Abstract
Trial-by-trial variability in local field potential (LFP), tissue partial pressure of oxygen (Po2), cerebral blood flow (CBF) and deoxyhemoglobin-weighted optical imaging of intrinsic signals (OIS) were tested in the rat somatosensory cortex while fixed electrical forepaw stimulation (1.0 ms pulses with amplitude of 1.2 mA at a frequency of 6 Hz) was repeatedly applied. The changes in the cerebral metabolic rate of oxygen (CMRO2) were also evaluated using a hypotension condition established by our group based on the administration of a vasodilator. Under normal conditions, CBF, Po2, and OIS showed positive signal changes (48%, 32%, and 0.42%, respectively) following stimulation. Over multiple trials, the CBF responses were well correlated with the integral of the LFP amplitudes (ΣLFP) (Rmean = 0.78), whereas a lower correlation was found between Po2 and ΣLFP (Rmean = 0.60) and between OIS and ΣLFP (Rmean = 0.54). Under the hypotension condition the LFP responses were preserved, but the CBF responses were suppressed and the Po2 and OIS changes were negative (−12% and −0.28%, respectively). In this condition, the trial-by-trial variations in Po2 and OIS were well correlated with the variability in ΣLFPs (Rmean = −0.77 and −0.76, respectively), indicating a single trial coupling between CMRO2 changes and ΣLFP. These findings show that CBF and CMRO2 signals are more directly correlated with neural activity compared to blood-oxygen sensitive methods such as OIS and BOLD fMRI.
Introduction
Since fMRI measures neural activity-induced changes in physiologic processes, i.e., local cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2), understanding the direct relationship between the fMRI signal and neural activity is fundamental. Interestingly, the variability in fMRI signals is relatively high even during repetition of simple tasks (Tegeler et al., 1999; Waldvogel et al., 2000). Many of these sources of variability are undesirable including some which are of physiologic origin, such as cardiac and respiratory fluctuations (Hu, et al., 1995; Glover, et al., 2000). In addition, the physiological baseline state has also been shown to impact the amplitude and shape of the BOLD fMRI response (Cohen, et al., 2002). Recent studies have also shown that trial-by-trial fMRI can allow the measurement of trial-by-trial fluctuations in neural activity (Eichler, et al., 2005; Debener, et al., 2005). For example, slow fluctuations in the fMRI signal have been used to map known brain systems (e.g. sensory) in the absence of stimulation (Biswal, et al., 1995), and they have been shown to be linearly superimposed with stimulation-related responses (Fox, et al., 2006). Simultaneous EEG and fMRI studies strongly suggest that a significant or at least measurable amount of variability also stems from stimulus-related neural activities. To validate the capability of trial-by-trial fMRI measurements to encode trial-by-trial variations in neural responses, it is important to investigate how these variabilities translate through the principal pathways of the hemodynamic response, namely neuro-metabolic (CMRO2) and neuro-vascular (CBF) coupling.
Optical imaging of intrinsic signals (OIS) can measure deoxyhemoglobin-weighted changes (Frostig et al., 1990; Malonek and Grinvald, 1996), and can be combined with recordings of electrical activity and tissue oxygen tension with relative ease compared to BOLD fMRI. It has been shown that the spatial specificity of deoxyhemoglobin-weighted OIS induced by neural activity (i.e., the increase in light reflectance) coincides with that of BOLD responses (Kennerley et al., 2005), suggesting that deoxyhemoglobin-weighted OIS can be used as a BOLD analogue. To test the variability in CMRO2 induced by neural activity, our laboratory adapted a vasodilator-induced hypotension model in which the vascular response to neural activity is specifically suppressed (Nagaoka et al., 2006). In that study, the cerebral blood volume (CBV) and BOLD fMRI responses to visual stimulation were measured and typical positive responses (i.e., increase in BOLD signal and CBV) were reported under control conditions. After infusion of the vasodilator sodium nitroprusside, the CBV response was suppressed and the BOLD fMRI response was negative. In another study by Fukuda, et al., spiking activity and deoxyhemoglobin-weighted OIS were measured in response to visual stimulation under control and hypotension conditions in the anesthetized cat and the neural response was shown to be preserved under the hypotension condition (Fukuda et al., 2006). Similarly, OIS showed activity-induced decreases in intensity (i.e., increase in light absorption), indicating that it predominantly represents the dynamic CMRO2 changes. By measuring the tissue partial pressure of oxygen (Po2), the absolute changes in CMRO2 can be determined from hypotension condition measurements. Further, the dynamic measurement of CMRO2 change can be compared with the dynamic changes in CBF obtained from control condition measurements in the same subject. This direct comparison allows the investigation of a neurovascular mechanism issue, which is whether the increases in CBF are controlled by elevations in CMRO2.
