Abstract
Our laboratory previously reported that uremic levels of urea inhibit l-arginine (l-Arg) transport into endothelial cells. The present study further investigated this effect. We measured l-Arg transport in cultured bovine aortic endothelial cells with normal or high urea (25 mM). The urea transport inhibitor phloretin abolished the inhibitory effect of urea on l-Arg transport, suggesting a role for urea transporters (UTs). We screened bovine aortic endothelial cells and several other endothelial cell types for the presence of UTs by using Western blot analysis. UT-B was present in all endothelial cells, irrespective of species or location of derivation, whereas UT-A distribution was variable and sparse. UT-B was also abundant in rat aorta, mesenteric blood vessels, and spinotrapezius muscle, whereas UT-A distribution was, again, variable and sparse. Chronic elevation of urea had variable, inconsistent effects on UT abundance. This study showed that urea must enter endothelial cells, probably by UT-B, to inhibit l-Arg transport. In view of the wide distribution of UT-B in rat vasculature, elevated blood urea nitrogen may lead to endothelial l-Arg deficiency in vivo.
Keywords: uremia, nitric oxide, phloretin, blood vessels
Our laboratory previously reported that increases in blood urea nitrogen (BUN) to the uremic range (70 mg/dl = 25 mM) inhibit l-arginine (l-Arg) transport into cultured vascular endothelial cells (18). The mechanism of this inhibition is unknown but is specific to urea rather than being an osmotic effect. Urea transporters (UTs) are required for urea entry into any cell, and UT type B (UT-B) protein is present in endothelial cells of descending vasa recta from several species (4, 13, 20). In addition, our laboratory has recently observed that UT-B is present in cultured bovine aortic endothelial cells (BAEC) (13). Together, these observations suggest that UT-B may be widely expressed in vascular endothelium and that uremic levels of BUN might affect endothelial cell l-Arg availability, via inhibition of l-Arg transport, by means of an intracellular action.
The goals of the present study were to determine whether urea is required to enter the endothelial cell to inhibit l-Arg transport, whether UTs are present on cultured endothelia and in the nonrenal vasculature, and whether chronic elevation of extracellular urea alters the expression of UT-A or UT-B. We measured l-Arg transport in cultured BAEC in the presence and absence of 25 mM urea and the urea transport inhibitor phloretin. We also screened a range of cultured vascular endothelial cells and several parts of rat circulation for the presence of UT-A and UT-B. Finally, we investigated the effect of high BUN on vascular UT expression in BAEC and in rat vasculature by using a model of accelerated uremia (6).
METHODS
l-Arg transport was measured in confluent monolayers of BAEC (passages 3–5), as described previously by our laboratory (18). Briefly, cells were subcultured into 12-well plates, and when just confluent, the culture medium was replaced with MEM containing 25 mM urea (=70 mg/dl) + 5.5 mM glucose, or 30.5 mM glucose (as osmotic control) in the presence of the UT inhibitor phloretin (100 μM) or with vehicle (0.1% ethanol). Cells were incubated for 6 h, and the transport of l-Arg into endothelial cells was measured by the method of Gazzola et al. (3) as follows: cells were washed with Krebs-HEPES buffer, and then 0.5 ml Krebs-HEPES buffer containing 500 μM l-Arg + 2 μCi/ml 3H-labeled l-Arg (58.0 Ci/mmol) was added to each well. Transport was terminated at 3 min by removing media and washing cells with 10 mM of ice-cold unlabeled l-Arg in PBS. Cells were lysed with 0.5% Triton X-100 in 0.5 M NaOH, and radioactive l-Arg was assayed by liquid scintillation counting and background was subtracted. The total cell protein was determined by the Bio-Rad detergent method with bovine serum albumin as a standard (18).
