Summary
Objective
Previous studies have indicated that joint hypermobility may affect the development of clinical and radiological hand osteoarthritis (OA), but this question has not been addressed in epidemiological studies. Our objective was to investigate this relationship in a population-based study.
Patients and methods
The study group consisted of 384 unselected older participants in the Age, Gene/Environment Susceptibility–Reykjavik Study (161 males, median age 76, range 69–90, and 223 females median age 75, range 69–92). The criterion used for joint mobility was the single maximal degree of hyperextension of digits 2 and 5 on both hands (HYP°).
Results
HYP° was more prevalent in females and on the left hand in both men and women. Both genders had a positive association between the degree of mobility measured by HYP° and radiological scores for the first carpometacarpal joint (CMC1) OA. Thus, those with HYP° ≥70 had an odds ratio of 3.05 (1.69–5.5, P < 0.001) of having a Kellgren–Lawrence score of ≥3 in a CMC1 joint. There was also a trend towards a negative association between HYP° and proximal interphalangeal joint scores.
Conclusion
Hand joint mobility, defined as hyperextension in the metacarpophalangeal joints (HYP°) is more prevalent in females and on the left side. It was associated with more severe radiographic OA in the CMC1 joints in this population. The reasons for this relationship are not known, but likely explanations involve ligament laxity and CMC1 joint stability. These findings may relate to the left-sided predominance of radiographic OA in the CMC1 joints observed in many prevalence studies.
Keywords: Hand osteoarthritis, Radiology, Hypermobility, Epidemiology
Introduction
Previous clinical studies have indicated that joint hypermobility may affect the development of clinical and radiological hand osteoarthritis (OA), but this question has not been addressed in epidemiological studies. The initial observations that hypermobility was associated with more severe thumb base OA came from a member of this group1,2 but those studies were hampered by lack of radiological data1 and relatively small numbers2. Furthermore, they were based on patients receiving occupational therapy for hand OA and thus likely to have clinically recognized thumb problems. In a large study, Kraus and coworkers did not find any relation between general hypermobility and carpometacarpal (CMC1) OA, but instead they found a protective effect of hypermobility on proximal inter phalangeal joint (PIP) joint OA3. That study was not population-based but a genetic study, and included only patients with confirmed distal inter phalangeal joint (DIP) joint OA.
Other authors have speculated on the general relationship between hypermobility and OA in other joints4. There is some evidence that ligament laxity predisposes to the progression of knee OA5. However, others have found no relationship between generalized hypermobility and OA6.
The etiology of hand OA is still very unclear. There is evidence that ligament laxity and trapeziometacarpal subluxation are important early events in the development of CMC1 OA7,8. There is also a strong genetic component which is currently the subject of several studies. Given the importance of phenotype definition in hand OA, it is important to clarify possible confounders affecting the expression of the disease.
The Age Gene/Environment Susceptibility Reykjavik study (AGES-Reykjavik Study) is a population-based study of older subjects9. In the present investigation, we used an unselected sample of the study population to investigate the relationship between hand joint mobility and radiological hand OA.
Patients and methods
The AGES-Reykjavik Study is a follow-up study of 5674 individuals from the 40-year long Reykjavik study. The participants in the AGES-Reykjavik Study, aged between 66 and 92, were randomly recruited from the Reykjavik Study survivors. To ensure representative samples at different times during the study, the recruitment of individual subjects was also random during the 5 year study period.
In the spring of 2005, consecutive AGES-Reykjavik participants attending the research center were invited to participate in an ancillary study involving a clinical hand examination and a standard hand radiograph in addition to other study investigations. All participants gave informed consent. A total of 389 individuals participated. The final sample was similar to the AGES-Reykjavik Study population with regard to age and gender. Five subjects were subsequently excluded, four due to evidence of erosive inflammatory arthritis and one due to extensive contractures of both hands which prohibited reading of radiographs for OA. The remaining patients comprised 161 males (median age 76, range 69–90), and 223 females (median age 75, range 69–92).
All participants had a clinical examination by the same examiner (HJ) and 69 of those had a second examination (LA) for interobserver comparison. The clinical examination included hand joint mobility by a measurement of passive dorsiflexion of the second and fifth fingers on both hands, and scored as the highest degree of extension in any of the four fingers. This measurement is referred to as HYP°. Hyperextension of the fifth finger has been widely used to measure hypermobility and is included in the Beighton criteria for hypermobility5. The second finger on both hands were added to guard against bias by Dupuytrens contractures, a common condition in this age group. This measurement had acceptable interobserver reproducibility with an intra-class coefficient (ICC) of 0.8–0.89.
