus, F(1,10) = 22.915, P = 0.0007; cortex, F(1,10) = 34.39, P = 0.0002). Post-hoc Scheffe test showed that animals treated with ketanserin displayed significant lower levels of 5-HT compared with animals injected with vehicle plus 25 mg/kg 5-HTP.
3.4. Involvement of NMDA receptors
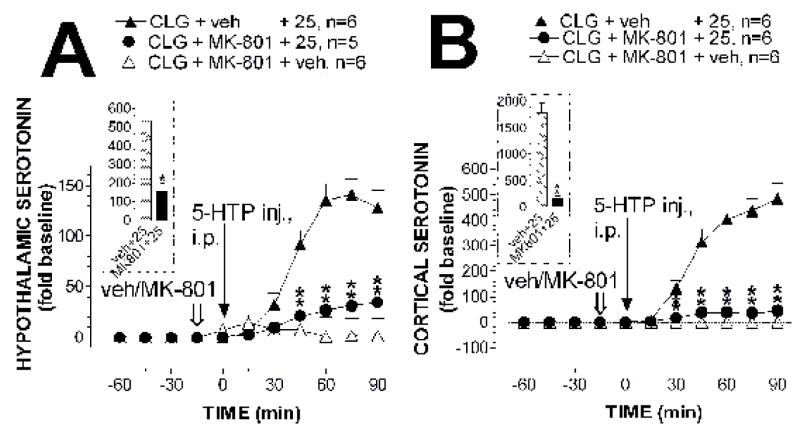
In control experiments, injection of (+)MK-801 alone (0.5 mg/kg, s.c.) produced approximately 7-fold and 1-fold increases in the hypothalamus and cortex, respectively, in rats pretreated with clorgyline. Despite this, (+)MK-801 pretreatment significantly attenuated the increases in 5-HT efflux induced by 25 mg/kg 5-HTP. Fig. 6A shows the effect of (+)MK-801 on hypothalamic 5-HT. The two-way factorial ANOVA revealed a significant main effect of (+)MK-801 pretreatment (F(2,14) = 40.11, P < 0.0001) and time course (F(5,70) = 16.935, P < 0.0001). In addition, there was a significant interaction of (+)MK-801 × sampling time (F(10,70) = 15.559, P < 0.0001). The post-hoc Scheffe test showed that the significant differences (P < 0.001) were reached in 60 min after (+)MK-801 injection (or 45 min after 5-HTP). The inset in fig. 6A depicts the overall efflux during the 90-min collection. The unpaired t-test showed a significant difference in 5-HT efflux in rats treated with MK-801 (t = 5.225, P = 0.0005) compared with the control group.
Fig. 6. Effect of (+)MK-801 on 5-HT efflux.
All rats were pretreated with clorgyline (CLG; 2 mg/kg, i.p.) 12 h before the experiment. Open arrows indicate the time for vehicle or (+)MK-801 pretreatment (0.5 mg/kg, s.c.) and solid arrows for 5-HTP administration at time zero (25 mg/kg, i.p.). 5-HT efflux was measured using dual-probe microdialysis with one probe in the hypothalamus (A) and another in the cortex (B). ** P < 0.01 vs. vehicle + 5-HTP examined by the two-way factorial ANOVA followed by the post-hoc Scheffe test. The inset shows a total of 5-HT efflux over 90 min expressed as the AUC. * P < 0.05 examined by the unpaired t-test.
Similar to that in the hypothalamus, (+)MK-801 reduced the increase in 5-HT efflux in the cortex induced by 25 mg/kg 5-HTP (fig. 6B). The two-way factorial ANOVA indicated a significant main effect of (+)MK-801 pretreatment (F(2,15) = 87.342, P < 0.0001) and time course (F(5,75) = 29.895, P < 0.0001). An interaction of (+)MK-801 × sampling time was also significant (F(10,75) = 23.668, P < 0.0001). Time for statistically significant reduction, examined by post-hoc Scheffe analysis, was apparent as early as 45 min after (+)MK-801 injection (or 30 min after 5-HTP). The inset in fig. 6B shows AUC between animals treated with (+)MK-801 and vehicle during a 90-min observation. Compared to vehicle group, there was a 95% reduction in 5-HT efflux in rats treated with (+)MK-801.