In the present study, single trial neuro-metabolic and neuro-vascular couplings were directly measured using an oxygen microelectrode, laser-Doppler flowmetry (LDF), and OIS concurrently performed with LFP recordings in the rat somatosensory cortex. Fixed forepaw stimulation was repeatedly applied under normal (control) and vasodilator-induced hypotension conditions. The dynamic CMRO2 change was evaluated from the hypotension condition data obtained in the same subject. In summary, we found that trial-by-trial variations in CMRO2, CBF and oxygenation level changes are all well correlated with trial-by-trial variations in LFP changes.
Materials and methods
Animal preparation
Six male Sprague-Dawley rats (400 to 560 g; Charles River Laboratories, Wilmington, MA) were used with experimental protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee in accordance with the standards for humane animal care and use as set by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals. The animals were anesthetized with isoflurane (5% for induction and 1.5 to 2% during surgery) via a vaporizer with a mixture of O2 (35 to 50%) and N2O (50 to 65%). Endotracheal intubation was performed, and catheters were placed into the femoral vein and artery. Minute ventilation volume and respiratory rate were adjusted with a ventilator (TOPO™, Kent Scientific Corp., Torrington, CT). Arterial blood sampling was periodically performed to measure systemic arterial blood Po2 (PaO2), PCO2 (PaCO2), pH, and hemoglobin concentrations (Hb) with a blood gas analyzer (Stat Profile®, Nova Biomedical Corporation, Waltham, MA). Systemic arterial blood pressure, heart rate, and respiratory parameters were monitored with polygraph data-acquisition software (AcqKnowledge, BIOPAC systems, Inc., Goleta, CA). Rectal temperature was maintained at 37.0 °C with a DC temperature control module (40-90-8C, FHC, Inc., Bowdoinham, ME).
The animal was placed in a stereotaxic frame (Narishige International USA, Inc., East Meadow, NY) and an area (5 mm × 7 mm) on the left skull, centered at 3.5 mm lateral and 0.5 mm rostral from the Bregma (Hall and Lindholm, 1974) was thinned with a drill to make a thinned skull preparation (Lindauer et al., 1993). After the completion of the animal preparation, the inspired gas was converted to a mixture of air and O2 (30 to 35% total O2) and the end-tidal isoflurane concentration was adjusted to 1.3 ± 0.1% (Antognini et al., 1999; Masamoto et al., 2007a). At first, preliminary mapping with OIS (pre-mapping) was performed for the localization of the forepaw area in the primary somatosensory cortex. Based on this OIS map, the probes were arranged. The concurrent recordings of LFP, CBF, Po2, and OIS were then performed. The vasodilator drug was intravenously injected, and the same experiment was duplicated under vasodilator-induced hypotension conditions. In some animals (N = 3), multiple sets of control and hypotension experiments were repeatedly conducted to increase the number of trials.
