Abstract
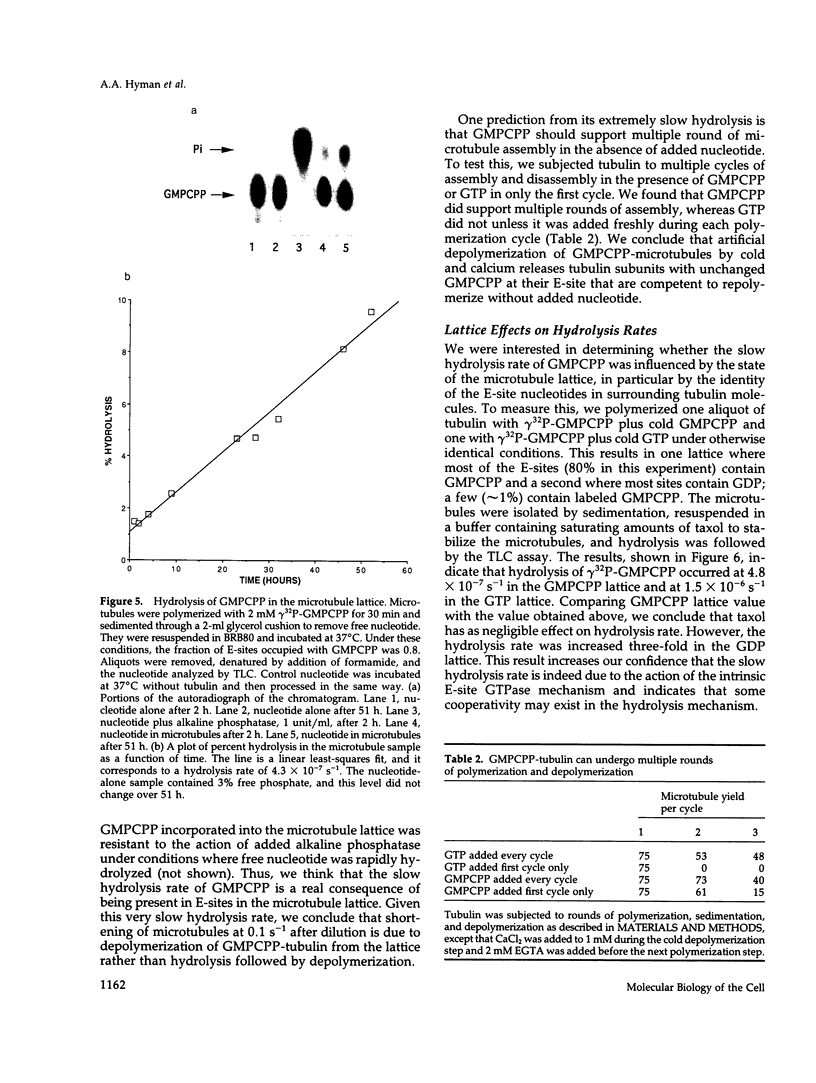
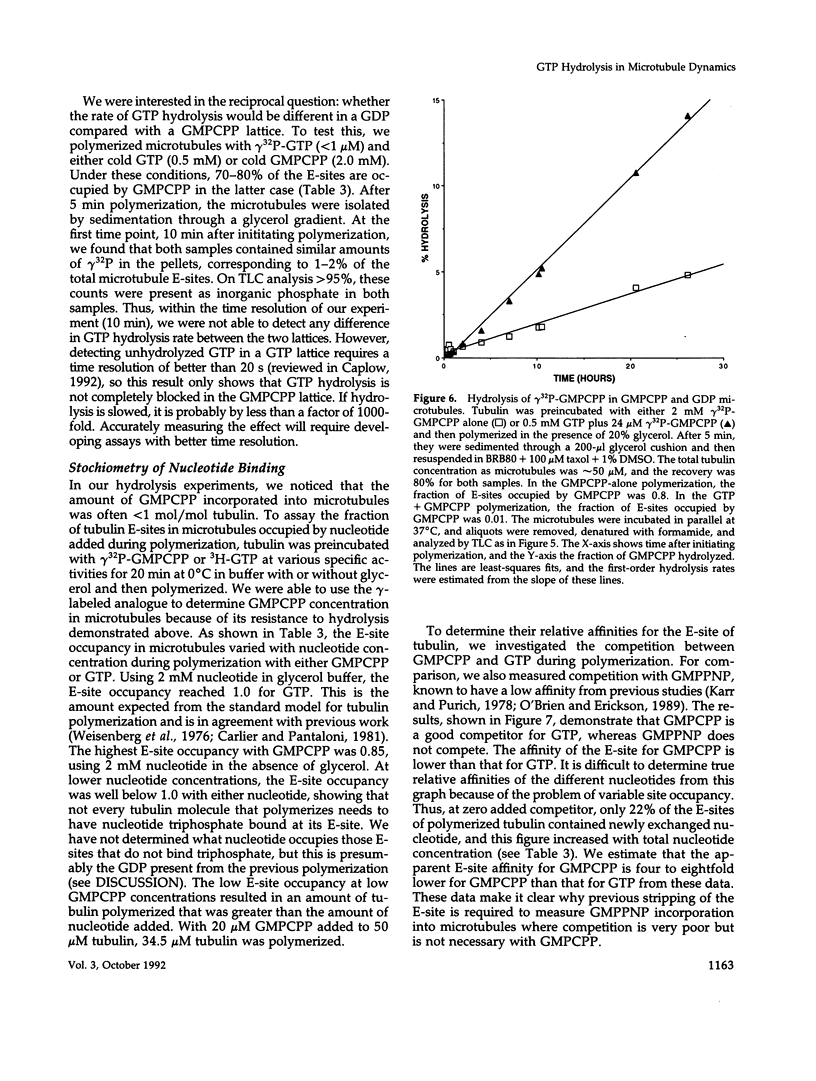
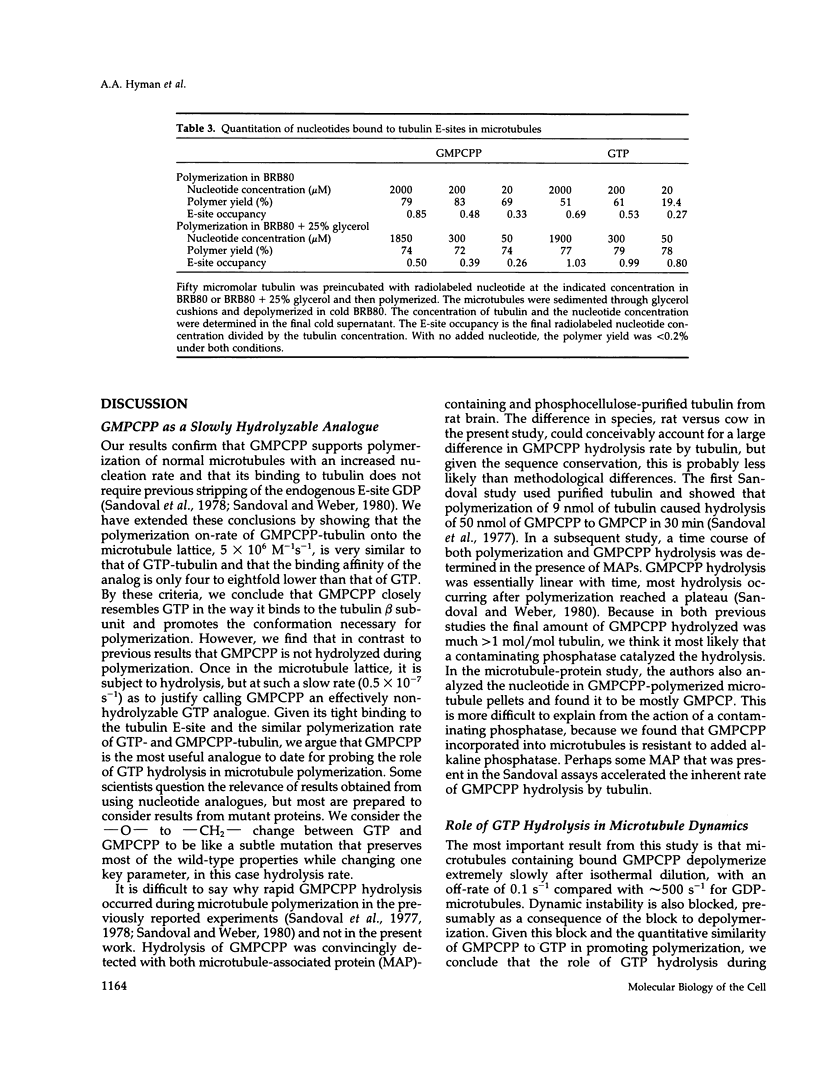
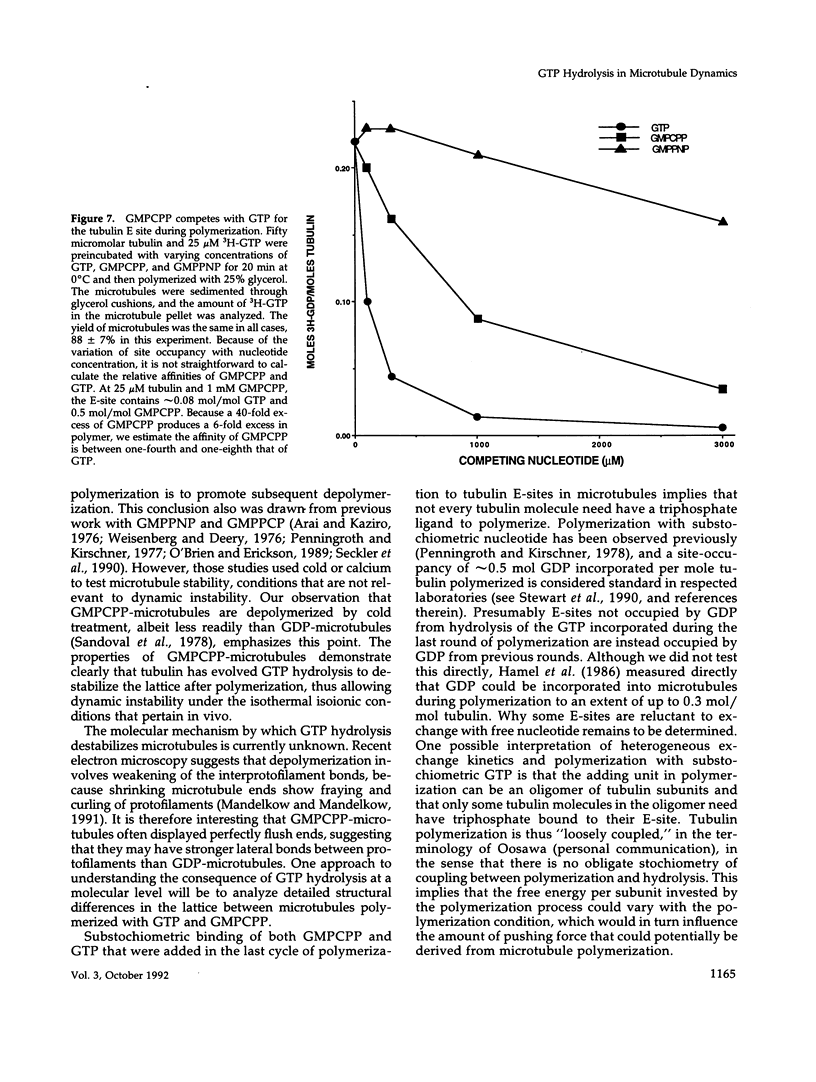
The role of GTP hydrolysis in microtubule dynamics has been reinvestigated using an analogue of GTP, guanylyl-(alpha, beta)-methylene-diphosphonate (GMPCPP). This analogue binds to the tubulin exchangeable nucleotide binding site (E-site) with an affinity four to eightfold lower than GTP and promotes the polymerization of normal microtubules. The polymerization rate of microtubules with GMPCPP-tubulin is very similar to that of GTP-tubulin. However, in contrast to microtubules polymerized with GTP, GMPCPP-microtubules do not depolymerize rapidly after isothermal dilution. The depolymerization rate of GMPCPP-microtubules is 0.1 s-1 compared with 500 s-1 for GDP-microtubules. GMPCPP also completely suppresses dynamic instability. Contrary to previous work, we find that the beta--gamma bond of GMPCPP is hydrolyzed extremely slowly after incorporation into the microtubule lattice, with a rate constant of 4 x 10(-7) s-1. Because GMPCPP hydrolysis is negligible over the course of a polymerization experiment, it can be used to test the role of hydrolysis in microtubule dynamics. Our results provide strong new evidence for the idea that GTP hydrolysis by tubulin is not required for normal polymerization but is essential for depolymerization and thus for dynamic instability. Because GMPCPP strongly promotes spontaneous nucleation of microtubules, we propose that GTP hydrolysis by