For Western blot analysis measurements, the following endothelial cells were grown to confluence in T-75 flasks: BAEC, rat thoracic aortic cells (RTAEC), rat mesenteric arteriolar cells (RMAEC), and human glomerular capillary cells (HGEC). Characteristics and culture of these cells have been previously described by our laboratory (15, 18). Cell lysate was prepared, and proteins were separated on 10% SDS-PAGE, transferred to polyvinylidene difluoride membrane, and probed with our affinity-purified polyclonal anti-UT-A1,2,4 or anti-UT-B antibodies (11, 13). Our laboratory previously confirmed the specificity of protein recognition by these antibodies by probing with preimmune serum and by performing peptide competition studies (11, 13), and this was further confirmed in the present study. Bands were visualized by using enhanced chemiluminescence and quantitated by densitometry, expressed as integrated optical density (IOD). Details of these techniques have been previously published by our laboratory (6, 11, 13).
In animal studies, 16 male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) aged 3–5 mo were used. Eight rats were subjected to right nephrectomy and infarction of two-thirds of the left kidney (by ligating branches of renal artery), and the rest were sham operated 1 day later as controls. Immediately after recovery from surgery, rats were given 0.225% NaCl instead of drinking water, which they remained on for the duration of the study. Thirteen days later, rats were placed on a high-protein diet (40% protein) in place of their regular rat chow (24% protein). Controls were paired to uremic rats and were pair fed to their uremic counterpart once the 40% protein diet was started. Uremic rats were killed at 17–18 days after surgery and controls within 1–3 days, plasma was collected, and spinotrapezius muscle, thoracic aorta, and mesenteric vessels were dissected, harvested, and prepared for Western blot analysis as described previously (6, 11, 13). The abundance of UT protein was quantitated by densitometry and expressed as IOD units. Plasma was analyzed for BUN, and the remainder was used for 6-h incubations in cell culture studies (see RESULTS).
Data were analyzed by paired and unpaired Student’s t-test (P < 0.05 = statistical significance).
RESULTS
As shown in Fig. 1, for a representative experiment, 25 mM (70 mg/dl) urea (+5.5 mM glucose) reduced l-Arg transport vs. the osmotic control of 30.5 mM glucose, as previously reported by our laboratory (18). The UT inhibitor phloretin prevented this effect of urea to inhibit l-Arg transport, whereas phloretin alone (control) had little effect on l-Arg transport. Variations in baseline l-Arg transport rates between different assays prevent presentation of averaged absolute data (n = 5), but when expressed as a percentage of control (30.5 mM glucose = 100%), 25 mM urea reduced l-Arg transport to 82 ± 1% (P < 0.001 vs. control), whereas l-Arg transport was not significantly different from control with urea + phloretin (105 ± 2%) or the value with phloretin alone (109 ± 2%).
Fig. 1.
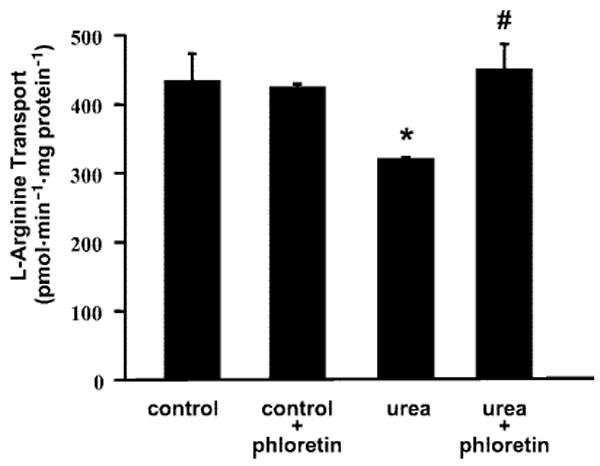
Example experiment (n = 3 wells for each condition) showing l-arginine transport after 6-h incubation of bovine aortic endothelial cells (BAEC) with control medium (containing 30.5 mM glucose + 0.1% ethanol), control medium + phloretin (100 μM; urea transport inhibitor), medium containing 25 mM urea with 0.1% ethanol, and medium containing 25 mM urea + 100 μM phloretin. *P < 0.05 vs. control; #P < 0.05 vs. urea alone.