Forepaw stimulation
Two needle electrodes were inserted between digits two and four of the right forepaw (Silva et al., 1999). Electrical pulse stimulation (1.0 ms pulse width, 1.2 mA current) was applied with a pulse generator and isolator (Master 8 and ISO-Flex, A.M.P.I, Israel). For pre-mapping, twenty runs of forepaw stimulation were performed with stimulus duration of 3 sec and frequency of 3 Hz with an inter-run time of 6 sec. For the remaining experiments, ten to sixty runs of forepaw stimulation were performed with stimulus duration of 10 sec and frequency of 6 Hz with an inter-run time of 80 sec. The stimulation protocol was previously refined to induce robust LFP, CBF, and BOLD responses (Masamoto et al., 2007a).
Hypotension condition
Sodium-nitroprusside (0.5 mg/ml in saline), a rapid-acting vasodilator, was used to suppress the vascular responses to neural activity (Fukuda et al., 2006; Nagaoka et al., 2006). Its pharmacological action is principally the relaxation of vascular smooth muscle, leading to a dilation of peripheral arteries and veins, and a consequent decrease in the arterial blood pressure (Auer, 1978; Roos et al., 1994). While the drug was continuously injected (0.03 to 0.11 ml/min, i.v.), the mean arterial blood pressure was stabilized between 35 and 45 mmHg. The recording commenced about 2 min after onset of injection. Upon completion of the recordings (~20 min), the drug administration was terminated. The total dosage was 2.2 ± 0.6 mg/kg (N = 6).
Pre-mapping
For localization of the forepaw area in the primary somatosensory cortex, deoxyhemoglobin-weighted OIS was performed with the irradiation of 620 ± 10 nm filtered light. The field of view was 4.9 mm × 3.7 mm and the matrix size was 320 × 240 after spatial binning of 2 × 2 pixels. The images were acquired at 30 frames per second and 15 consecutive frames (0.5 sec) were averaged during off-line analysis. The average of two pre-stimulation images was used as the control image (R) and the average of four images (from 0.5 sec to 2.5 sec) after the onset of stimulation was used as the activation image (R’). The activation focus was determined to be the area surrounding the largest decrease in light reflectance in the map (Narayan et al., 1994; Nemoto et al., 2004; Masamoto et al., 2007a).
Simultaneous recordings of LFP, CBF, Po2 and OIS
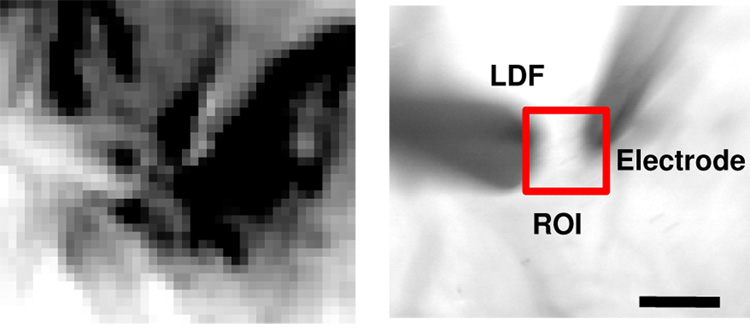
The LFP and Po2 were simultaneously recorded with a double-barrel type microelectrode: a Clark-type electrode for oxygen measurements and a platinum electrode for neural activity measurements (APOX, Unisense A/S, Aarhus N, Denmark). The electrode tip (30 µm outside diameter) was positioned at a depth of about 0.3 mm from the cortical surface on the activation focus (Masamoto et al., 2007a,b). The oxygen electrode was calibrated before and after each experiment with 0%, 21%, and 100% oxygen in saline solution (37 °C). The CBF around the activation focus was measured with a laser-Doppler flowmetry system (LDF; PeriFlux 4001Master, Perimed, Sweden) equipped with a needle-type probe (Probe 411, Perimed, Sweden). The probe was placed on the surface of the thinned skull covering the activation focus (see Fig. 1). Based on the OIS-determined activation area (Fig. 1A; dark pixels indicate an increase in light absorption due to an increase in deoxy-hemoglobin), the dual-barrel microelectrode and LDF probe were positioned within 0.5 mm of each other (Fig. 1B). The time constant of LDF system was 0.03 sec. The LFP, Po2 and LDF signals were recorded on a PC with BIOPAC data-acquisition software (AcqKnowledge, BIOPAC systems, Inc., Goleta, CA) at a rate of 1 kHz. OIS was also simultaneously performed at a rate of 30 frames per sec, as described above (see pre-mapping section).
