Abstract
Peripheral stimulation and physical therapy can promote neurovascular plasticity and functional recovery after CNS disorders such as ischemic stroke. Using a rodent model of whisker-barrel cortex stroke, we have previously demonstrated that whisker activity promotes angiogenesis in the penumbra of the ischemic barrel cortex. This study explored the potential of increased peripheral activity to promote neurogenesis and neural progenitor migration toward the ischemic barrel cortex.
Three days after focal barrel cortex ischemia in adult mice, whiskers were manually stimulated (15 min × 3 times/day) to enhance afferent signals to the ischemic barrel cortex. 5-bromo-2′-deoxyuridine (BrdU, i.p.) was administered once daily to label newborn cells. At 14 days after stroke, whisker stimulation significantly increased vascular endothelial growth factor (VEGF) and stromal derived factor-1 (SDF-1) expression in the penumbra. The whisker stimulation animals showed increased doublecortin (DCX) positive and DCX/BrdU-positive cells in the ipsilateral corpus of the white matter but no increase in BrdU-positive cells in the subventricular zone, suggesting a selective effect on neuroblast migration. Neurogenesis indicated by neuronal nuclear protein (NeuN) and BrdU double staining was also enhanced by whisker stimulation in the penumbra at 30 days after stroke. Local cerebral blood flow was better recovered in mice that received whisker stimulation. It is suggested that the enriched microenvironment created by specific peripheral stimulation increases regenerative responses in the post-ischemic brain and may benefit long-term functional recovery from ischemic stroke.
Keywords: Neurogenesis, Ischemic stroke, Barrel cortex, Whisker stimulation, Cell migration
Introduction
Ischemic stroke is a devastating injury caused by interruption of the blood supply to regions of the brain. In humans, this type of injury strongly correlates with paralysis, sensory deficits, impairments in learning and memory, high risk of dementia and elevated incidence of depression (Asplund et al., 1998), however, there are limited effective therapies for the treatment of ischemic stroke (Alberts and Ovbiagele, 2007; Goldstein, 2007). While triggering massive cell death in the ischemic region, cerebral ischemia also stimulate regenerative responses in the tissue adjacent and remote to the impaired area (Wei et al., 2003; Fan and Yang, 2007). Recent evidence suggests that cerebral ischemia induces the proliferation of endogenous neural stem/progenitor cells in the subventricular zone (SVZ) (Jin et al., 2001; Tonchev et al., 2005), and after stroke these SVZ cells migrate laterally toward the striatal ischemic boundary with distinct migratory behaviors and retained capacity for cell division (Zhang et al., 2007). These neuroblasts in the striatum form elongated chain-like cell aggregates similar to those in the normal SVZ, and these chains were observed to be closely associated with thin astrocytic processes and blood vessels. The SVZ-derived neuroblasts differentiated into mature neurons in the striatum, expressing neuronal specific nuclear protein and forming synapses with neighboring striatal cells (Yamashita et al., 2006). Taken together, these studies indicate the exciting possibility that some degree of tissue repair after stroke may be induced through endogenous neurogenesis (Wei et al., 2003).
Angiogenesis is coupled with neurogenesis in the brain, and neurogenesis occurs within an angiogenic niche (Palmer et al., 2000). The aggregation of neuroblasts around astrocytic processes and blood vessels (Fasolo et al., 2002), suggests that the blood vessels may play an important role in neuroblast migration to injured regions. One common thread that connects angiogenesis and neurogenesis is the vascular endothelial growth factor (VEGF), which was identified on the basis of its vascular effects, but has since been recognized as an important signaling molecule in neurogenesis as well (Jin et al., 2002; Wei et al., 2005). Another link between angiogenesis and neurogenesis is that after angiogenic stimulation, endothelial cells secrete brain-derived neurotrophic factor (BDNF), which induces neurogenesis (Greenberg and Jin, 2005).
The chemokine stromal cell derived factor-1 (SDF-1, also known as CXCL12) is a constitutively expressed and inducible chemokine that regulates multiple physiological and pathological processes in the central nervous system (CNS) via interaction with its CXC chemokine receptor 4 (CXCR4) (Li and Ransohoff, 2008). Extensive evidence supports the idea that the SDF-1/CXCR4 pathway plays an important role after cerebral ischemia, both in directing the migration of neuroblasts to lesioned sites (Ceradini et al., 2004; Shyu et al., 2008), and in augmenting ischemic neovascularization in the damaged tissue (Yamaguchi et al., 2003; Hiasa et al., 2004). Thus, SDF-1/CXCR4 signaling plays key roles in regenerative responses in the post-ischemic brain.
Peripheral stimulation and activity have significant ‘use-dependent’ impacts on functional and morphologic alterations in the CNS and on outcomes of CNS disorders. Using the rodent model of focal ischemia in the whisker barrel cortex, we have previously shown post-ischemic endothelial cell proliferation and neurovascular remodeling in and around the ischemic barrel cortex. Enhanced whisker activity created an “enriched environment” that attenuated ischemia-induced endothelial cell death, increased the expression of angiogenic factors, promoted angiogenesis, and helped to restore local blood flow to the ischemic and penumbra regions (Whitaker et al., 2007). In the present investigation, we tested the hypothesis that in addition to its angiogenic action, the enriched environment of increased peripheral activity can also promote neuroblast migration and neurogenesis in the post ischemic brain.