For calculations, the study group was dichotomized by the traditional cutoff of HYP° ≥ 902,6. This criterion had a very low prevalence in males (n = 3) and therefore we added a less stringent criterion, HYP° ≥ 70, that enabled a sufficient number of males to be considered for odds-ratio calculations.
All hand radiographs were read for evidence of OA and scored by the Kellgren–Lawrence radiological scale by two experienced radiologists (GJE, AJ). Discordant scores were subsequently examined and consensus scores determined.
Statistics
All statistics were calculated with the SPSS software package. The Spearman rank correlaton (Rs) was used for correlations, Chi-square and Mann–Whitney U test for comparisons between groups and Mantel-Haenszel statistics for odds-ratio calculations. Logistic regression analysis was used to check for possible confounders such as age.
Results
As expected, the prevalence of radiographic OA in this elderly population was high, with more than 85% having definite (K-L ≥ 2) OA in a DIP joint (Table I). In most joints, there was a nonsignificant tendency towards higher radiological scores on the right side, most apparent in the metacorpophalangeal joint (MCP) joints. In the CMC1 and scophotrapezial joint (STT) joints however, the trend was towards slightly higher scores on the left side (data not shown). Females had more OA in all joints except the MCP joints compared with males (Table I). Radiographic scores had a low but significant correlation with age (RS 0.17, P = 0.001).
Table I.
Radiological characteristics of the study group by Kellgren–Lawrence scores
| Males (n = 163) (%) | Females (n = 221) (%) | |
|---|---|---|
| DIP ≥ 2 | 82 | 92 |
| DIP bilateral ≥ 2 | 63 | 77 |
| DIP ≥ 3 | 28 | 46 |
| PIP ≥ 2 | 40 | 57 |
| PIP bilateral ≥ 2 | 26 | 36 |
| PIP ≥ 3 | 9 | 14 |
| MCP (2–5) ≥ 2 | 21 | 18 |
| CMC1 ≥ 2 | 27 | 34 |
| CMC bilateral ≥ 2 | 14 | 21 |
| CMC ≥ 3 | 9 | 18 |
| STT ≥ 2 | 15 | 26 |
Joint mobility measured by HYP° was significantly more common on the left side in both males and females. It was also much more prevalent in females than in males (Table II). It also had a nonsignificant negative correlation with age (RS −0.08, P = 0.12).
Table II.
Joint mobility measured as the single highest degree of passive dorsiflexion in two fingers on each hand (HYP°)
| Males (n = 163) (%) | Females (n = 221) (%) | P | |
|---|---|---|---|
| HYP ≥ 70 | 13 | 41 | <0.001 |
| Left* | 12 | 38 | |
| Right* | 4 | 20 | |
| HYP ≥ 90 | 2 | 17 | <0.001 |
| Left* | 2 | 16 | |
| Right* | 0 | 4 | |
| Mean HYP° (SD) | 43 (17.9) | 61 (19.9) | <0.001 |
| Left* | 41 (18.3) | 59 (20.6) | |
| Right* | 34 (15.1) | 49 (18.1) |
P < 0.001 for the three left vs right comparisons.
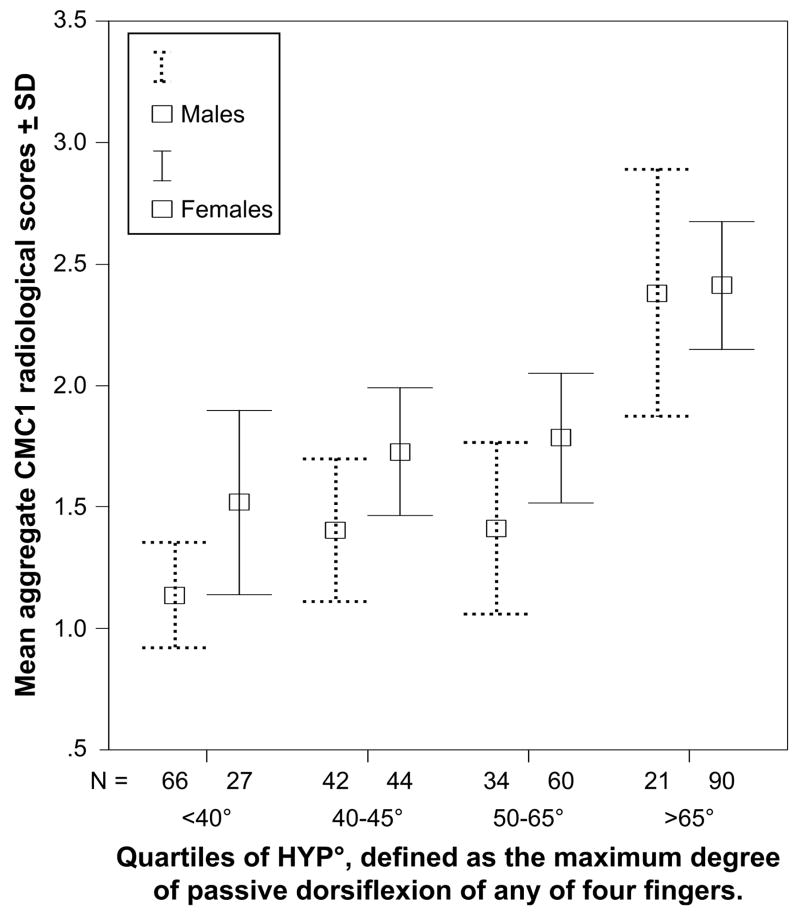