UT-B is densely present ~40–45 kDa in cultured RMAEC, RTAEC, HGEC (Fig. 2), and BAEC (Fig. 3). A 98-kDa band is also seen in all cell lines, similar to the 98-kDa band that our laboratory previously detected in several rat tissues (13). This band is specifically detected by the UT-B antibody in rat tissues (13) and BAEC (as confirmed by the preadsorption and preimmune controls in Fig. 3), although the molecular explanation for this band is uncertain (see Ref. 13). In addition, the RMAEC show a band at 32 kDa and a smeared band from 40 to 60 kDa that are consistent with the size of nonglycosylated and glycosylated UT-B, respectively, present in red blood cells and kidney medulla (13).
Fig. 2.
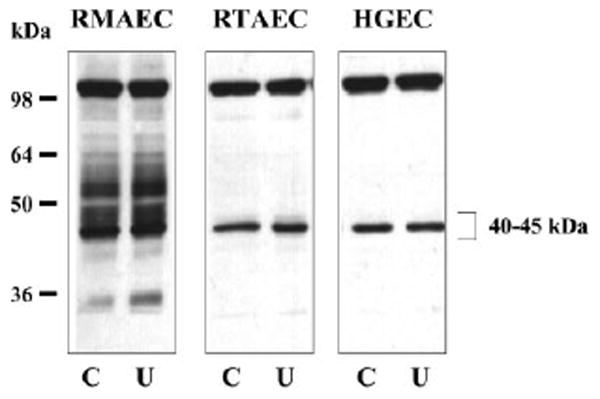
Representative Western blots showing the presence of UT-B in different types of cultured vascular endothelial cells. Cells were incubated for 6 h in the absence (control, C) or presence of 25 mM urea (U) in the culture medium. Total protein loaded was 20 μg/lane. RMAEC, rat mesenteric arteriolar endothelial cells; RTAEC, rat thoracic aortic endothelial cells; HGEC, human glomerular endothelial cells.
Fig. 3.
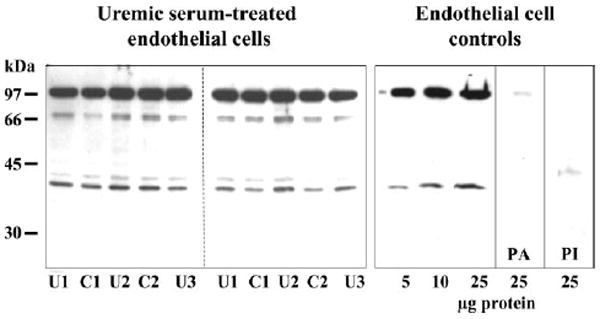
Representative Western blots showing presence of UT-B in BAEC. Left: BAEC that were cultured in normal DMEM supplemented with either 10% serum from a uremic rat (U1, U2, U3) or with 10% serum from a normal rat (C1, C2). The protein load was 20 μg tissue lysate/lane. Right: from left to right, a loading dose-response curve for antibody recognition of UT-B from these cells, a preadsorption control in which 25 μg of cell lysate were probed with antibody that was preadsorbed with immunizing peptide (PA), and an identical sample probed with preimmune serum (PI).
Incubation of RMAEC, RTAEC, and HGEC with 25 mM urea (Fig. 2) and BAEC with 20% uremic rat plasma, BUN = 136 ± 15 mg/dl (Fig. 3) for 6 h, had no effect on the abundance of UT-B vs. respective controls of 30.5 mM glucose or 20% control plasma, BUN = 21 ± 2 mg/dl. Seven days incubation of BAEC with 25 mM urea was also without effect compared with either 30.5 mM glucose or 30.5 mM mannitol as osmotic controls (424 ± 48, 457 ± 32, and 406 ± 24 IOD, respectively). The presence of UT-A was more variable: BAEC exhibited none (data not shown); the rat cells (RMAEC and RTAEC) showed faint bands at ~ 31 and 49 kDa, which are consistent with the size of UT-A2 and/or UT-A4; and the HGEC showed a doublet band centered around 117 kDa and a single band at 49 kDa (Fig. 4), which are consistent with the size of UT-A1 and UT-A2, respectively. There was no effect on the abundance of any UT-A protein in any cell line with 6-h exposure to 25 mM urea (Fig. 4).
Fig. 4.