Materials and methods
Animals and focal ischemia of the whisker-barrel cortex
Adult male 129S2/Sv mice weighing between 25 and 30 g and in the age range of 2.5 to 3 months were used in this study. The focal barrel cortex ischemic stroke was induced as previously described (Whitaker et al., 2007). Briefly, animals were subjected to 4% chloral hydrate intraperitoneal (i.p.) anesthesia, and the right middle cerebral artery (MCA) supplying the barrel cortex, was permanently ligated by a 10-0 suture (Surgical Specialties CO., Reading, PA, USA). This was accompanied by 10-min bilateral common carotid artery (CCA) ligation. During surgery and recovery periods, body temperature was monitored and maintained at 37 °C using a temperature control unit and heating pads. In sham-operated animals, the same procedure was performed without ligation of MCA and CCAs. Before and after the surgery, animals were housed in standard mice cages (27×17×13 cm), at 3 to 4 mice per cage. Animal had free access to food and water. They were sacrificed by decapitation 14 or 30 days after ischemic stroke. The brain was immediately removed and preserved in optimal cutting temperature compound (Sakura Finetek, Inc., Torrance, CA, USA) at −80 °C for further processing. All animal experiments and surgery procedures were approved by the University Animal Research Committee and met the NIH standard.
Experimental design
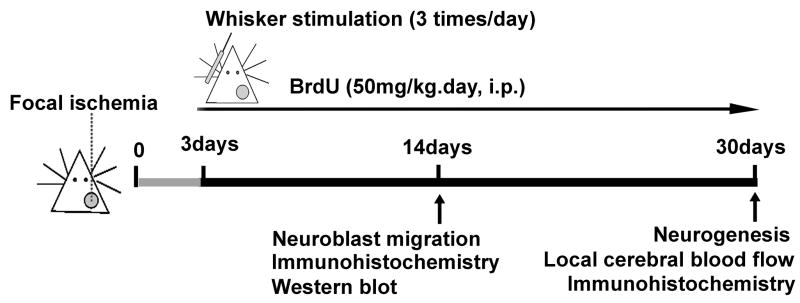
The design of the experiments is illustrated in Figure 1. Three days after ischemia, mice were randomly divided into ischemic stroke-only group and stroke plus whisker stimulation group. For whisker stimulation, whiskers on the mouse’s left face were swiped in a rostro-caudal direction using a stick for 15 min at about 140 strokes per minute, three times per day. During and after the stimulation, animals were allowed move freely in the cage and no anxious behavior was observed. To label proliferating cells, 5-bromo-2′-deoxyuridine (BrdU) (Sigma, St Louis, MO, USA) was administered to all animals (50 mg/kg/day, intraperitoneal injection) beginning on day 3 after ischemia and continued once daily until sacrifice.
Figure 1. Experimental protocol outline.
Adult mice were subjected to MCA/CAA ligations that induced focal ischemic stroke to the right sensorimotor cortex or sham operation. The gray circle represents the ischemic region including the barrel cortex in the right hemisphere. Three days after ischemia, all mice received BrdU injection (50mg/kg/day, i.p.) and continued once daily until sacrifice. Ischemic mice were randomly divided into ischemic stroke-only group and stroke plus whisker stimulation group. For whisker stimulation group, whiskers on the left (contralateral) face were manually stimulated for 15 min, three times per day. Western blot and immunohistochemistry were performed 14 days after stroke to analyze proteins expression, cell proliferation, and neuroblast migration. Immunohistochemistry and laser Doppler scanner measurement were performed 30 days after stroke to test neurogenesis and local cerebral blood flow.
Western blot analysis
The peri-infarct region was defined as previously described by a 500 μm boundary extending from the edge of the infarct core, medial and lateral to the infarct (Ohab et al., 2006). Tissue samples were taken from the peri-infarct region of the cortex and proteins were extracted by homogenization in protein lysis buffer (25mM Tris-HCl (pH 7.4), 150mM NaCl, 5mM EDTA, 0.1% SDS, 2mM sodium orthovanadate, 100 mM NaF, 1% Triton, leupeptin, aprotinin, and pepstatin). Tissue was centrifuged at 13,000 rpm for 20 minutes to pellet insoluble fraction and supernatant was collected. Protein concentration of each sample was determined using the Bicinchoninic Acid Assay (Sigma, St Louis, MO, USA). Proteins from each sample (50 mg) were separated by SDS-polyacrylamide gel electrophoresis in a Hoefer Mini-Gel system (Amersham Biosciences, Piscataway, NJ, USA) and transferred in the Hoefer Transfer Tank (Amersham Biosciences, Piscataway, NJ, USA) to a PVDF membrane (BioRad, Hercules, CA, USA). Membranes were blocked in 7% evaporated milk diluted in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) at room temperature for at least 2 h, and then incubated overnight at 4 °C with one of the following primary antibodies: VEGF, BDNF, SDF-1 (1:500–1:4000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Mouse β-actin antibody (Sigma) was used for protein loading control. After primary antibody incubation, membranes were washed with TBS-T and incubated with alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgG antibodies (Promega, Madison, WI, USA) for 2 h at room temperature. Finally, membranes were washed with TBST followed by three washes with TBS. The signal was detected by the addition of 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (BCIP/NBT) solution (Sigma), quantified and analyzed by the imaging software ImageJ (NIH Image, USA) and Photoshop Professional (Adobes Photoshops CS 8.0, San Jose, CA, USA). The intensity of each band was measured and subtracted by the background. The expression ratio of each target protein was then normalized against β-actin.
Immunohistochemistry and cell counting
Fresh frozen brains were sliced into coronal sections at 14-μm thick using a cryostat vibratome (Ultapro 5000, St Louis, MO, USA). Immunohistochemistry was performed as previously described (Whitaker et al., 2007). Primary antibodies used for single- or double-staining were as follows: rabbit anti-Glut-1 (1:1,000; Chemicon, Temecula, CA, USA) for endothelial cells, mouse anti-NeuN (1:400; Chemicon) for mature neurons, rat anti-BrdU (1:400; Abcam, Cambridge, UK) for cell proliferation, goat anti-doublecortin (DCX; 1:200; Santa Cruz Biotechnology) for migrating neuroblasts, and rabbit polyclonal anti-glial fibrillary acidic protein (GFAP; 1:500; Sigma) for astrocytes. Additional antibodies included mouse anti-SDF-1 (1:200; Santa Cruz Biotechnology), mouse anti-CXCR4 (1:200; Santa Cruz Biotechnology), rabbit anti-VEGFR2/Flk-1 (1:200; Chemicon) and, secondary antibody Alexa Fluor 488 anti-rabbit, anti-goat, anti-mouse IgG (1:200; Invitrogen, Carlsbad, CA), or Cy3-conjugated anti-rat, anti-rabbit IgG (1:1000; Invitrogen). Staining was visualized by fluorescent and confocal microscopy (BX61; Olympus, Tokyo, Japan).