On bivariate analysis of the relationship between joint mobility and radiological scores in individual joints, both genders had a positive association between HYP° and radiological CMC1 scores (Fig. 1). Some calculations showed a slight negative association between HYP° and interphalangeal (DIP and PIP) joint scores (Table III) but this was not a consistent finding across the field. Multiple logistic regression analysis including age and gender confirmed an association between HYP° and CMC1 radiological scores (P < 0.001). Table III shows the odds ratio calculations for this relationship. There were only 3 males that fulfilled the HYP° ≥ 90 criterion, but males with HYP° ≥ 70 had increased CMC1 OA, comparable to that observed in females. There was no relation between STT joint OA and HYP°.
Fig. 1.
The relationship between aggregate radiological scores for both CMC1 joints and joint mobility measured in quartiles of HYP°. (N = Number of subjects in each group).
Table III.
Radiographic OA in relation to joint mobility measured as the highest degree of extension in any of 4 fingers tested (HYP°)
| Females with HYP°≥ 90° (n = 38) |
All subjects with HYP°≥ 70° (n = 111) |
Females with HYP°≥ 70° (n = 90) |
Males with HYP°≥ 70° (n = 21) |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| DIP scores | ||||||||
| ≥ 2 (n = 338) | 0.46 (0.15–1.4) | ns | 1.58 (0.76–3.31) | ns | 0.76 (0.28–2.04) | ns | 2.33 (0.51–10.6) | ns |
| ≥ 2 bilat. (n = 275) | 0.57 (0.26–1.23) | ns | 1.32 (0.84–2.07) | ns | 0.61 (0.33–1.16) | ns | 1.19 (0.45–3.14) | ns |
| ≥ 3 (n = 148) | 0.48 (0.23–1.0) | 0.051 | 0.99 (0.61–1.61) | ns | 1.04 (0.6–1.77) | ns | 1.02 (0.37–2.81) | ns |
| PIP scores | ||||||||
| ≥ 2 (n = 192) | 0.73 (0.36–1.46) | ns | 0.89 (0.58–1.39) | ns | 0.74 (0.43–1.28) | ns | 0.55 (0.2–1.49) | ns |
| ≥ 2 bilat. (n = 122) | 0.42 (0.18–0.97) | 0.042 | 0.88 (00.54–1.41) | ns | 0.71 (0.4–1.25) | ns | 0.86 (0.29–2.5) | ns |
| ≥ 3 (n = 45) | 0.3 (0.07–1.29) | ns | 0.67 (0.54–1.41) | ns | 0.46 (0.2–1.08) | 0.07 | 1.14 (0.24–5.5) | ns |
| MCP 2–5 scores | ||||||||
| ≥ 2 (n = 75) | 1.03 (0.42–2.53) | ns | 0.67 (0.37–1.21) | ns | 0.65 (0.32–1.34) | ns | 0.84 (0.26–2.69) | ns |
| CMC1 scores | ||||||||
| ≥ 2 (n = 120) | 1.49 (0.73–3.05) | ns | 2.01 (1.27–3.19) | 0.003 | 1.65 (0.94–2.89) | 0.08 | 2.89 (1.13–7.39) | 0.03 |
| ≥ 2 bilat. (n = 68) | 2.03 (0.94–4.41) | 0.076 | 1.97 (1.14–3.39) | 0.015 | 1.8 (0.94–3.47) | 0.08 | 1.62 (0.49–5.36) | ns |
| ≥ 3 (n = 53) | 2.26 (1.01–5.06) | 0.049 | 3.05 (1.69–5.5) | <0.001 | 2.47 (1.22–5) | 0.012 | 3.11 (0.88–11) | 0.08 |
Discussion
In this population-based study of 384 elderly subjects, hand joint mobility measured by passive dorsiflexion of digits two and five was more prevalent on the left side and in females. High mobility was associated with the presence of radiological CMC1 OA. The degree of hand mobility also showed a linear association with the severity of OA in the CMC1 joints (Fig. 1). In addition, there was a trend towards less OA in the proximal interphalangeal joints in those with high hand mobility.