Figure 1. OIS map and ROI selection for simultaneous recordings of LFP, Po2, CBF, and OIS.
A double-barrel type electrode (used to record LFP and Po2) and LDF probe were placed at the activation focus (left) determined by mapping the forepaw area of rat somatosensory cortex using OIS (pre-mapping). The electrode tip was positioned at a depth of ~0.3 mm from the cortical surface (right) and around the center of the LDF detection volume (~1 mm3). The position of electrode and LDF probe was within 0.5 mm of each other (right). The OIS time-series data were calculated by averaging the intensity over a 0.5 mm × 0.5 mm region of interest (ROI; within the red square) centered at the activation focus (scale bar = 0.5 mm).
Data processing
Among the six animals studied, five animals were studied using the same dual-barrel microelectrode, and the remaining animal was not. Due to different electrode sensitivities, this specific animal was not included in the population analysis. All population data are reported as mean ± SD (N = 5), unless otherwise specified. All the data processing was performed using Matlab (Mathworks Inc, Natick, MA).
For each trial, the LFP amplitude responding to each individual stimulation pulse was measured by the difference in amplitude between the negative peak and the positive peak observed within 20 msec after each stimulus. Then, the LFP amplitudes of all 60 pulses were summed to obtain the integrated LFP response of each trial (ΣLFP) (Masamoto et al., 2007a). To examine the variations in LFP responses, the coefficient of variance (CV) was determined for the each individual LFP amplitude (pulses #1 to #60 in a pulse train) across all trials in each subject. LDF (CBF), Po2, and OIS data were then downsampled to 5 Hz (i.e., 5 samples per second) by averaging consecutive data points to improve the signal-to-noise ratio. The OIS time series data were obtained from a 0.5 mm × 0.5 mm region of interest (ROI) centered on the activation focus (Fig. 1) for comparison with the LFP, CBF and Po2, data. The size of the ROI was selected in order to approximately cover the response volume of the Po2 and LDF measurements. The fractional changes in CBF and OIS were calculated by the difference between activation and baseline data divided by the baseline data, while absolute Po2 changes were determined by subtracting the baseline data from the activation data. The baseline data was defined as the average over 3 sec prior to the stimulation onset. Similarly, the stimulation data was defined as the mean of 3-sec period around the response maxima; specifically, 6–9 sec, 8–11 sec, and 6–9 sec after stimulation onset for the CBF, Po2, and OIS data under the control condition, and 6–9 sec, 9–12 sec, and 8–11 sec for the CBF, Po2, and OIS under the hypotension condition, respectively. Trial-by-trial changes in CBF, Po2 and OIS were then compared with corresponding ΣLFP data using linear regression within each subject tested. The significance of the slope being different from zero was assessed and reported using a t-test (their appropriate correlation coefficient was also calculated). Finally, a paired t-test was performed to compare the correlation results from the two experimental conditions performed in each subject.
The CBF, Po2 and OIS time courses were then averaged across all trials for each experiment in individual subjects to examine their dynamic properties. The time-to-%peak was determined as the time it takes for the signal to reach 10%, 50%, 90% and 100% of the peak amplitude, except for CBF under the hypotension since there was no detectable response peak in this condition.