For systematic random sampling in design-based stereological cell counting, three coronal brain sections per mouse were selected, spaced 276 μm apart across the same region of interest in each animal (from bregma + 0.5mm to bregma − 0.5mm). For multi-stage random sampling, four fields per brain section were randomly chosen in ischemic border region under 20× magnification of a light microscope or in confocal images. Neurogenesis in the ischemic border region was evaluated by counting the number of NeuN/BrdU-colabeled cells. BrdU-positive cells were counted in the SVZ of ischemic hemispheres. Neuroblast migration was evaluated by counting the number of DCX-positive and DCX/BrdU-colabeled cells in white matter between the SVZ and ischemic cortex. All counting assays were performed under blind condition.
Local Cerebral Blood Flow Measurement (LCBF)
Laser scanning imaging was used to measure LCBF as previously described (Li et al., 2007) at three time points: immediately before ligation, right after occlusion, and 30 days after ischemia. Briefly, under anesthesia, a crossing skin incision was made on the head to expose the whole skull. Laser scanning imaging measurements and analysis were performed using the PeriScans system and LDPIwin 2s (Perimed AB, Stockholm, Sweden) on the intact skull. The scanning region had a center point of ML+ 4.1mm, and the four edges of the infarct area were ML+ 2.9mm, ML+ 5.3mm, AP−1.5mm, and AP+ 2.0mm, respectively. In laser scanning imaging, the ‘single mode’ with medium resolution was used to scan the photo image of LCBF. The laser beam was pointed to the center of the ischemic core (ML + 4.1 mm, AP 0 mm), the scan range parameter was set up as 5×5 and the intensity was adjusted to 7.5 to 8.0. The conventional ‘duplex mode’ was used to record the Doppler image with the laser beam pointed to exact the same point on the border of the stroke core (ML- 0, 5 mm, AP 0 mm). Corresponding areas in the contralateral hemisphere were similarly surveyed as internal controls.
Statistical Analysis
Multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by LSD test for post hoc analysis. Changes were identified as significant if P value was less than 0.05. Mean values were reported together with the standard error of mean (SEM).
Results
Whisker stimulation increased expression of neurovascular regulatory proteins in the ischemic barrel cortex
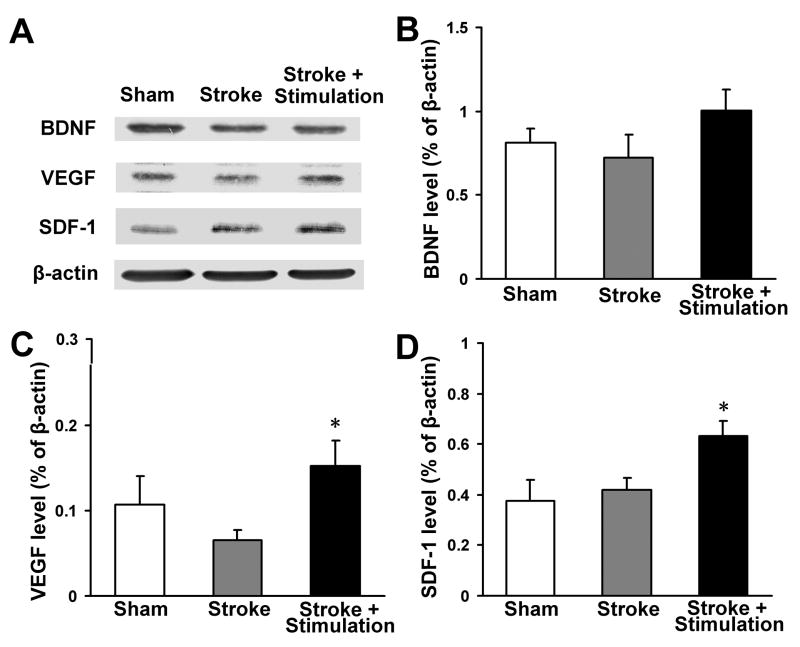
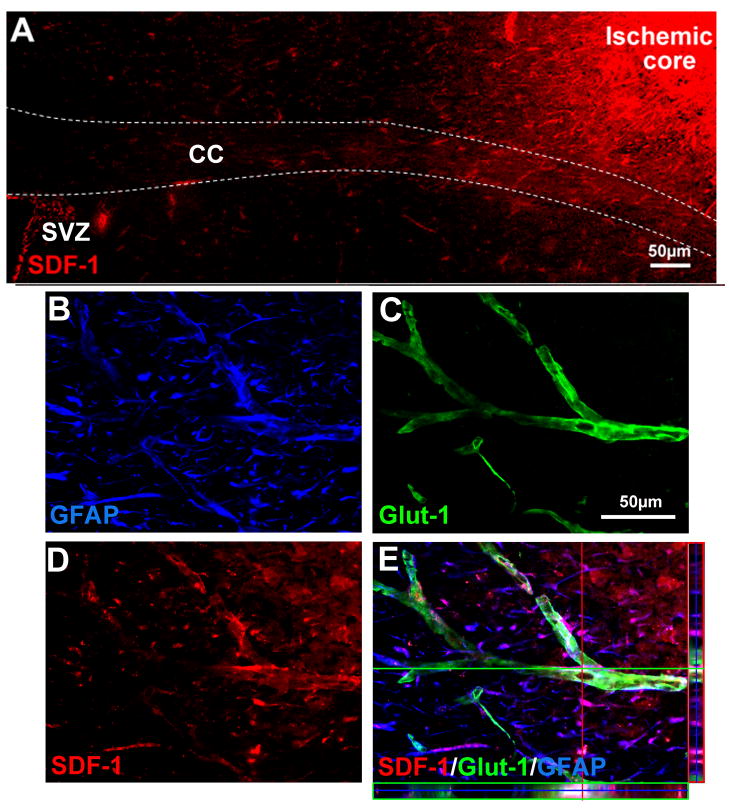
In the ischemic peri-infarct region, expression of VEGF and key factors in neurogenesis and neuroblast migration, SDF-1 and BDNF, were analyzed using Western blot. In stroke-only mice, the protein level of VEGF was transiently increased at 7 days after stroke (data not shown), but returned to the basal level 14 days after stroke (Fig. 2A and 2C). In stroke plus whisker stimulation animals, however, VEGF remained at higher levels, which is consistent with our previous observation (Whitaker et al., 2007) (Fig. 2A and 2C). In the case of SDF-1 expression, there was also significant increase in whisker stimulation group compared with stroke-only and sham-operated groups (Fig. 2A and 2D). Meanwhile, whisker stimulation showed a noticeable trend of increasing BDNF expression (Fig. 2A and 2B). Immunohistochemical assessment revealed that SDF-1 immunoreactivity was mainly located in the infarct core and peri-infarct regions and, interestingly, SDF-1 expression was extended from the infarct core to the SVZ (Fig. 3A). Confocal microscopy showed that most SDF-1-positive cells were also stained with Glut-1 or GFAP, suggesting they were vessel endothelial cells or astrocytes (Fig. 3B, 3C and 3D).