4. Discussion
In an effort to better understand the syndrome, our focus in this study was to unveil the relationship between 5-HT efflux and degrees of the syndrome caused by administration of 5-HTP in rats pretreated with clorgyline, an animal model of 5-HT syndrome as described in the previous reports (Ma et al., 2008; Shioda et al., 2004). By measuring changes in neuromuscular responses (e.g., forepaw treading, hindlimb abduction, myoclonus and head shakes), body-core temperature and lethality, we found that the severity of syndrome was strongly correlated with extracellular levels of 5-HT in the CNS. In particular, a 2-fold increase in 5-HT efflux following drug administration did not induce the syndrome. While the increase was over 5-fold above baseline, a head shaking behavior was induced. Animals developed a mild syndrome as 5-HT was increased by 11-fold. The syndrome became severe and malignant when the increase was over 55-fold. We conclude that the behavioral symptoms of the syndrome is apparent when extracellular 5-HT in the CNS is increased over 11-fold above baseline under our experimental conditions.
Furthermore, by examining the effect of 5-HT2A and NMDA receptor antagonists, our data suggest that extracellular 5-HT in the syndrome was derived from at least two distinct mechanisms. Initial efflux was caused by a direct effect of serotonergic drugs on 5-HT metabolic pathways, for instance, increases in biosynthesis and decreases in breakdown at serotonergic nervous endings. Once the efflux reached a threshold, the syndrome was elicited. The initial efflux then activated a 5-HT2A and NMDA receptor-containing neural circuit, the effect resulting in a further increase in 5-HT in the CNS. Altogether, we conclude that extracellular 5-HT in the course of the syndrome consists of primary (direct drug effect) and secondary efflux (indirect drug effect).
4.1. Determination of 5-HT efflux (threshold) at different degrees of the syndrome
The first set of this study was to reveal degrees of the syndrome by recording the time course of neuromuscular responses including head shaking, myoclonus, forepaw treading and hindlimb abduction. The rationale behind the experiments is based on clinical observations that human patients with the syndrome show a diagnostic clinical triad of neuromuscular hyperactivity, autonomic dysfunction and mental disorder (Boyer and Shannon, 2005; Sternbach, 1991). The neuromuscular responses in the current study are similar to those described in the study of 8-OH-DPAT (Borsini et al., 2001; Darmani and Zhao, 1998), consistent with the view that 5-HT1A receptors are involved in the behavioral syndrome (Isbister and Buckley, 2005). It should be kept in mind that 8-OH-DPAT in a normal dose range of 0.1 – 0.2 mg/kg (Hjorth, 1985; Hjorth and Sharp, 1991) does not produce any neuromuscular response relevant to the syndrome. The acquired syndrome was caused by at least 1 – 2 mg/kg 8-OH-DPAT, a 10-times greater amount than the pharmacological dose (Borsini et al., 2001; Darmani and Zhao, 1998). Theoretically, one would expect that 5-HT efflux in a syndrome should be at least 10 times greater than pre-drug levels. In this study, it was found the mild syndrome was induced in response to 11-fold increases in 5-HT efflux in the CNS (see table 2), consistent with the expectation. However, it is unlikely that under a normal condition antidepressants could evoke such a high level of 5-HT efflux in the CNS. It has been demonstrated that antidepressants cause only 1- to 5-fold increases for therapeutic purposes (Fuller, 1994; Hervas et al., 2000; Rutter and Auerbach, 1993). This agrees with our results that 5-HT at a 5-fold increase, which was induced by 5 mg/kg 5-HTP in rats pretreated with clorgyline, had no visible effect on behavior except for a low frequency (< 5 times/15 min) of head shaking (see table 2; also Ma et al., 2008). Altogether, there exists a strong relationship between 5-HT efflux and degrees of the syndrome. The results of our data suggest that 5-HT threshold for a mild syndrome, measured by neuromuscular responses, is an approximate 11-fold increase in efflux in the CNS.