The relationship between hand joint mobility and hand OA has not been addressed before in a population-based epidemiological study. The current results indicate that joint mobility affects the expression of hand OA with increased severity of radiological CMC1 OA. This is consistent with our previous reports1,2, but contrasts somewhat with the findings of Kraus and coworkers who found no relation of general hypermobility to CMC1 OA but instead found a protective effect of hypermobility on PIP joint OA3. Several explanations are possible for this discrepancy, the most likely one relating to the mobility criteria used, which in this study were confined to the hand. Patient selection bias may also have contributed to the different findings. Kraus et al. studied hand OA patients and DIP joint OA was an inclusion criterion whereas our study involved a population recruited from a community-based population not recruited for OA. Other factors must also be considered, such as age; if there is a protective effect of hypermobility on PIP joint OA delaying its development, this effect may be more apparent in younger subjects.
The pathomechanic events leading to the development of CMC1 OA are not clear, but Pellegrini has demonstrated that both degenerative and traumatic conditions compromize ligamentous structures and result in translational instability of the CMC1 joint. Ligament laxity, particularly laxity of the beak ligament, appears to be an early event leading to OA in the joint7. It is easy to postulate that individuals with hypermobile joints are more likely to develop such instability. The finding by Hunter and coworkers that trapeziometacarpal subluxation predisposes incident CMC1 OA, lends further support to a pathomechanic pathway involving ligament integrity and joint instability8. The biomechanics of the PIP joints, however, are quite different from those of the CMC1 joint. The development of PIP joint OA is positively correlated to grip strength indicating the importance of mechanical forces10. If high mobility is protective for OA in that joint, a possible explanation relates to the creation of less forces on the joint margins in those with more pliable periarticular tissues.
The left side predominance of hypermobility has been observed by others11–14. It raises the question whether dominant hand use functions as a stabilizing factor, perhaps through improved proprioception or activation of other neuromuscular pathways. In this study 13 participants reported left handedness and 12 equal handedness. Seven subjects in each group were more mobile on the left side, but the numbers are too small for conclusions. Proprioception has been found to be impaired in the hand joints of hypermobile subjects, and to be improved by exercise15,16. Impaired proprioception has also been associated with knee OA17. One of the mysteries of OA epidemiology has been the regular finding of a left predominance for the CMC1 joint in contrast with other hand joints18–21. There have been speculations that particular opposing high strain functions are more commonly performed by the left hand, but this study suggests a much more likely explanation, that the deleterious effect of high joint mobility or laxity on the CMC1 joint is stronger on the left side.
One limitation of this study is its cross-sectional nature, its size and the age of our subjects. A larger study might have given more definite answers regarding a possible protective effect of hypermobility on interphalangeal joint OA. The natural course of joint mobility not well known but available evidence suggests that joint hypermobility is a genetic condition and that joint mobility slowly decreases with age. The possibility that the current results could be due to a particular genetic subset, of hypermobility associated CMC1 OA, in the Icelandic population must be considered. However, the prevalence of CMC1 OA in this study is similar to other prevalence studies and almost the exact replica of the prevalence recently observed by Wilder in Florida residents18. Lastly, to report a possible bias, joint mobility assessment is a clinical finding and could theoretically be affected by the examiners in question. In this study however, the relationship between joint mobility and CMC1 OA was similar for both observers and the measurements of the second observer were significant despite the smaller number of examined subjects (data not shown).
The present study shows an association between high joint mobility and the presence and severity of CMC1 OA, the likely explanation being that lack of stability induces or at least contributes to the progression of OA in that joint. This has relevance for phenotype definitions of hand OA. The fact that hypermobility is more prevalent on the left side also provides a plausible explanation for the left-sided predominance of CMC1 OA found in epidemiological studies.
Acknowledgments
This study has been funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Ice-landic Parliament). The study is approved by the Icelandic National Bioethics Committee, VSN: 00-063, the Icelandic Data Protection Authority and the Institutional Review Board serving NIA. The study was also supported by the Icelandic Osteoarthritis Fund. We would also like to thank Lauren Abbate for her contribution to the clinical examination for hypermobility.