Figure 2. Effects of whisker stimulation on expression of neurovascular regulatory factors.
The protein levels of VEGF, BDNF and SDF-1 were detected using Western blot analysis. (A) Representative electrophoresis gels show the expression level of VEGF, BDNF and SDF-1 in the ischemic peri-infarct region at 14 days after stroke. (B–D) Densitometry analysis for comparisons of each factor. Gray intensity was normalized against β-actin and quantified using ImageJ software. Whisker stimulation enhanced the expression of VEGF (C) and SDF-1 (D) compared with stroke-only group. There was a trend of increased BDNF expression in animals treated with whisker stimulation (B). N=4 animals for each test. Data are expressed as mean ± SEM. *. P<0.05 compared with stroke-only controls.
Figure 3. SDF-1 expression in the ischemic penumbra region.
SDF-1 expression was detected 14 days after ischemia using immunostaining. Glut-1 (green) and GFAP (blue) staining revealed microvessels and astrocytes, respectively. (A) SDF-1 (red) expression mainly located in the infarct core and ischemic boundary regions and extended to the SVZ. (B–E) Three-dimensional confocal image showed GFAP-positive astrocytes (B), Glut-1-positive microvessels (C), SDF-1-positive cells (D) and the merged image of SDF-1/Glut-1/GFAP (E). The colocalization suggested that much of SDF-1 expression was located in microvessels and astrocytes. SVZ: subventricular zone; CC, corpus callosum.
Whisker stimulation enhanced neuroblast migration after barrel cortex ischemia
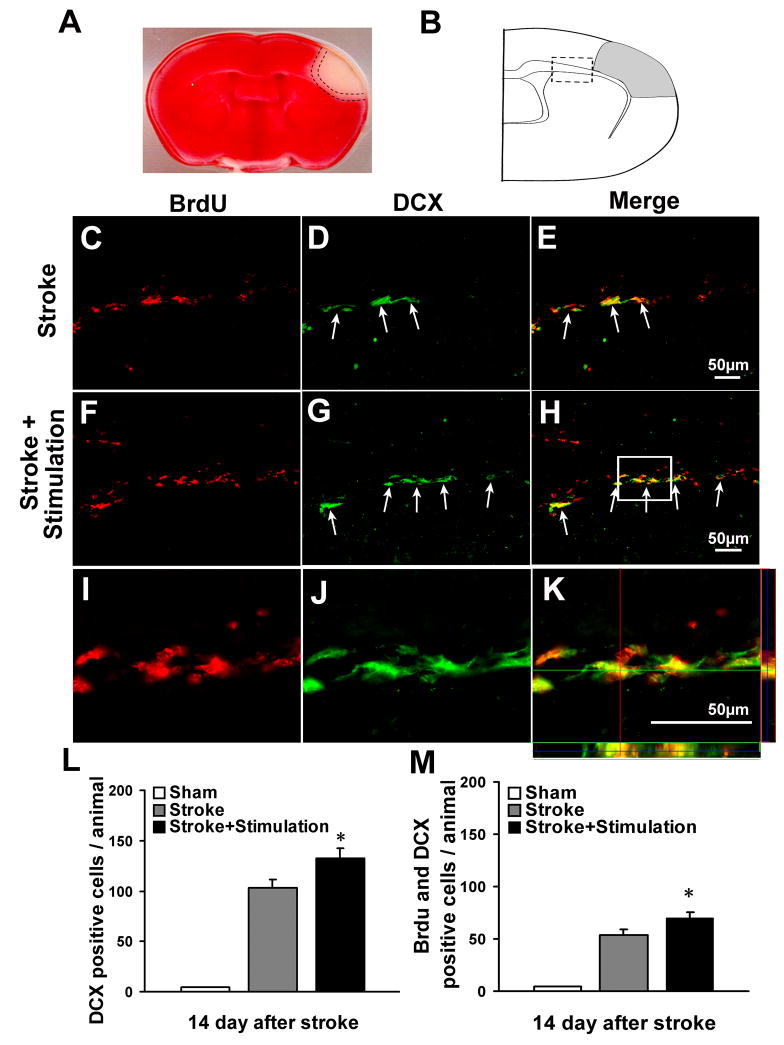
After focal ischemia, SVZ derived neural progenitor cells or neuroblasts tend to migrate towards the injured areas of the brain. Migrating cells can be identified by expression of the microtubule-associated protein doublecortin (DCX) (Couillard-Despres et al., 2005). To determine the effect of whisker stimulation on neuroblast migration, we performed immunofluorescence double labeling of DCX and BrdU at 14 days after stroke. Large numbers of DCX-positive cells were interspersed in the white matter between ipsilateral SVZ and ischemic cortex after ischemia (Fig. 4A, 4B and 4C), whereas in the sham-operated group, DCX-positive cells were mainly located in SVZ. Whisker stimulation group showed a significant increase in the number of DCX-positive cells and these cells moved further away from SVZ compared with stroke-only group (Fig. 4B and 4D). We observed about 50% of the DCX-positive cells were incorporated with BrdU, indicating the recent proliferative activity of these migrating cells. There were more DCX/BrdU-positive cells in mice that received stroke plus whisker stimulation than in stroke-only mice (Fig. 4E). Although ischemia increased BrdU-positive cells in the ipsilateral SVZ, there was a trend of decrease of BrdU-positive cells in the ipsilateral SVZ in whisker stimulation group, which was consistent with the enhanced cell migration from SVZ (Fig. 5).