In addition to the neuromuscular responses, previous studies have provided evidence that autonomic response, especially changes in body-core temperature could be used to assess degrees of the syndrome (Ma et al., 2008; Nisijima et al., 2001). Animals with a mild, moderate or severe syndrome exhibited hypothermia, normothermia or hyperthermia, respectively (Abdel-Fattah et al., 1995; Ma et al., 2008). To confirm and extend the previous findings, the present study also revealed the relationship between changes in body-core temperature and 5-HT efflux in the animal model of the syndrome. It is well known that neurons regulating autonomic responses in the CNS are distributed mainly in the preoptic/anterior hypothalamus (Lin et al., 1998; Romanovsky, 2007). Additionally, 5-HT1A receptors are known to be the mechanism responsible for hypothermia (Goodwin et al., 1986; Lin et al., 1998; Millan et al., 1993) while 5-HT2A receptors are responsible for hyperthermia (Lin et al., 1998; Matuszewich and Yamamoto, 2003; Mazzola-Pomietto et al., 1995). Although it is not clear whether two receptors execute their actions on the same neurons for the temperature regulation, there are convincing data in the literature suggesting that the body-core temperature is in fact determined by their net effect (Ootsuka and Blessing, 2006; Salmi and Ahlenius, 1998). In this study, it was found that hypothermia occurred to 11-fold increases in hypothalamic 5-HT and hyperthermia to 55-fold following 10 mg/kg and 20 mg/kg 5-HTP, respectively. It appeared that 5-HT1A receptors were activated by a relatively small amount of 5-HT efflux to mediate hypothermia, whereas 5-HT2A receptors were functioned by a large amount of efflux for hyperthermia. This is consistent with in vitro studies that 5-HT1A receptors retain a high affinity to their natural ligands compared to that of 5-HT2A receptors (Almaula et al., 1996; Dalpiaz et al., 1995; Peroutka et al., 1981). It was not surprising to see that body-core temperature was not altered (normothermia) by a 33-fold increase in 5-HT because it was likely that there was a net zero effect of 5-HT1A and 5-HT2A receptors at this level of 5-HT under our experimental condition. Thus, measurements of body-core temperature could be used to estimate not only the degrees of the syndrome (Ma et al., 2008) but also the relative activity between 5-HT1A and 5-HT2A receptors.
Relevant to the present study is a debate in literature whether a syndrome could be malignant (Garside and Rosebush, 2003; Gillman, 2005). Through measuring lethality, it was found that animals began to die when the 5-HT efflux reached a 55-fold increase, a possible threshold of 5-HT level for a malignant response. However, after pretreatment with cyproheptadine, ketanserin or (+)MK-801, animals could survive such high 5-HT levels. Our data suggest that the malignant response is determined not only by the level of extracellular 5-HT, but also by activity of 5-HT2A and NMDA receptors in the CNS.
4.2. 5-HT efflux derived from two distinct mechanisms
The present results are consistent with previous reports that 5-HTP or clorgyline alone produced a very small increase in 5-HT efflux, <1 fold above their baseline (Bel and Artigas, 1995; Gartside et al., 1992). In contrast, there were hundreds of fold increases following a combined injection of 5-HTP and clorgyline (Shioda et al., 2004; also in this study). Although efflux to such an enormous level was beyond our expectation and probably of irrelevance to antidepressants, mechanisms behind this observation were worthwhile for investigation. From a classic point of view, the syndrome is elicited due to over-accumulation of intracellular 5-HT by increases in synthesis and decreases in breakdown (Grahame-Smith and Green, 1974). However, there was only a 2 – 4 fold increase in 5-HT content, as examined by ex vivo studies with homogenized brain tissues (Abdel-Fattah et al., 1997; Grahame-Smith, 1971). Due to a limit of storage capacity in the vesicles, intracellular 5-HT is reversely transported to extracellular space (Gobbi et al., 1993; Levi and Raiteri, 1993). As a result, there is a marked and sustained increase in extracellular 5-HT for mediating post-synaptic receptors. Therefore, determination of 5-HT efflux as measured by microdialysis is crucial, and it is also an appropriate approach to estimate the syndrome.
We observed that cyproheptadine at the dose that blocked hyperthermia and death (Ma et al., 2008; Nisijima et al., 2001) was able to reduce 50 – 60% of the increases in 5-HT efflux evoked by 25 mg/kg 5-HTP. Likewise, ketanserin, another 5-HT2A receptor antagonist elicited the same reduction, suggesting that increases in 5-HT efflux are enabled through mechanisms involving not only 5-HT metabolic enzymes but also 5-HT2A receptors. However, since neither 5-HTP nor clorgyline has a direct effect on 5-HT2A receptors, our data suggest that 5-HT2A receptor-mediated increases in 5-HT must be indirect via 5-HT that was initially induced by the direct effect of drugs on the metabolic pathways. We also demonstrated that, similar to 5-HT2A receptors, NMDA receptors were involved in an increase in 5-HT during the syndrome, as examined by (+)MK-801. Our data lead us to propose that increases in 5-HT efflux for the syndrome can be theoretically divided into two interrelated processes: first, an initial increase caused by a direct effect of 5-HT drugs on pre-synaptic enzymes; second, a secondary increase induced by the primary increase through activation of a 5-HT2A and NMDA receptor-containing circuitry that controls serotonergic neuronal activity and subsequently 5-HT release in the forebrain. Therefore, the secondary efflux is an indirect effect of the drugs, temporally lagging behind the primary efflux.