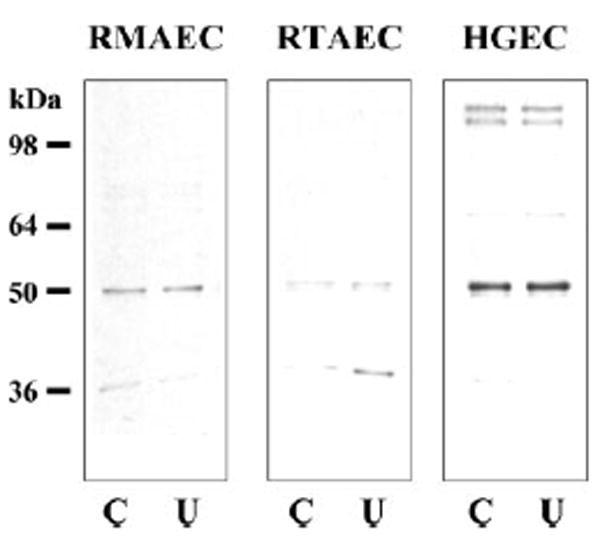
Representative Western blots showing the presence of UT-A in different types of cultured vascular endothelial cells. Cells were incubated in the absence (C) or presence of 25 mM urea (U) in the culture medium. Total protein loaded was 20 μg/lane.
In tissue, UT-B bands were present in aorta, spinotrapezius muscle, and mesenteric vessels (Fig. 5). The ~45- and 98-kDa bands are similar to the bands detected in the cultured cell lines (Figs. 2 and 3) and other rat tissues (13). The ablation/infarction + 0.225% NaCl and 40% protein feeding was successful in raising BUN from 21 ± 2 in controls to 136 ± 15 mg/dl. Uremia had no effect (vs. control) on the ~45-kDa UT-B band in either aorta or mesenteric vessels (159 ± 12 vs. 128 ± 19 IOD and 57 ± 11 vs. 47 ± 6 IOD, respectively; all not significant); however, this band was reduced in abundance in spinotrapezius from uremic rats (358 ± 57 vs. 507 ± 16 IOD, P < 0.025). Each of the three tissues contained the higher molecular mass band (98 kDa) reported earlier (13), and this band was unaffected by uremia (aorta, 574 ± 38 and 602 ± 41; mesenteric artery, 433 ± 23 and 401 ± 24; and spinotrapezius, 629 ± 26 and 462 ± 104 IOD for control and uremia, respectively; all not significant). In addition, a novel band at 88 kDa was seen in spinotrapezius and aorta and was markedly upregulated by uremia in aorta (203 ± 54 vs. 45 ± 10 IOD, P < 0.012), although not in spinotrapezius (169 ± 66 vs. 273 ± 43 IOD, not significant). All UT-B bands, including the novel 88-kDa band, were completely ablated by peptide competition (Fig. 5), indicating specificity of the primary antibodies.
Fig. 5.
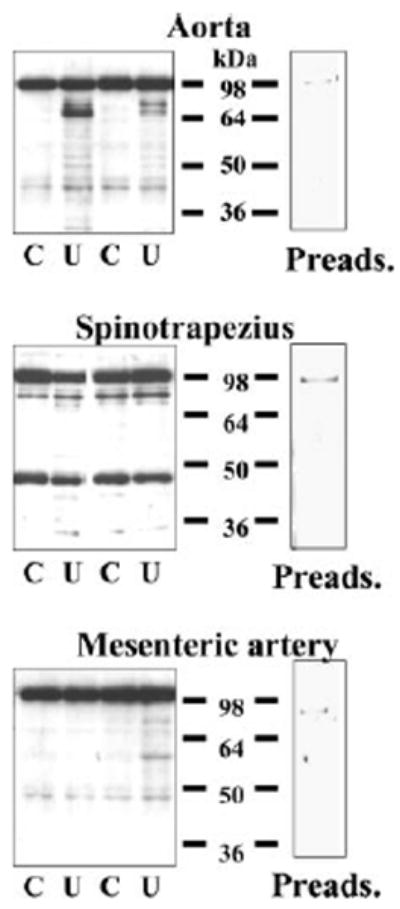
Representative Western blots showing presence of UT-B in aorta, spinotrapezius muscle, and mesenteric artery. Each blot shows an example of UT-B abundance in control (C) and uremic (U) animals. Right: identical control rat sample probed with antibody that was preadsorbed with immunizing peptide (Preads.). In each lane, 20 μg of tissue lysate protein were loaded.