Figure 4. Whisker stimulation enhanced neuroblast migration.
The effects of whisker stimulation on neuroblasts migration were examined by immunostaining of BrdU (red) and DCX (green) 14 days after the onset of ischemia. (A) Selective damage occurred to the right barrel cortex region shown as a negative (white) area in TTC staining (red) 3 days after ischemia. Dotted lines represent penumbra region. (B) Schematic diagram of coronal brain section for immunostaining, gray area indicates ischemia region. Analysis of neuroblasts migration was focused on the white matter (boxed area) between ipsilateral SVZ and the ischemic region, the total number of cells in 3 sections per animal was counted under fluorescent microscopy according to the stereological principal. (C–H) Double-labeling of BrdU (C, F), DCX (D, G), and the merged image of DCX/BrdU (E, H) in white matter of ischemic stroke-only (C, D, E) and whisker stimulation mice (F, G, H) 14 days after ischemia. Whisker stimulation promoted more DCX-positive and DCX/BrdU-positive cells moving toward the ischemic cortex. (I–K) High-power confocal image of colocalization of DCX (I), BrdU (J) and the merged image of DCX/BrdU from the frame in H. (L–M) Summarized data of neuroblast migration. The total numbers of DCX-positive and DCX/BrdU-positive cells in 3 sections of each animal were counted. There were more DCX-positive (L) and DCX/BrdU-positive cells (M) in whisker stimulation mice 14 days after stroke, while no DCX-positive or DCX/BrdU-positive cells were detected in sham-operated mice. N=6 animals in each group. Data are expressed as mean ± SEM. *. P<0.05, compared with ischemic stroke-only group by one-way analysis of variance (ANOVA).
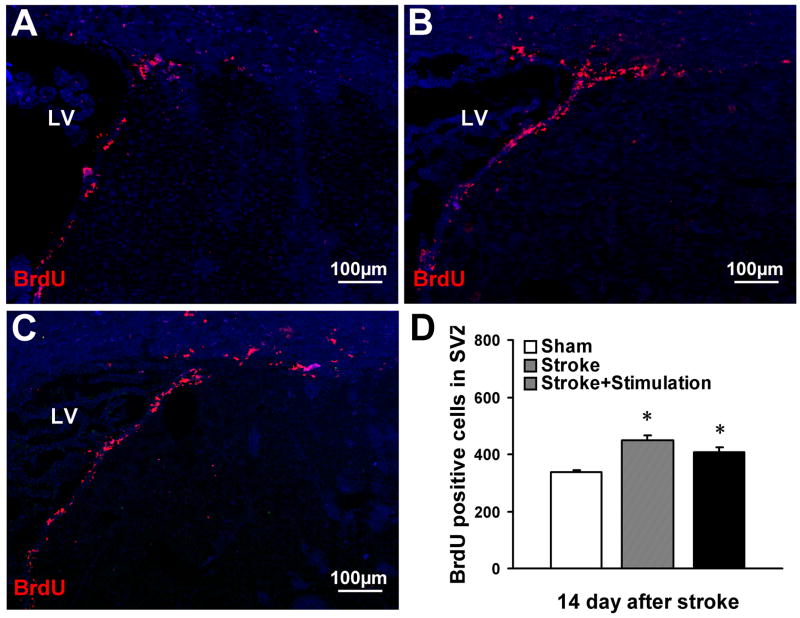
Figure 5. The effect of whisker stimulation on cell proliferation in the SVZ.
Proliferation of stem cells and progenitor cells in the SVZ was evaluated by BrdU-positive cells 14 days after stroke in sham-operated mice (A), stroke-only mice (B) and whisker stimulation mice (C). (D) Ischemia significantly increased the number of BrdU-positive cells in the ipsilateral SVZ, measured 14 days post-ischemia, in both ischemic stroke-only mice and whisker stimulation mice compared with sham-operated mice. The number of BrdU-positive cells had no significant difference between stroke-only mice and whisker stimulation mice. N=6 animals in each group. Data are expressed as mean ± SEM. *. P<0.05, compared with sham-operated group by one-way ANOVA. LV: lateral ventricle.
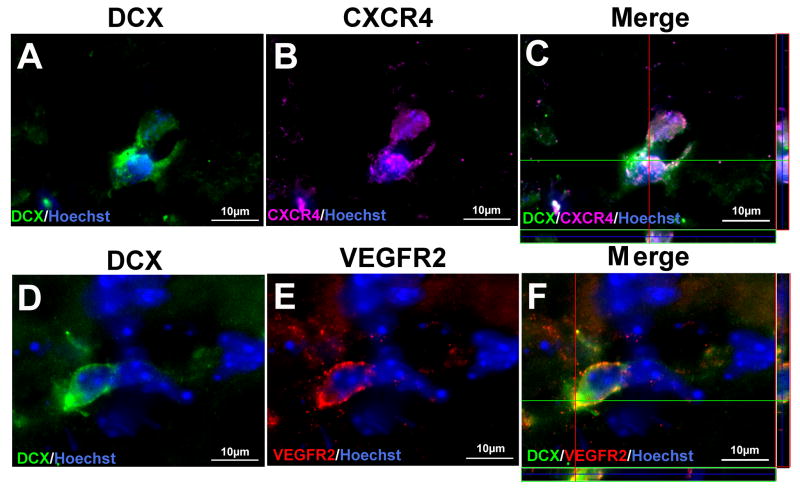
Immunofluorescent double labeling for DCX and the SDF-1 receptor CXCR4, or DCX and VEGFR2 showed that both CXCR4 and VEGFR2 colocalized in DCX positive cells along the migration path (Fig. 6).