Results
Neural and vascular responses under control and hypotension conditions
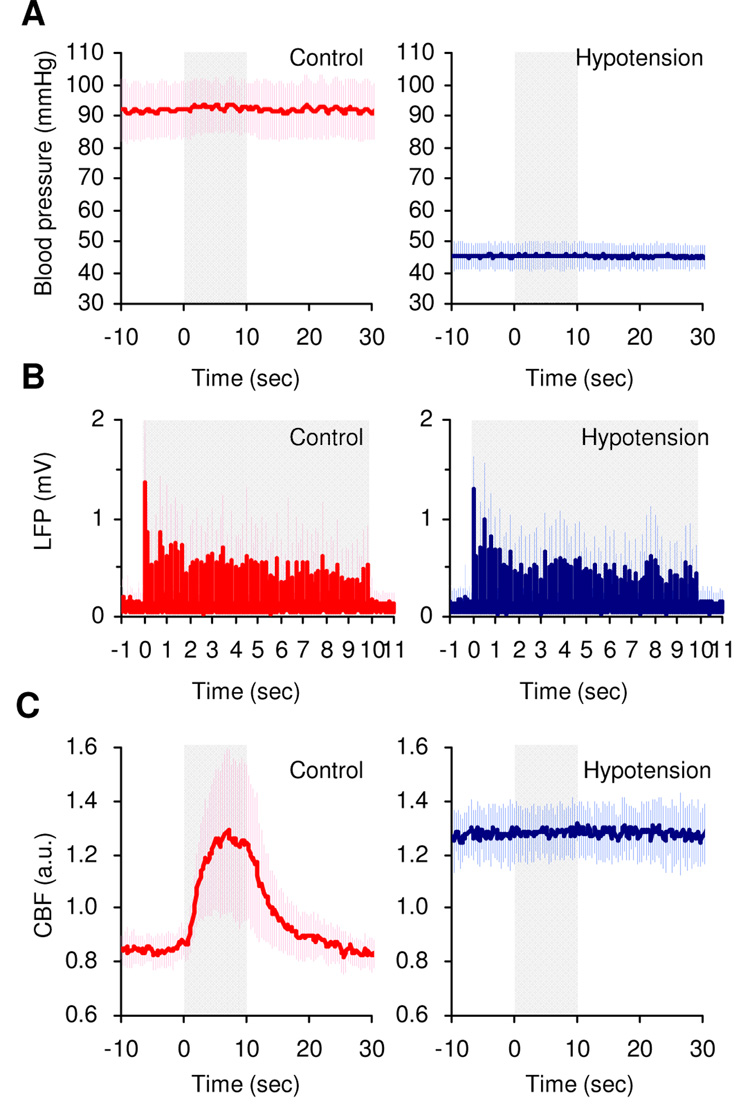
No significant differences in the arterial blood conditions were observed between control and vasodilator-induced hypotension conditions (pH = 7.465 ± 0.017 and 7.454 ± 0.018, PaCO2 = 39 ± 2 and 38 ± 3 mmHg, PaO2 = 114 ± 11 and 114 ± 13 mmHg, and Hb = 13 ± 1 and 13 ± 1 g/dL, respectively, N = 6 animals). Thus, we can compare data obtained during normal and hypotension conditions. Typical time-series of mean arterial blood pressure, LFP and CBF were compared between control and hypotension conditions in one representative animal (Figs. 2A–2C). A robust increase in CBF was observed under normal blood pressure conditions (~ 92 mmHg), while the CBF response was suppressed under the vasodilator-induced low blood pressure condition (~ 44 mmHg). However, no detectable difference in LFP activity was observed between control and hypotension conditions (Fig. 2B). Therefore, the neuro-vascular coupling in the normal condition can be directly compared with the neuro-metabolic coupling in the hypotension condition.
Figure 2. Comparison between control and hypotension conditions.
The effect of the vasodilator (right) was compared to the control condition (left; no vasodilator) in one representative animal. A. A significant drop in systemic arterial blood pressure was observed due to the effect of the vasodilator. Note that a stable blood pressure level was maintained during recording period and over all trials. The shaded area represents the forepaw stimulation period (10 sec). The error bars show the s.d. (n = 15 trials). B. LFP activity simultaneously recorded in the same trials are presented and no detectable differences in the LFP amplitude were observed between control and hypotension experiments. C. CBF responses simultaneously recorded in the same trials are presented. Under control conditions, a robust increase in CBF was evident (left). The CBF response was suppressed under hypotension conditions (right), while the CBF baseline level increased due to vasodilation effects (a.u.: arbitrary units).