UT-A was barely detectable in mesenteric vessels, present in very low abundance in aorta, and present in low abundance in spinotrapezius muscle (Fig. 6), and as with the cultured cells, the distribution was more variable. The UT-A antibody detects a doublet band centered around 60 kDa (Fig. 6), which is slightly larger than the size of UT-A2 in rat heart (2). In aorta, there was no change in the UT-A bands with uremia (142 ± 27, 115 ± 14 and 156 ± 25, 122 ± 15 IOD, control vs. uremia). Similarly, these bands in mesenteric vessels were unaffected by uremia (18 ± 9, 17 ± 3 and 32 ± 11, 23 ± 5 IOD, respectively). In spinotrapezius, a doublet band centered around 40 kDa is detected (Fig. 6), which is consistent with the size of the smaller UT-A protein found in heart and liver (2). These UT-A bands were downregulated by uremia (106 ± 25 vs. 183 ± 28 IOD, P = 0.06; 150 ± 25 vs. 237 ± 12 IOD, P < 0.01).
Fig. 6.

Representative Western blot showing presence of UT-A in aorta, spinotrapezius muscle, and mesenteric artery. Each blot shows examples of UT-A abundance in control (C) and uremic (U) animals. In each lane, 20 μg of tissue lysate protein were loaded.
DISCUSSION
The main, novel findings in the present study are the following: 1) the inhibitory action of urea on l-Arg transport depends on urea transport into the endothelial cell; 2) UT-B protein is present on cultured endothelial cells from several species and from conduit, arteriolar vessels and capillaries; and 3) UT-B protein is also present in rat aorta, mesenteric vessels, and the highly vascularized spinotrapezius muscle. Uremic levels of urea do not have predictable effects on abundance of UTs, which vary with the UT subtype and tissue/cell type.
Our laboratory previously showed that uremic levels of urea inhibit l-Arg entry into cultured vascular endothelial cells (18). There is evidence that the l-Arg entry step delivers substrate to endothelial nitric oxide synthase (NOS) at the caveolar complex (10). Together, this suggests a novel mechanism by which uremia may contribute to impaired vasorelaxation and glomerular function, specifically by means of substrate limitation of NOS. Our laboratory’s earlier work with an in vivo model of high BUN with normal renal function does not suggest any inhibitory effect of the elevated urea alone on the NO system (16). However, in uremia, when renal function is impaired, many other substances accumulate in the plasma in addition to urea. For example, guanidines and methylated arginines accumulate, which can compete with l-Arg for NOS and inhibit NO synthesis (9, 14). Indeed, our laboratory has reported that plasma from end-stage renal disease and some chronic renal disease patients contains endothelial NOS-inhibitory substances when it is incubated with cultured cells (17, 19). These findings, together with our present results, suggest that the balance between the availability of l-Arg and NOS inhibitors changes in uremia to inhibit eNOS NO production.
The present study suggests that urea has to enter the cell via a UT to exert its inhibitory effect, because the UT inhibitor phloretin abolishes the inhibition of l-Arg transport by high urea concentrations. Our laboratory has confirmed the presence of UT-B in cultured BAEC (13) and now reports that UT-B is also abundant in cultured human and rat endothelial cells derived from various locations throughout the circulation. Because immunofluorescence studies suggest a membrane location for UT-B in BAEC (13), this is consistent with the hypothesis that uremic levels of urea may influence membrane transport events. There is variability in UT-B protein band size in different endothelial cells and different tissues, as shown here and previously (13), but all cells and tissues studied show the ~45-kDa band. Distribution of UT-A-selective bands is even more heterogenous between the different cells and rat tissues and is also present in very low abundance in vivo. We have focused on UT-B in this study because uremia inhibits l-Arg transport into BAEC and HGEC (18) and the one UT common to these cells is 40- to 45-kDa UT-B.