4.3. Neural circuitry responsible for the secondary efflux: a positive-feedback hypothesis
(+)MK-801, as a non-competitive antagonist, is known to block cation channels (e.g., Ca2+) on NMDA receptors (Rosenmund and Westbrook, 1993). We showed that, although (+)MK-801 alone caused an increase in 5-HT efflux (Lopez-Gil et al., 2007; also in this study), it attenuated 70 – 90% of the 5-HTP-evoked increase in 5-HT efflux. The observation is consistent with the previous studies demonstrating that (+)MK-801, similar to cyproheptadine and ketanserin, blocked 5-HTP-evoked hyperthermia and death in the severe syndrome (Ma et al., 2008; Nisijima et al., 2004). However, there is no direct ligand-receptor relationship between 5-HT and (+)MK-801-mediated NMDA receptors, suggesting that the antagonistic effect must be through a neural circuit involving multiple neuronal systems. The results shed lights into a better understanding of the indirect effect of 5-HTP and clorgyline in the course of the syndrome.
It has been demonstrated that 5-HT2A receptors are widely distributed on glutamatergic neurons in the forebrain, especially the prefrontal cortex (de Almeida and Mengod, 2007). Moreover, the direct effect of 5-HT2A receptors is excitatory (Amargos-Bosch et al., 2003). Thus, it is likely that 5-HT2A receptors on glutamatergic neurons are activated in response to the primary efflux of 5-HT, for instance 11-fold above baseline as shown in this study, and in turn cause glutamate release to regions with glutamatergic innervations. This pathway has already been described to involve the mechanistic effect of hallucinogenic and antipsychotic drugs, showing that raphe serotonergic neurons are a prominent target of glutamatergic innervations in controlling mental stability and behavioral expressions (Bortolozzi et al., 2003; Martin-Ruiz et al., 2001). Interestingly, raphe serotonergic neurons also send projections to the prefrontal cortex on the glutamatergic neurons (Aghajanian and Marek, 1999; 2000). Thus, there exist likely reciprocal innervations between raphe and cortex, in which serotonergic fibers project to the cortex which contains glutamatergic neurons feeding back to the raphe nuclei. Furthermore, evidence strongly suggests that NMDA and 5-HT2A receptors are integrated within this neural circuit in the regulation of 5-HT release (Aghajanian and Marek, 1999; Bortolozzi et al., 2003; Martin-Ruiz et al., 2001; Puig et al., 2003). Since both receptors are excitatory, the circuit is theoretically a positive feedback loop. Therefore, activation of 5-HT2A receptors by primary efflux causes a depolarization-dependent release of glutamate in the circuit, and the response would in turn result in a secondary efflux further activating 5-HT2A receptors. Based on this hypothesis, one would expect that activation of the circuit should cause a spiral increase in secondary efflux. Concerning the low affinity of 5-HT2A receptors to 5-HT, one would also expect that the circuit should be more active in the severe syndrome compared to the mild cases. In fact, several lines of evidence obtained from our laboratory and others (Nisijima et al., 2001, 2004; Shioda et al., 2004) had met the expectations. For instance, 5-HT2A receptor antagonists reduced 50–60% of the increase in 5-HT in the severe syndrome following a high dose of 5-HTP, suggesting that the increase consists of a primary efflux caused by the direct drug effect on the enzymes and a secondary efflux associated with the 5-HT2A and NMDA receptor-containing circuitry. It was also demonstrated that, consistent with the hypothesis, activation of the positive feedback loop could ultimately be vicious and associated with death in the severe syndrome. In conclusion, our positive feedback hypothesis provides an insight into understanding the observations on why 5-HT2A receptor antagonists are shown to be so potent in the treatment of the severe and malignant syndrome (Isbister and Buckley, 2005; Nisijima et al., 2001).