tubulin also plays the important biological role of inhibiting spontaneous microtubule nucleation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Kaziro Y. Effect of guanine nucleotides on the assembly of brain microtubles: ability of 5'-guanylyl imidodiphosphate to replace GTB in promoting the polymerization of microtubules in vitro. Biochem Biophys Res Commun. 1976 Mar 22;69(2):369–376. doi: 10.1016/0006-291x(76)90531-3. [DOI] [PubMed] [Google Scholar]
- Caplow M. Microtubule dynamics. Curr Opin Cell Biol. 1992 Feb;4(1):58–65. doi: 10.1016/0955-0674(92)90059-l. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Kinetic analysis of guanosine 5'-triphosphate hydrolysis associated with tubulin polymerization. Biochemistry. 1981 Mar 31;20(7):1918–1924. doi: 10.1021/bi00510a030. [DOI] [PubMed] [Google Scholar]
- Carlier M. F. Role of nucleotide hydrolysis in the dynamics of actin filaments and microtubules. Int Rev Cytol. 1989;115:139–170. doi: 10.1016/s0074-7696(08)60629-4. [DOI] [PubMed] [Google Scholar]
- Chen Y. D., Hill T. L. Monte Carlo study of the GTP cap in a five-start helix model of a microtubule. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1131–1135. doi: 10.1073/pnas.82.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., De Mey J. Microtubule assembly in living cells after release from nocodazole block: the effects of metabolic inhibitors, taxol and PH. Cell Biol Int Rep. 1981 Sep;5(9):913–920. doi: 10.1016/0309-1651(81)90206-x. [DOI] [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand V. I., Bershadsky A. D. Microtubule dynamics: mechanism, regulation, and function. Annu Rev Cell Biol. 1991;7:93–116. doi: 10.1146/annurev.cb.07.110191.000521. [DOI] [PubMed] [Google Scholar]