Figure 6. Migrating neuroblasts express chemotactic factors receptors.
Immunofluorescent double labeling for DCX (green) and CXCR4 (purple) or DCX (green) and VEGFR2 (red) were performed 14 days after stroke. (A–C) Three-dimensional confocal images of DCX (A), SDF-1 receptor: CXCR4 (B), and the merged image of DCX/CXCR4 (C), showing that DCX-positive cells co-expressed CXCR4. (D–F) Three-dimensional confocal images of DCX (D), VEGFR2 (C), and the merged image of DCX/VEGFR2 (F), showing colocalization of VEGFR2 and DCX. Nuclear staining with Hoechst 33258 is shown in blue.
Whisker stimulation enhanced neurogenesis in the ischemic barrel cortex
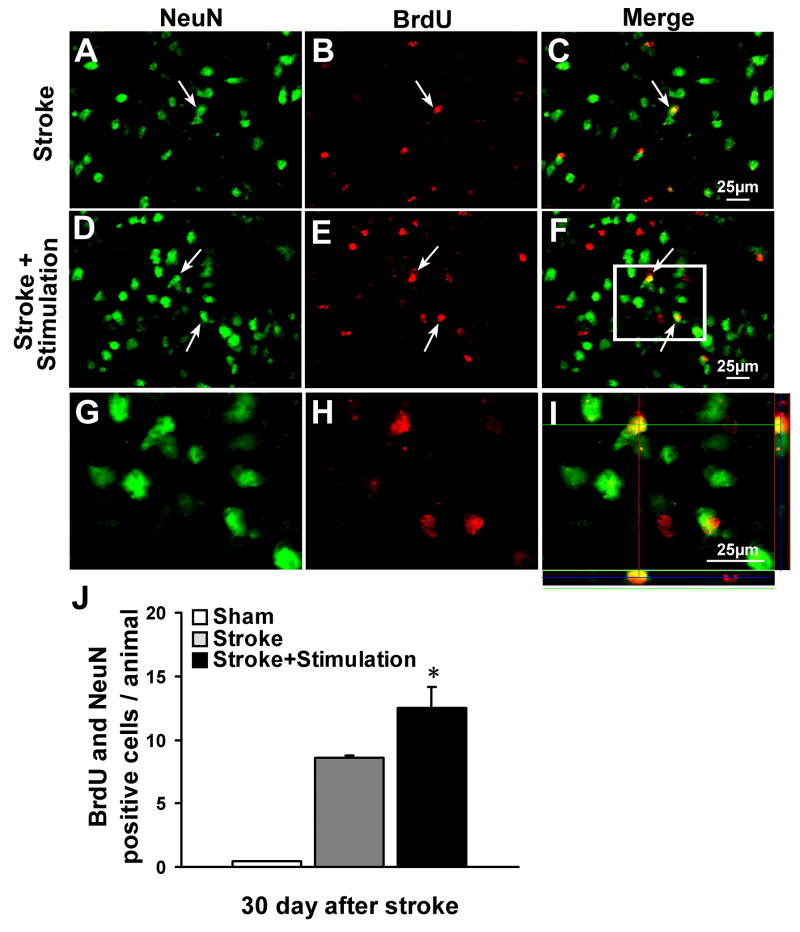
To determine whether newly generated neuroblasts differentiated into mature neurons in the peri-infarct region where they are needed for tissue repair, NeuN and BrdU were co-stained 30 days after stroke. In ischemic mice that received whisker stimulation, there was a much greater increase in the number of NeuN/BrdU-positive cells compared with the stroke-only controls. No NeuN/BrdU-positive cells were detected in sham-operated mice (Fig. 7).
Figure 7. Whisker stimulation enhanced neurogenesis.
Neurogenesis in the ischemic border region was examined by the colocalization of the neuronal marker NeuN (green) and the proliferation marker BrdU (red) 30 days after stroke. Double-labeling of NeuN-positive cells (A, D), BrdU -positive cells (B, E), and the merged image of NeuN/BrdU (C, F) in the ischemic peri-infarct region of ischemic stroke-only (A, B, C) and stroke plus whisker stimulation mice (D, E, F). There were more NeuN/BrdU-positive cells in whisker stimulation group compared with stroke-only group. (G–I) Enlarged confocal images of colocalization of NeuN (G), BrdU (H) and the merged image of NeuN/BrdU (I) from the frame in F. (J) Cell count was performed in four randomly chosen fields in the penumbra region per section. The total number of cells in three sections was summarized for each animal. Cell counts show increased numbers of NeuN/BrdU-positive cells in whisker stimulation group compared with stroke-only group; there were little NeuN/BrdU-positive cells in sham-operated mice. N=6 animals in each group. Data are expressed as mean ± SEM. *. P<0.05 compared with ischemic stroke-only group by one-way ANOVA.