In addition to ubiquitous distribution of UT-B in cultured endothelial cells, we now report that UT-B and UT-A are distributed in nonrenal vasculature. There is evidence for a doublet UT-A band in aorta and mesenteric artery, although the abundance is very low in the latter. In addition, spinotrapezius skeletal muscle, which is highly vascularized, also contains a doublet UT-A band. The size of these doublet bands is similar to singlet bands present in rat heart and liver (2). We previously showed that the 56-, 51-, and 39-kDa UT-A bands in rat heart result from UT-A2 mRNA (2), suggesting that UT-A2 may yield several protein sizes in different tissues. Future studies will be needed to determine whether the 60- and 40-kDa doublet UT-A bands in the nonrenal vasculature are both the result of UT-A2 mRNA expression.
The prominent presence of UT-B in blood vessels may explain the widespread distribution of UT-B subtypes in other organs, including heart, brain, liver, lung, and testis (13). It is reasonable to expect the presence of UTs in endothelial cells because they possess arginase (7) and are thus capable of urea synthesis. Presumably, the primary physiological function of endothelial UTs is the export of urea from endothelium. Perhaps uremia may provide a pathological stimulus to urea entry by driving the transporter in the reverse direction.
We also examined the effect of uremic levels of urea on UT expression in the vasculature and cultured endothelial cells. We previously observed marked upregulation of the liver and heart UT-A in this model (2, 6). On the basis of this finding, we speculated that if widespread vascular endothelial UT upregulation also occurred, the rate of urea entry would increase, which could amplify l-Arg deficiency at the caveolus. However, in the present study, we found very variable regulation of UT-B in uremia. The 45-kDa UT-B band was unaffected by uremia in aorta and mesenteric vessels and downregulated in spinotrapezius. We had seen widespread distribution of a 98-kDa UT-B band in many organs, including heart, brain, and lung (13), and this was also evident in aorta and was not affected by uremia. However, there was marked upregulation by uremia of a novel 88-kDa UT-B band in aorta. We do not yet know whether this band represents a functional UT, perhaps a dimer of 45 kDa. Of note, this band was not evident in cultured BAEC grown for 7 days in medium containing 25 mM urea, so this may reflect a nonendothelial response to uremia.
This study demonstrates that endothelial UTs are functionally active, because phloretin prevents the inhibitory effect of high extracellular urea concentrations on l-Arg transport. l-Arg is transported via a membrane transporter, and at least 80% of transport into endothelial cells is via the cationic amino acid transporter family (1). There is also evidence that under uremic concentrations, urea can inhibit other membrane transport processes, including several important Na transporters, such as Na+-K+-ATPase (5, 8, 12). If this inhibitory action of high urea levels on membrane transport persists in vivo, then the elevated BUN of uremia may have previously unsuspected, direct, and deleterious actions.
In conclusion, this study shows that the UTs located in cultured endothelial cells are functional and can transport urea into the cell when extracellular levels are high, leading to inhibition of l-Arg transport. When urea transport is inhibited, high levels of extracellular urea have no effect on l-Arg transport in vitro. Cultured endothelium exhibits considerable heterogeneity of UTs but always shows UT-B. In vivo, UT-B is widely distributed in nonrenal blood vessels, whereas UT-A is sparse. Chronically high levels of extracellular urea do not have consistent effects on expression of any vascular UT, either in vivo or in vitro.
Acknowledgments
The technical assistance of Lennie Samsell, Patricia Rouillard, and Addison Sears-Collins is gratefully acknowledged.
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-56843 and DK-45517 (to C. Baylis) and DK-41707 and P01-DK-50268 (to J. M. Sands).