- Hamel E., Batra J. K., Lin C. M. Direct incorporation of guanosine 5'-diphosphate into microtubules without guanosine 5'-triphosphate hydrolysis. Biochemistry. 1986 Nov 4;25(22):7054–7062. doi: 10.1021/bi00370a045. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Mitchison T. J. Modulation of microtubule stability by kinetochores in vitro. J Cell Biol. 1990 May;110(5):1607–1616. doi: 10.1083/jcb.110.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence. J Cell Sci Suppl. 1991;14:125–127. doi: 10.1242/jcs.1991.supplement_14.25. [DOI] [PubMed] [Google Scholar]
- Hyman A., Drechsel D., Kellogg D., Salser S., Sawin K., Steffen P., Wordeman L., Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Joshi H. C., Palacios M. J., McNamara L., Cleveland D. W. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992 Mar 5;356(6364):80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Karr T. L., Purich D. L. Examination of tubulin-nucleotide interactions by protein fluorescence quenching measurements. Biochem Biophys Res Commun. 1978 Oct 30;84(4):957–961. doi: 10.1016/0006-291x(78)91676-5. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W. Microtubule assembly and nucleation. Int Rev Cytol. 1978;54:1–71. doi: 10.1016/s0074-7696(08)60164-3. [DOI] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Mitchison T. J., Kirschner M. W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988 Feb 11;331(6156):499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- Kristofferson D., Mitchison T., Kirschner M. Direct observation of steady-state microtubule dynamics. J Cell Biol. 1986 Mar;102(3):1007–1019. doi: 10.1083/jcb.102.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS T. C., NAKAMURA K., DANIELZADEH A. B. PHOSPHONIC ACID ANALOGS OF NUCLEOSIDE PHOSPHATES. 3. THE SYNTHESIS OF ADENOSINE-5'-METHYLENEDIPHOSPHONATE, A PHOSPHONIC ACID ANALOG OF ADENOSINE-5'-DIPHOSPHATE. J Org Chem. 1965 May;30:1517–1520. doi: 10.1021/jo01016a043. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E., Milligan R. A. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol. 1991 Sep;114(5):977–991. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejillano M. R., Barton J. S., Nath J. P., Himes R. H. GTP analogues interact with the tubulin exchangeable site during assembly and upon binding. Biochemistry. 1990 Feb 6;29(5):1208–1216. doi: 10.1021/bi00457a017. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- O'Brien E. T., Erickson H. P. Assembly of pure tubulin in the absence of free GTP: effect of magnesium, glycerol, ATP, and the nonhydrolyzable GTP analogues. Biochemistry. 1989 Feb 7;28(3):1413–1422. doi: 10.1021/bi00429a070. [DOI] [PubMed] [Google Scholar]
- Penningroth S. M., Kirschner M. W. Nucleotide binding and phosphorylation in microtubule assembly in vitro. J Mol Biol. 1977 Oct 5;115(4):643–673. doi: 10.1016/0022-2836(77)90108-5. [DOI] [PubMed] [Google Scholar]