Group-averaged CBF, Po2 and OIS responses
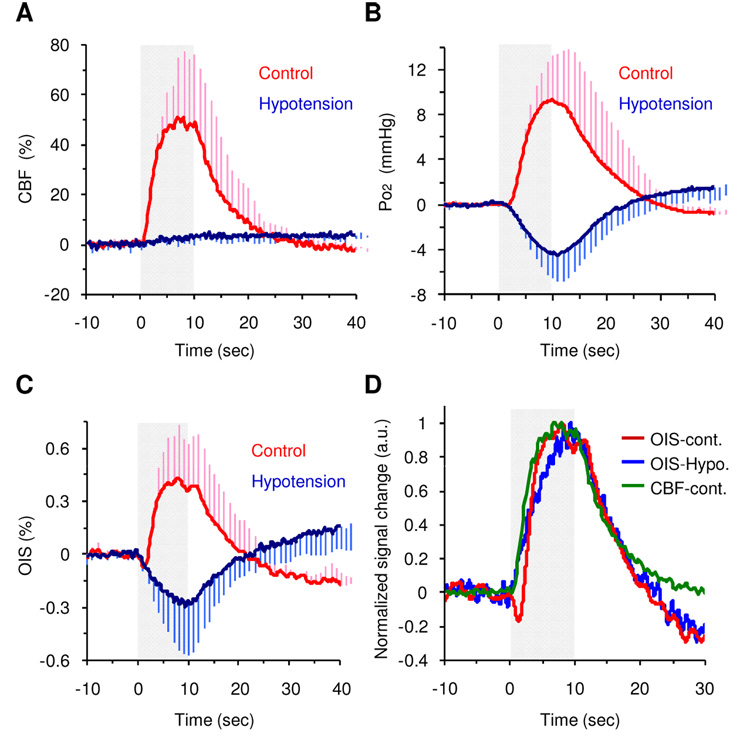
Averaged time courses of CBF, pO2 and OIS are shown in Figure 3A–C, and the magnitude of functional signal changes is shown in Table 1. During the control experiments, functional CBF, Po2 and OIS signals increased by 48 ± 23%, 32 ± 12% and 0.42 ± 0.29%, respectively. In contrast, under the hypotension condition, both Po2 and OIS decreased by 12 ± 9% and 0.28 ± 0.29%, respectively, while the CBF change was negligible (2 ± 2%). These results indicate that the Po2 and OIS changes under hypotension conditions were predominantly induced by a functional CMRO2 increase. Note that the baseline Po2, OIS (i.e., light reflectance) and CBF (LDF) were 47 ± 43% higher, 1.5 ± 2.0% lower, and 35 ± 18% higher under hypotension conditions compared to the respective control levels, which are due to vasodilator-induced effects. For OIS measurements, the temporal response obtained from a larger ROI was also examined (e.g. 2.0 mm × 2.0 mm), but the OIS time course was not significantly different from that of the selected ROI (data not shown), indicating that the functional response covered a sizable region.
Figure 3. Group averaged temporal dynamics.
Temporal dynamics of CBF (A), Po2 (B), and OIS (C) compared between control (red) and hypotension (blue) conditions. The signal was normalized by baseline (10-sec prestimulation data), and fixed forepaw stimulation (10 sec, gray shade) was repeatedly applied. For better visualization, error bars (s.d.) are shown in only one side. D. Normalized temporal dynamics of averaged OIS under control conditions (red), OIS under hypotension conditions (blue), and CBF under control conditions (green) compared. The signal was normalized by its peak point, and the magnitude of the OIS hypotension data is presented for easier comparison. Note that CBF and OIS-control signals peaked earlier than the OIS-hypotension signal.