We showed that increases in 5-HT efflux, particularly evoked by a high dose of 5-HTP (>15 mg/kg), were relatively smaller in the hypothalamus compared to those in the cortex. The exact mechanism for such a regional difference is not clear. Regardless of this, finding that the increase in 5-HT was also reduced in the hypothalamus after blocking 5-HT2A or NMDA receptors is consistent with the positive feedback hypothesis. To date, there is no evidence suggesting reciprocal innervations between raphe and hypothalamus involving glutamatergic neurons. It is plausible that the secondary efflux in the hypothalamus is also determined by the neural circuit between raphe and cortex. In this context it is worth mentioning that such an indirect regulation may explain the smaller efflux in the hypothalamus. Future experiments should explore this possibility.
In conclusion, our data indicate that there is a positive correlation between 5-HT efflux and degrees of the syndrome induced by 5-HTP in rats pretreated with clorgyline. Given that the syndrome in human patients is caused mainly by SSRIs in the course of either monotherapy (Terao and Hikichi, 2007) or combined therapies (Boyer and Shannon, 2005; Gillman, 2005), caution should be taken in use of interpretations obtained in this observational study. For example, although the present data demonstrate that the primary efflux of 5-HT in the pre-synaptic site may be the initial cause of the syndrome in animal models, causal mechanisms in patients remain elusive but likely due to molecular alterations at post-synaptic sites (Zhang and Tao, unpublished observations). Indeed, activity of post-synaptic 5-HT2A receptors can be sensitized in the CNS after repeated increases in 5-HT efflux using SSRIs examined in the experimental animals (Damjanoska et al., 2003; Napier and Istre, 2008; Zanoveli et al., 2005) and humans (Zanardi et al., 2001). The data of the current study also demonstrate the critical role of post-synaptic 5-HT2A receptors in the syndrome. More importantly, ongoing efforts on the neural circuitry between raphe serotonergic and cortex glutamatergic systems promise to elicit new insights into the commonalities among neural disorders related to excessive 5-HT in the CNS, insights which may guide future approaches in neural disorder treatment.
Acknowledgments
This research was supported by the public health grant (DA14541) and funds from the Charles E. Schmidt College of Biomedical Science. The Authors thank Ms. Wanda Dominger for secretary assistance.
Abbreviation
- aCSF
artificial cerebrospinal fluid
- AUC
area under the curve
- CNS
central nervous system
- HPLC
high-pressure liquid chromatography
- 5-HT
5-hydroxytryptamine, serotonin
- 5-HTP
5-hydroxy-L-tryptophan
- LD50
lethal dose at 50% death rate
- LD100
lethal dose at 100% death rate
- MAOI
monoamine oxidase inhibitor
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Fattah AF, Matsumoto K, el-Hady KA, Watanabe H. 5-HT1A and 5-HT2 receptors mediate hypo- and hyperthermic effects of tryptophan in pargyline-pretreated rats. Pharmacol Biochem Behav. 1995;52:379–384. doi: 10.1016/0091-3057(95)00122-d. [DOI] [PubMed] [Google Scholar]
- Abdel-Fattah AF, Matsumoto K, Murakami Y, Adel-Khalek Gammaz H, Mohamed MF, Watanabe H. Central serotonin level-dependent changes in body temperature following administration of tryptophan to pargyline- and harmaline-pretreated rats. Gen Pharmacol. 1997;28:405–409. doi: 10.1016/s0306-3623(96)00300-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Almaula N, Ebersole BJ, Ballesteros JA, Weinstein H, Sealfon SC. Contribution of a helix 5 locus to selectivity of hallucinogenic and nonhallucinogenic ligands for the human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors: direct and indirect effects on ligand affinity mediated by the same locus. Mol Pharmacol. 1996;50:34–42. [PubMed] [Google Scholar]
- Amargos-Bosch M, Adell A, Bortolozzi A, Artigas F. Stimulation of α1-adrenoceptors in the rat medial prefrontal cortex increases the local in vivo 5-hydroxytryptamine release: reversal by antipsychotic drugs. J Neurochem. 2003;87:831–842. doi: 10.1046/j.1471-4159.2003.02044.x. [DOI] [PubMed] [Google Scholar]
- Bel N, Artigas F. In vivo evidence for the reversible action of the monoamine oxidase inhibitor brofaromine on 5-hydroxytryptamine release in rat brain. Naunyn-Schmiedeberg’s Arch Pharmacol. 1995;351:475–482. doi: 10.1007/BF00171038. [DOI] [PubMed] [Google Scholar]