Restoration of local cerebral blood flow in the post-ischemic cortex by whisker stimulation
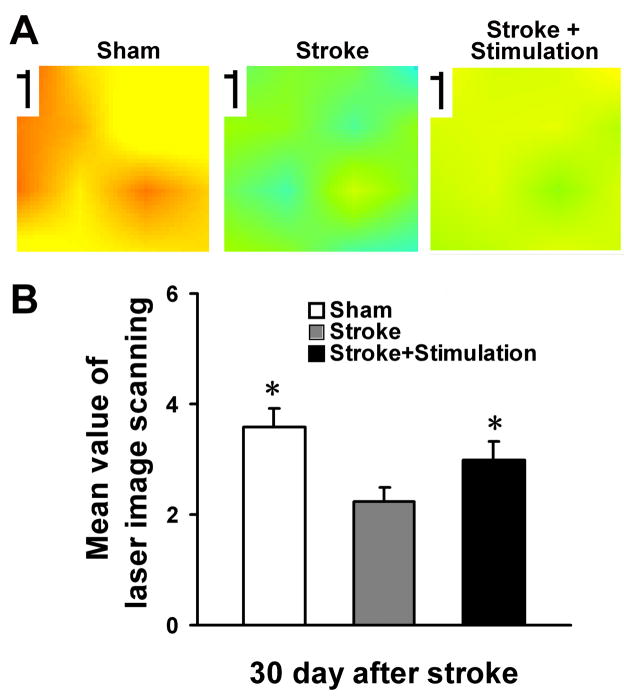
Local blood flow recovery is needed to ensure sustained cell survival and tissue repair after stroke. The local cerebral blood flow was measured using Laser Doppler imaging at 30 days after stroke. We observed that whisker stimulation enhanced higher local blood flow compared with the stroke-only group. The level of recovered flow reached near normal level with whisker stimulation (Fig. 8).
Figure 8. Restoration of local cerebral blood flow in the ischemic cortex.
Local cerebral blood flow was measured using the PeriScans laser image scanner. (A) Laser scanning images of LCBF in stroke region 30 days after ischemia in sham-operated mouse, stroke-only mouse and stroke plus whisker stimulation mouse. Marked LCBF recovery can be seen in the mouse received stroke and whisker stimulation. (B) Average of mean image values 30 days after ischemia. Whisker stimulation mice showed significant higher local blood flow compared with stoke only group. N=6 animals in each group. Data are expressed as mean ± SEM. *. P<0.05 compared with stroke-only group by one-way ANOVA.
Discussion
We report in this investigation that peripheral stimulation targeting the ischemic cortex helps to recruit more endogenous neuroblasts from the SVZ to migrate to the lesioned barrel cortex, presumably mediated by increased SDF-1/CXCR4 signaling (Imitola et al., 2004; Sun et al., 2004; Ohab et al., 2006; Robin et al., 2006). This effect combined with the increased VEGF expression and angiogenesis induced by whisker stimulation provide evidence that adequate peripheral activity as a means of physical therapy may benefit the post-ischemic tissue repair in the CNS.
The adult brain harbors two neuroproliferative zones, the subventricular zone (SVZ) and subgranular zone (SGZ), which normally supply neural progenitors to the olfactory bulb and the dentate gyrus of the hippocampus, respectively. Cerebral ischemia alters this normal pattern of adult neurogenesis in two ways: it enhances cell proliferation within the SVZ and SGZ and evokes migration of neuroblasts into areas of damage (Jin et al., 2003; Thored et al., 2006; Zhang et al., 2007). Enhancing this post-stroke neurogenesis and cell migration is expected to promote tissue repair and improve functional outcomes of stroke (Zheng and Chen, 2007). Peripheral stimuli and physical activity have significant ‘use-dependent’ impacts on the morphological and functional alterations in the adult brain after stroke, the mechanisms of which have been shown to be associated with enhanced angiogenesis and neurogenesis. For example, exercise preconditioning ameliorates inflammatory responses and brain damage in ischemic rats (Ding et al., 2005). Exposure to an enriched environment after focal cerebral ischemia resulted in enhanced proliferation of neural stem/progenitor cells and neurogenesis in SVZ and a better functional performance in a battery of sensorimotor tasks (Komitova et al., 2005). Specific rehabilitative training of an impaired forelimb effectively stimulates dentate gyrus neurogenesis and improves spatial learning after focal cortical infarcts (Wurm et al., 2007). Rehabilitative therapies, therefore, favor the reorganization in the peri-lesion tissue, and contribute to the compensation and/or recovery of the impaired function. As shown in our previous study, whisker-barrel ischemia is an ideal rodent model for testing the use-dependent mechanism, having the advantages of the well-defined whisker-barrel sensorimotor pathway and feasible identification of corresponding morphological and functional changes (Whitaker et al., 2007). Using the same animal model, the present study showed that although the specific peripheral stimulation did not further increase the number of proliferating cells in the SVZ, it significantly promoted the migration of neuroblasts from the SVZ to the damaged barrel cortex. We also found increased maturation of new neurons that reached the ischemic penumbra region in whisker stimulation group 30 days after stroke. Our results suggest that appropriately increased peripheral activity and afferent signals to the ischemic cortex can recruit more endogenous neural stem cells to migrate to injured region and differentiate into mature neurons, which may imply beneficial effects of specific physical therapy on long-term recovery from ischemic stroke.
CXCR4 is the receptor for the CXC chemokine family member SDF-1 (Matthys et al., 2001). SDF-1/CXCR4 signaling has been shown to play a major role in directing the migration of neural stem cells to lesioned sites (Imitola et al., 2004). Neural progenitor cells, derived from adult rat SVZ and cultured in vitro, expressed the CXCR4 receptor and migrated toward a gradient signal of SDF-1 (Sun et al., 2004). SDF-1 protein expression is clearly upregulated in the periphery of the damaged striatal area within 24 hrs after 2-hr MCA occlusion (Miller et al., 2005; Thored et al., 2006). Blocking SDF-1 by a n4eutralizing antibody against CXCR4 significantly attenuated stroke-enhanced cell migration (Ohab et al., 2006; Robin et al., 2006). Our study reports the novel observation that peripheral stimulation after stroke enhances the SDF-1/CXCR4 signaling pathway and migration of SVZ-derived neuroblasts toward the damaged cortex. In addition, SDF-1/CXCR4 signaling plays important roles in angiogenesis, which has a close relationship with neuroblast migration and neurogenesis. A recent study demonstrates that the SDF-1/CXCR4 signaling axis induce angiogenesis by increasing expression of VEGF through the activation of PI3K/Akt pathway (Liang et al., 2007). This is consistent with our observations that SDF-1 expression is detected in vascular endothelial cells and whisker stimulation increases VEGF expression and angiogenesis.