Table 1.
Mean signal level during baseline and activation periods and relative signal change to baseline (mean ± s.d., n = 5)
| CBF (a.u.) | Po2 (mmHg) | OIS (a.u.) | ||||
|---|---|---|---|---|---|---|
| control | hypotension | control | hypotension | control | hypotension | |
| baseline | 1.2 ± 0.4 | 1.7 ± 0.5 | 33 ± 18 | 43 ± 13 | 7.48 ± 1.17 | 7.37 ± 1.17 |
| activation | 1.8 ± 0.4* | 1.7 ± 0.5 | 42 ± 20* | 38 ± 13* | 7.51 ± 1.15* | 7.35 ± 1.19 |
| signal change (%) | 48 ± 23 | 2 ± 2 | 32 ± 12 | −12 ± 9 | 0.42 ± 0.29 | −0.28 ± 0.29 |
Significant differences (paired t-test) between baseline and activation data (P < 0.01).
Dynamics of CBF, Po2 and OIS signals
The group time-to-%peak data are summarized in Table 2. The dynamic changes in CBF and CMRO2 can be visualized by the normalized LDF and OIS time courses under normal and hypotension conditions, respectively, plotted in Fig. 3D. The CBF response under normal conditions peaked earlier than the apparent changes in CMRO2 as represented by OIS (Fig. 3D). The time-to-10% peak for the CBF response under control conditions was observed to be 0.9 ± 0.3 sec, while that of the OIS signal under hypotension conditions was 0.6 ± 0.4 sec. However, the CBF response peaked 7.8 ± 1.8 sec after the stimulation onset, whereas the OIS response under hypotension conditions peaked 9.1 ± 1.4 sec after stimulation onset (Fig. 3D). Corresponding to this result, the peak of the positive OIS change under control conditions was observed to occur earlier than that in the hypotension condition (Fig. 3D). It is worth noting that although the time-courses for the OIS and Po2 responses were very similar (Figs. 3B and 3C), a relative lag of 1.4 ± 0.6 sec and 1.5 ± 1.3 sec was observed under control and hypotension conditions, respectively (Table 2). This difference is likely due to the temporal characteristics of different recording systems.
Table 2.
Time to % peak of CBF, Po2, and OIS (mean ± s.d., n = 5)
| Time to % peak (sec) | ||||
|---|---|---|---|---|
| 10% | 50% | 90% | 100% | |
| CBF | ||||
| Control | 0.9 ± 0.3 | 2.2 ± 0.6 | 5.2 ± 1.7 | 7.8 ± 1.8 |
| Hypotension | ---— | ---— | ---— | ---— |
| Po2 | ||||
| Control | 2.9 ± 0.2§ | 4.7 ± 0.6§ | 7.6 ± 1.9§ | 9.6 ± 2.6§ |
| Hypotension | 2.1 ± 0.7 | 4.9 ± 0.6 | 7.9 ± 1.2 | 10.6 ± 1.5 |
| OIS | ||||
| Control | 2.4 ± 0.3§,¶ | 3.3 ± 0.5§,¶ | 6.2 ± 2.6¶ | 7.2 ± 2.4¶ |
| Hypotension | 0.6 ± 0.4¶ | 2.5 ± 1.3¶ | 6.6 ± 2.2 | 9.1 ± 1.4¶ |
Significant differences (P < 0.05)
vs. CBF data
vs. Po2 data.
Trial-by-trial correlations
Variation of the ΣLFP was significant even within subjects. The CV of the LFP response to pulse #1 ranged 0.1 – 0.3 across the trials. In contrast, the rest of the LFP responses (pulse #2 to #60) showed a higher level of variations (CV = 0.3 – 0.7). We observed that the correlation analysis was not biased against the temporal integration of LFP (data not shown). Thus, ΣLFP was used for comparison with hemodynamic and metabolic responses.