- Borsini F, Brambilla A, Cesana R, Grippa N. Lack of interaction between flibanserin and antidepressants in inducing serotonergic syndrome in rats. Int J Neuropsychopharmacol. 2001;4:9–15. doi: 10.1017/S1461145701002206. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Amargos-Bosch M, Adell A, Diaz-Mataix L, Serrats J, Pons S, Artigas F. In vivo modulation of 5-hydroxytryptamine release in mouse prefrontal cortex by local 5-HT2A receptors: effect of antipsychotic drugs. Eur J Neurosci. 2003;18:1235–1246. doi: 10.1046/j.1460-9568.2003.02829.x. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- Dalpiaz A, Gessi S, Borea PA, Gilli G. Binding thermodynamics of serotonin to rat-brain 5-HT1A, 5-HT2A and 5-HT3 receptors. Life Sci. 1995;57:PL141–146. doi: 10.1016/0024-3205(95)02072-q. [DOI] [PubMed] [Google Scholar]
- Damjanoska KJ, Van de Kar LD, Kindel GH, Zhang Y, D’Souza DN, Garcia F, Battaglia G, Muma NA. Chronic fluoxetine differentially affects 5-hydroxytryptamine2A receptor signaling in frontal cortex, oxytocin- and corticotropin-releasing factor-containing neurons in rat paraventricular nucleus. J Pharmacol Exp Ther. 2003;306:563–571. doi: 10.1124/jpet.103.050534. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Zhao W. Production of serotonin syndrome by 8-OH-DPAT in Cryptotis parva. Physiol Behav. 1998;65:327–331. doi: 10.1016/s0031-9384(98)00174-7. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT2A receptors in human and monkey prefrontal cortex. J Neurochem. 2007;103:475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- Fox MA, Jensen CL, Gallagher PS, Murphy DL. Receptor mediation of exaggerated responses to serotonin-enhancing drugs in serotonin transporter (SERT)-deficient mice. Neuropharmacology. 2007;53:643–656. doi: 10.1016/j.neuropharm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fuller RW. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 1994;55:163–167. doi: 10.1016/0024-3205(94)00876-0. [DOI] [PubMed] [Google Scholar]
- Garside S, Rosebush PI. Serotonin syndrome: not a benign toxidrome. CMAJ. 2003;169:543. [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Cowen PJ, Sharp T. Effect of 5-hydroxy-L-tryptophan on the release of 5-HT in rat hypothalamus in vivo as measured by microdialysis. Neuropharmacology. 1992;31:9–14. doi: 10.1016/0028-3908(92)90154-h. [DOI] [PubMed] [Google Scholar]
- Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95:434–441. doi: 10.1093/bja/aei210. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Frittoli E, Uslenghi A, Mennini T. Evidence of an exocytotic-like release of [3H]5-hydroxytryptamine induced by d-fenfluramine in rat hippocampal synaptosomes. Eur J Pharmacol. 1993;238:9–17. doi: 10.1016/0014-2999(93)90499-8. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Wood AJ, Green AR. The enhancement by lithium of the 5-HT1A mediated serotonin syndrome produced by 8-OH-DPAT in the rat: evidence for a post-synaptic mechanism. Psychopharmacology (Berl) 1986;90:488–493. doi: 10.1007/BF00174066. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971;18:1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG, Green AR. The role of brain 5-hydroxytryptamine in the hyperactivity produced in rats by lithium and monoamine oxidase inhibition. Br J Pharmacol. 1974;52:19–26. doi: 10.1111/j.1476-5381.1974.tb09682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas I, Queiroz CM, Adell A, Artigas F. Role of uptake inhibition and autoreceptor activation in the control of 5-HT release in the frontal cortex and dorsal hippocampus of the rat. Br J Pharmacol. 2000;130:160–166. doi: 10.1038/sj.bjp.0703297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm. 1985;61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 1991;48:1779–1786. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol. 2005;28:205–214. doi: 10.1097/01.wnf.0000177642.89888.85. [DOI] [PubMed] [Google Scholar]
- Izumi T, Iwamoto N, Kitaichi Y, Kato A, Inoue T, Koyama T. Effects of co-administration of a selective serotonin reuptake inhibitor and monoamine oxidase inhibitors on 5-HT-related behavior in rats. Eur J Pharmacol. 2006;532:258–264. doi: 10.1016/j.ejphar.2005.12.075. [DOI] [PubMed] [Google Scholar]