VEGF is an angiogenic factor and exerts biologic functions through two closely related receptor tyrosine kinases VEGFR1 (flt-1) and VEGFR2 (flk-1). Most of the VEGF properties are mediated by its interaction with VEGFR2 (Waltenberger et al., 1994). VEGF/VEGFR2 has been shown to be essential for endothelial cell proliferation and formation of new microvessels (Breier et al., 1992). Very recent studies have shown that neuroblasts need the blood vessels to act as a scaffold to migrate to injured regions (Ohab et al., 2006; Thored et al., 2007). In addition to its role in inducing angiogenesis, VEGF also stimulates neurogenesis and axonal outgrowth, and promotes the growth and survival of neurons (Sun et al., 2003; Wang et al., 2007). VEGF is an attractive guidance cue for the migration of undifferentiated neural progenitors through VEGFR2 signal pathway (Zhang et al., 2003). We have found that whisker stimulation can increase VEGF expression, angiogenesis and the restoration of local cerebral blood flow (Wei et al., 2005; Whitaker et al., 2007). The increase in VEGF gene expression inspected using Western blot was relatively moderate, we assume that multiple gene regulations contribute to the observed neurogenesis and cell migration. It is expected that more intensive whisker stimulation should induce more elevated VEGF expression. We also report for the first time that VEGFR2 was specifically expressed in migrating neuroblasts, which provides the molecular basis for responding to increased VEGF upon peripheral stimulation. Likewise, BDNF regulates neuronal survival, cell migration, and synaptic function (Aguado et al., 2003; Gorski et al., 2003), which also have a trend of upregulation by whisker stimulation.
In summary, peripheral whisker activities may optimize the microenvironment of the barrel cortex by upregulating different factors to recruit more endogenous neural stem cells from SVZ, as well as create a more favorable microenvironment for cells survival, differentiation and guided neural network connections, which benefit the tissues repair and function recovery after ischemia. Further studies should be done to explore the molecular and cellular mechanisms involved in the regulation of neurovascular plasticity by peripheral stimulation or physical therapy, which may lead to the development of more specific and efficient therapeutic approaches for stroke patients.
Acknowledgments
This work was supported by NIH grants NS 37372, NS 045155, and NS 045810. The work was also supported by the NIH grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
References
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- Alberts MJ, Ovbiagele B. Current strategies for ischemic stroke prevention: role of multimodal combination therapies. J Neurol. 2007;254:1414–1426. doi: 10.1007/s00415-007-0569-9. [DOI] [PubMed] [Google Scholar]
- Asplund K, stegmayr B, Pelltonen M. From the twentieth to the twenty-first century: A public health perspective on stroke. In: Ginsberg M, Bogousslavsky J, editors. Cerebrovascular Disease: Pathophysiology, Diagnosis, and Management. Malden, MA: Blackwell Science; 1998. pp. 901–908. [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Ding YH, Young CN, Luan X, Li J, Rafols JA, Clark JC, McAllister JP, 2nd, Ding Y. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005;109:237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: a brief review. J Neuroimmune Pharmacol. 2007;2:284–289. doi: 10.1007/s11481-007-9073-3. [DOI] [PubMed] [Google Scholar]
- Fasolo A, Peretto P, Bonfanti L. Cell migration in the rostral migratory stream. Chem Senses. 2002;27:581–582. doi: 10.1093/chemse/27.6.581. [DOI] [PubMed] [Google Scholar]
- Goldstein LB. Acute ischemic stroke treatment in 2007. Circulation. 2007;116:1504–1514. doi: 10.1161/CIRCULATIONAHA.106.670885. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys P, Hatse S, Vermeire K, Wuyts A, Bridger G, Henson GW, De Clercq E, Billiau A, Schols D. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol. 2001;167:4686–4692. doi: 10.4049/jimmunol.167.8.4686. [DOI] [PubMed] [Google Scholar]
- Miller JT, Bartley JH, Wimborne HJ, Walker AL, Hess DC, Hill WD, Carroll JE. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yen PS, Su CY, Chen DC, Wang HJ, Li H. Stromal cell-derived factor-1 alpha promotes neuroprotection, angiogenesis, and mobilization/homing of bone marrow-derived cells in stroke rats. J Pharmacol Exp Ther. 2008;324:834–849. doi: 10.1124/jpet.107.127746. [DOI] [PubMed] [Google Scholar]
- Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Sawamoto K, Okano H. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. J Neurosci Res. 2005;81:776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Wei L, Keogh CL, Whitaker VR, Theus MH, Yu SP. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12:47–62. doi: 10.1016/j.pathophys.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Wei L, Yin K, Lee JM, Chao JY, Yu SP, Lin TN, Hsu CY. Restorative potential of angiogenesis after ischemic stroke. In: Maiese K, editor. Neuronal and Vascular Plasticity: Elucidating Basic Cellular Mechanisms for Future Therapeutic Discovery. Boston, Dordrecht, London: Kluwer Academic Publishers; 2003. pp. 75–95. [Google Scholar]
- Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- Wurm F, Keiner S, Kunze A, Witte OW, Redecker C. Effects of skilled forelimb training on hippocampal neurogenesis and spatial learning after focal cortical infarcts in the adult rat brain. Stroke. 2007;38:2833–2840. doi: 10.1161/STROKEAHA.107.485524. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Chen B. Effects of Pravastatin on neuroprotection and neurogenesis after cerebral ischemia in rats. Neurosci Bull. 2007;23:189–197. [PubMed] [Google Scholar]