- Penningroth S. M., Kirschner M. W. Nucleotide specificity in microtubule assembly in vitro. Biochemistry. 1978 Feb 21;17(4):734–740. doi: 10.1021/bi00597a028. [DOI] [PubMed] [Google Scholar]
- Purich D. L., Kristofferson D. Microtubule assembly: a review of progress, principles, and perspectives. Adv Protein Chem. 1984;36:133–212. doi: 10.1016/s0065-3233(08)60297-1. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., Jameson J. L., Niedel J., MacDonald E., Cuatrecasas P. Role of nucleotides in tubulin polymerization: effect of guanosine 5'-methylene diphosphonate. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3178–3182. doi: 10.1073/pnas.75.7.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval I. V., MacDonald E., Jameson J. L., Cuatrecasas P. Role of nucleotides in tubulin polymerization: effect of guanylyl 5'-methylenediphosphonate. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4881–4885. doi: 10.1073/pnas.74.11.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval I. V., Weber K. Guanasone 5'-(alpha,beta-methylene)triphosphate enhances specifically microtubule nucleation and stops the treadmill of tubulin protomers. J Biol Chem. 1980 Jul 25;255(14):6966–6974. [PubMed] [Google Scholar]
- Seckler R., Wu G. M., Timasheff S. N. Interactions of tubulin with guanylyl-(beta-gamma-methylene)diphosphonate. Formation and assembly of a stoichiometric complex. J Biol Chem. 1990 May 5;265(13):7655–7661. [PubMed] [Google Scholar]