References
- 1.Deves R, Boyd CAR. Transporters for cationic amino acids in animal cells. Discovery, structure and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 2.Duchesne R, Klein JD, Velotta JB, Doran JJ, Rouillard P, Roberts BR, McDonough AA, Sands JM. UT-A urea transporter protein in heart: increased abundance during uremia, hypertension, and heart failure. Circ Res. 2001;89:139–145. doi: 10.1161/hh1401.093293. [DOI] [PubMed] [Google Scholar]
- 3.Gazzola GC, Dall’Asta V, Franchi-Gazzola R, White MF. The cluster tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal Biochem. 1981;115:368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- 4.Hu MC, Bankir L, Michelet S, Rousselet G, Trinh-Trang-Tan MM. Massive reduction of urea transporters in remnant kidney and brain of uremic rats. Kidney Int. 2000;58:1202–1210. doi: 10.1046/j.1523-1755.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaji DM, Lim J, Shilkoth W, Zaid W. Urea inhibits the Na-K pump in human erthyrocytes. J Membr Biol. 1998;165:125–131. doi: 10.1007/s002329900426. [DOI] [PubMed] [Google Scholar]
- 6.Klein JD, Timmer RT, Rouillard P, Bailey JL, Sands JM. UT-A urea transporter protein expressed in liver: up-regulation by uremia. J Am Soc Nephrol. 1999;10:2076–2083. doi: 10.1681/ASN.V10102076. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 8.Lim J, Gasson C, Kaji DM. Urea inhibits NaK2Cl cotransport in human erythrocytes. J Clin Invest. 1995;96:2126–2132. doi: 10.1172/JCI118266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marescau BG, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM, Lornoy W, De Deyn PP. Guanidino compounds in serum and urine of nondialysed patents with chronic renal insufficiency. Metabolism. 1997;46:1024–1031. doi: 10.1016/s0026-0495(97)90273-0. [DOI] [PubMed] [Google Scholar]
- 10.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox.”. J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 11.Naruse M, Klein JD, Ashkar ZM, Jacobs JD, Sands JM. Glucocorticoids down-regulate the vasopressin-regulated urea transporter in rat terminal IMCDs. J Am Soc Nephrol. 1997;8:517–523. doi: 10.1681/ASN.V84517. [DOI] [PubMed] [Google Scholar]
- 12.Parker JC. Urea alters set point volume for K-Cl cotransport, Na+-H exchange and Ca-Na+ exchange in dog red blood cells. Am J Physiol Cell Physiol. 1993;265:C447–C452. doi: 10.1152/ajpcell.1993.265.2.C447. [DOI] [PubMed] [Google Scholar]
- 13.Timmer RT, Klein JD, Bagnasco SM, Doran JJ, Verlander JW, Gunn RB, Sands JM. Localization of the urea transporter UT-B protein in human and rat erythrocytes and tissues. Am J Physiol Cell Physiol. 2001;281:C1318–C1325. doi: 10.1152/ajpcell.2001.281.4.C1318. [DOI] [PubMed] [Google Scholar]
- 14.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 15.Wagner L, Erdely A, Hoey JG, Boegehold MA, Baylis C. The nitric oxide pathway is amplified in venular vs. paired arteriolar cultured rat mesenteric endothelial cells. Microvasc Res. 2001;62:401–409. doi: 10.1006/mvre.2001.2359. [DOI] [PubMed] [Google Scholar]
- 16.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal Physiol. 2001;280:F996–F1000. doi: 10.1152/ajprenal.2001.280.6.F996. [DOI] [PubMed] [Google Scholar]
- 17.Xiao S, Schmidt RJ, Baylis C. Plasma from ESRD patients inhibits nitric oxide synthase (NOS) activity in cultured human and bovine endothelial cells. Acta Physiol Scand. 2000;168:175–179. doi: 10.1046/j.1365-201x.2000.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao S, Wagner L, Mahaney J, Baylis C. Uremic levels of urea inhibit l-arginine transport in cultured endothelial cells. Am J Physiol Renal Physiol. 2001;280:F989–F995. doi: 10.1152/ajprenal.2001.280.6.F989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao S, Wagner L, Schmidt RJ, Baylis C. Circulating eNOS inhibitory factor in some patients with chronic renal disease. Kidney Int. 2001;59:1466–1472. doi: 10.1046/j.1523-1755.2001.0590041466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Olives B, Bailly P, Fischer E, Ripoche P, Ronco P, Cartron JP, Rondeau E. Endothelial cells of the kidney vasa recta express the urea transporter HUT11. Kidney Int. 1997;51:138–146. doi: 10.1038/ki.1997.17. [DOI] [PubMed] [Google Scholar]