- Levi G, Raiteri M. Carrier-mediated release of neurotransmitters. Trends Neurosci. 1993;16:415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- Lin MT, Tsay HJ, Su WH, Chueh FY. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am J Physiol. 1998;274:R1260–1267. doi: 10.1152/ajpregu.1998.274.5.R1260. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhang G, Jenney C, Krishnamoorthy S, Tao R. Characterization of serotonin-toxicity syndrome (toxidrome) elicited by 5-hydroxy-L-tryptophan in clorgyline-pretreated rats. Eur J Pharmacol. 2008;588:198–206. doi: 10.1016/j.ejphar.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Long-lasting effects of chronic stress on DOI-induced hyperthermia in male rats. Psychopharmacology (Berl) 2003;169:169–175. doi: 10.1007/s00213-003-1498-7. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Aulakh CS, Wozniak KM, Hill JL, Murphy DL. Evidence that 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced hyperthermia in rats is mediated by stimulation of 5-HT2A receptors. Psychopharmacology (Berl) 1995;117:193–199. doi: 10.1007/BF02245187. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Rivet JM, Canton H, Le Marouille-Girardon S, Gobert A. Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J Pharmacol Exp Ther. 1993;264:1364–1376. [PubMed] [Google Scholar]
- Napier TC, Istre ED. Methamphetamine-induced sensitization includes a functional upregulation of ventral pallidal 5-HT2A/2C receptors. Synapse. 2008;62:14–21. doi: 10.1002/syn.20460. [DOI] [PubMed] [Google Scholar]
- Nisijima K, Shioda K, Yoshino T, Takano K, Kato S. Memantine, an NMDA antagonist, prevents the development of hyperthermia in an animal model for serotonin syndrome. Pharmacopsychiatry. 2004;37:57–62. doi: 10.1055/s-2004-815526. [DOI] [PubMed] [Google Scholar]
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res. 2001;890:23–31. doi: 10.1016/s0006-8993(00)03020-1. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Lett. 2006;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain stereotaxic coordinates. New York: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder SH. Two distinct central serotonin receptors with different physiological functions. Science. 1981;212:827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Rundown of N-methyl-D-aspartate channels during whole-cell recording in rat hippocampal neurons: role of Ca2+ and ATP. J Physiol. 1993;470:705–729. doi: 10.1113/jphysiol.1993.sp019884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter JJ, Auerbach SB. Acute uptake inhibition increases extracellular serotonin in the rat forebrain. J Pharmacol Exp Ther. 1993;265:1319–1324. [PubMed] [Google Scholar]
- Salmi P, Ahlenius S. Evidence for functional interactions between 5-HT1A and 5-HT2A receptors in rat thermoregulatory mechanisms. Pharmacol Toxicol. 1998;82:122–127. doi: 10.1111/j.1600-0773.1998.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Shioda K, Nisijima K, Yoshino T, Kato S. Extracellular serotonin, dopamine and glutamate levels are elevated in the hypothalamus in a serotonin syndrome animal model induced by tranylcypromine and fluoxetine. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:633–640. doi: 10.1016/j.pnpbp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Speiser Z, Fine T, Litinetsky L, Eliash S, Blaugrund E, Cohen S. Differential behavioral syndrome evoked in the rats after multiple doses of SSRI fluoxetine with selective MAO inhibitors rasagiline or selegiline. J Neural Transm. 2008;115:107–116. doi: 10.1007/s00702-007-0811-8. [DOI] [PubMed] [Google Scholar]
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- Terao T, Hikichi T. Serotonin syndrome in a case of depression with various somatic symptoms: the difficulty in differential diagnosis. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:295–296. doi: 10.1016/j.pnpbp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Artigas F, Moresco R, Colombo C, Messa C, Gobbo C, Smeraldi E, Fazio F. Increased 5-hydroxytryptamine-2 receptor binding in the frontal cortex of depressed patients responding to paroxetine treatment: a positron emission tomography scan study. J Clin Psychopharmacol. 2001;21:53–58. doi: 10.1097/00004714-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Zanoveli JM, Nogueira RL, Zangrossi H., Jr Chronic imipramine treatment sensitizes 5-HT1A and 5-HT2A receptors in the dorsal periaqueductal gray matter: evidence from the elevated T-maze test of anxiety. Behav Pharmacol. 2005;16:543–552. doi: 10.1097/01.fbp.0000179280.05654.5a. [DOI] [PubMed] [Google Scholar]