Footnotes
Conflict of interest
There is no conflict of interest by any of the authors.
References
- 1.Jonsson H, Valtysdottir ST. Hypermobility features in patients with hand osteoarthritis. Osteoarthritis Cartilage. 1995;3:1–5. doi: 10.1016/s1063-4584(05)80032-9. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson H, Valtysdottir ST, Kjartansson O, Brekkan A. Hypermobility associated with osteoarthritis of the thumb base: a clinical and radiological subset of hand osteoarthritis. Ann Rheum Dis. 1996;55:540–3. doi: 10.1136/ard.55.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraus VB, Li YJ, Martin ER, Jordan JM, Renner JB, Doherty M, et al. Articular hypermobility is a protective factor for hand osteoarthritis. Arthritis Rheum. 2004;50:2178–83. doi: 10.1002/art.20354. [DOI] [PubMed] [Google Scholar]
- 4.Hakim A, Grahame R. Joint hypermobility. Best Pract Res Clin Rheumatol. 2003;17:989–1004. doi: 10.1016/j.berh.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42:861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Dolan AL, Hart DJ, Doyle DV, Grahame R, Spector TD. The relationship of joint hypermobility, bone mineral density, and osteoarthritis in the general population: the Chingford Study. J Rheumatol. 2003;30:799–803. [PubMed] [Google Scholar]
- 7.Pellegrini VD., Jr Pathomechanics of the thumb trapeziometacarpal joint. Hand Clin. 2001;17:175–84. [PubMed] [Google Scholar]
- 8.Hunter DJ, Zhang Y, Sokolove J, Niu J, Aliabadi P, Felson DT. Trapeziometacarpal subluxation predisposes to incident trapeziometacarpal osteoarthritis (OA): the Framingham Study. Osteoarthritis Cartilage. 2005;13:953–7. doi: 10.1016/j.joca.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik study: multidiscipliary applied phenomics. Am J Epidemiol. 2007 doi: 10.1093/aje/kwk115. [Epub March 2007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaisson CE, Zhang Y, Sharma L, Kannel W, Felson DT. Grip strength and the risk of developing hand osteoarthritis: results from the Framingham study. Arthritis Rheum. 1999;42:33–8. doi: 10.1002/1529-0131(199901)42:1<33::AID-ANR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Qvindesland A, Jonsson H. Articular hypermobility in Icelandic 12-year-olds. Rheumatology (Oxford) 1999;38:1014–6. doi: 10.1093/rheumatology/38.10.1014. [DOI] [PubMed] [Google Scholar]
- 12.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32:413–8. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pountain G. Musculoskeletal pain in Omanis, and the relationship to joint mobility and body mass index. Br J Rheumatol. 1992;31:81–5. doi: 10.1093/rheumatology/31.2.81. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rawi ZS, Al-Aszawi AJ, Al-Chalabi T. Joint mobility among university students in Iraq. Br J Rheumatol. 1985;24:326–31. doi: 10.1093/rheumatology/24.4.326. [DOI] [PubMed] [Google Scholar]
- 15.Mallik AK, Ferrell WR, McDonald AG, Sturrock RD. Impaired proprioceptive acuity at the proximal interphalangeal joint in patients with the hypermobility syndrome. Br J Rheumatol. 1994;33:631–7. doi: 10.1093/rheumatology/33.7.631. [DOI] [PubMed] [Google Scholar]
- 16.Ferrell WR, Tennant N, Sturrock RD, Ashton L, Creed G, Brydson G, et al. Amelioration of symptoms by enhancement of proprioception in patients with joint hypermobility syndrome. Arthritis Rheum. 2004;50:3323–8. doi: 10.1002/art.20582. [DOI] [PubMed] [Google Scholar]
- 17.Sharma L. Proprioceptive impairment in knee osteoarthritis. Rheum Dis Clin North Am. 1999;25:299–314. doi: 10.1016/s0889-857x(05)70069-7. [DOI] [PubMed] [Google Scholar]
- 18.Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953–7. doi: 10.1016/j.joca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Lane NE, Bloch DA, Jones HH, Simpson U, Fries JF. Osteoarthritis in the hand: a comparison of handedness and hand use. J Rheumatol. 1989;16:637–42. [PubMed] [Google Scholar]
- 20.Bagge E, Bjelle A, Eden S, Svanborg A. Osteoarthritis in the elderly: clinical and radiological findings in 79 and 85 year olds. Ann Rheum Dis. 1991;50:535–9. doi: 10.1136/ard.50.8.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64:682–7. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]