- Simon J. R., Parsons S. F., Salmon E. D. Buffer conditions and non-tubulin factors critically affect the microtubule dynamic instability of sea urchin egg tubulin. Cell Motil Cytoskeleton. 1992;21(1):1–14. doi: 10.1002/cm.970210102. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Salmon E. D. The structure of microtubule ends during the elongation and shortening phases of dynamic instability examined by negative-stain electron microscopy. J Cell Sci. 1990 Aug;96(Pt 4):571–582. doi: 10.1242/jcs.96.4.571. [DOI] [PubMed] [Google Scholar]
- Stewart R. J., Farrell K. W., Wilson L. Role of GTP hydrolysis in microtubule polymerization: evidence for a coupled hydrolysis mechanism. Biochemistry. 1990 Jul 10;29(27):6489–6498. doi: 10.1021/bi00479a022. [DOI] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
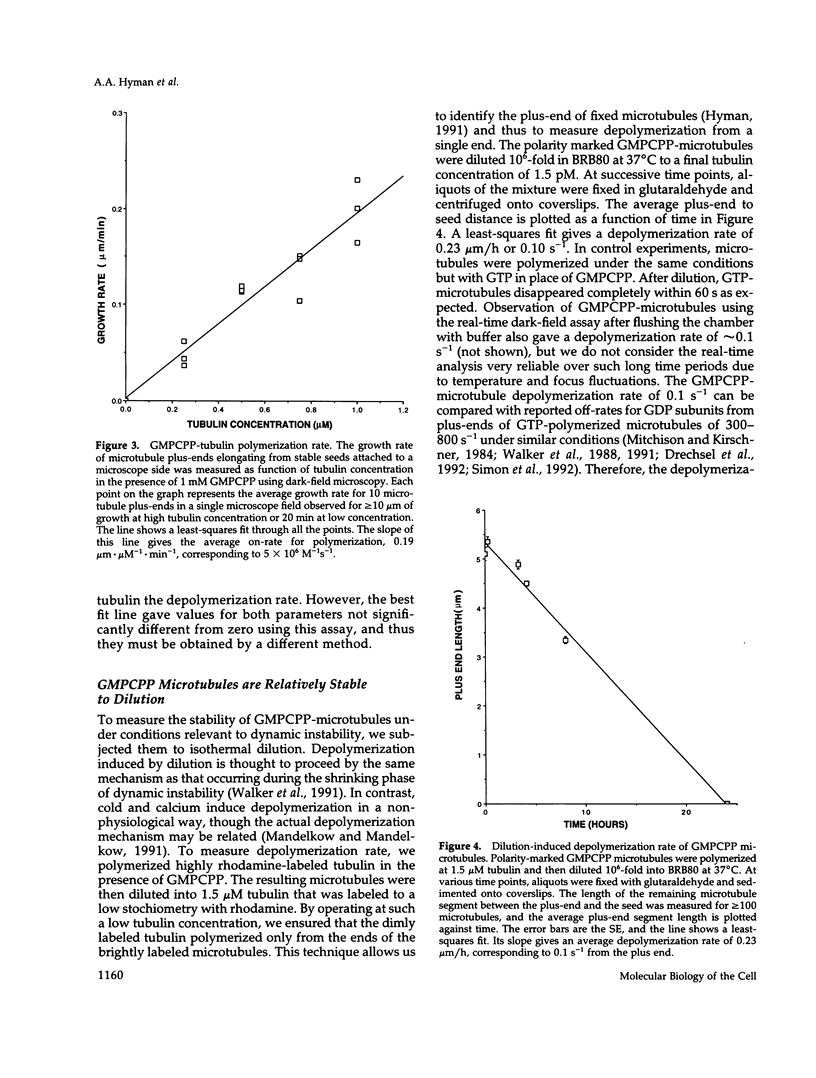
- Walker R. A., Pryer N. K., Salmon E. D. Dilution of individual microtubules observed in real time in vitro: evidence that cap size is small and independent of elongation rate. J Cell Biol. 1991 Jul;114(1):73–81. doi: 10.1083/jcb.114.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Sandoval I. V. Cells injected with guanosine 5'-[alpha, beta-methylene]triphosphate, an alpha, beta-nonhydrolyzable analog of GTP, show anomalous patterns of tubulin polymerization affecting cell translocation, intracellular movement, and the organization of Golgi elements. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1938–1941. doi: 10.1073/pnas.80.7.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J. Role of nucleotide hydrolysis in microtubule assembly. Nature. 1976 Oct 28;263(5580):792–793. doi: 10.1038/263792a0. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]