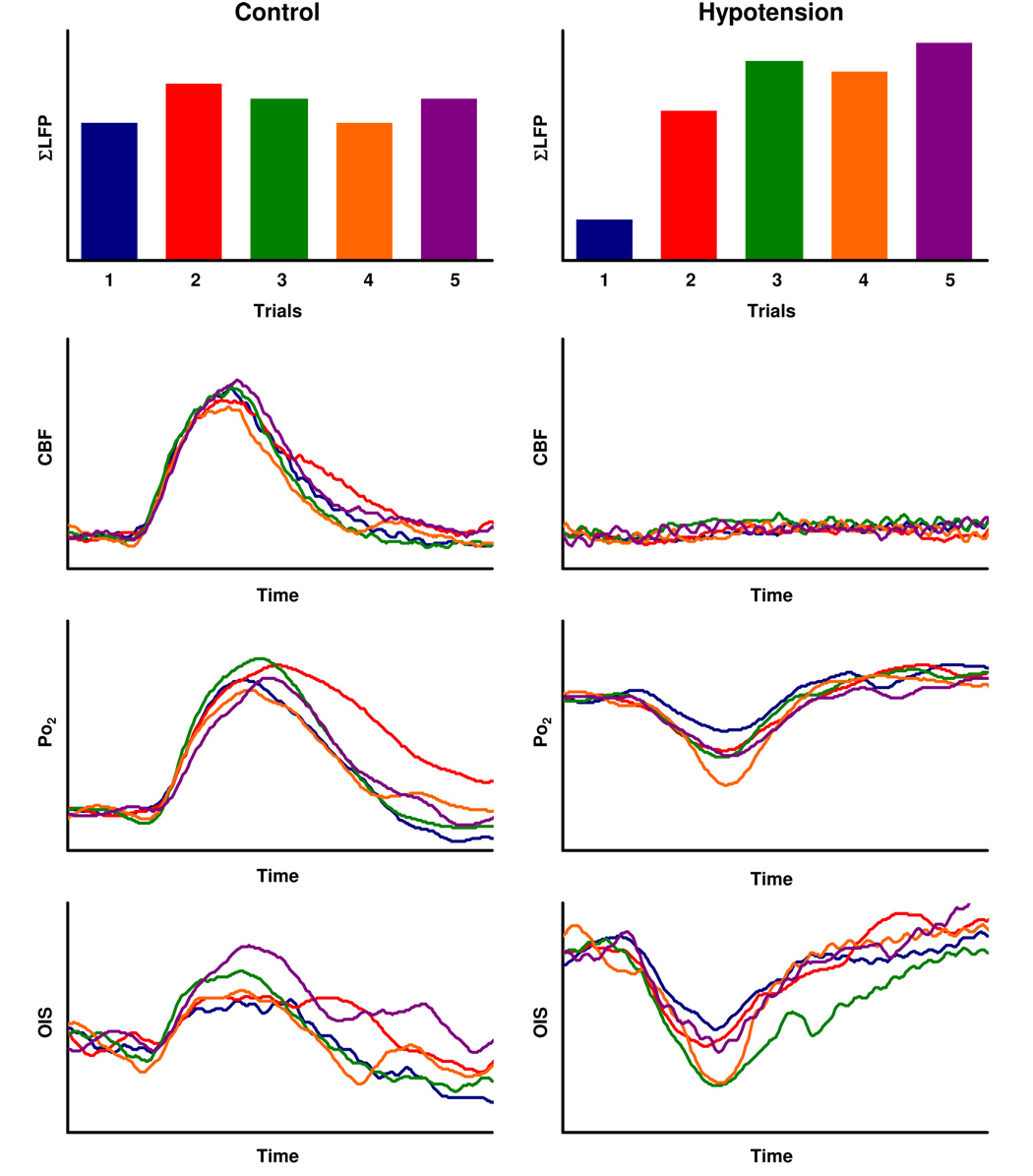
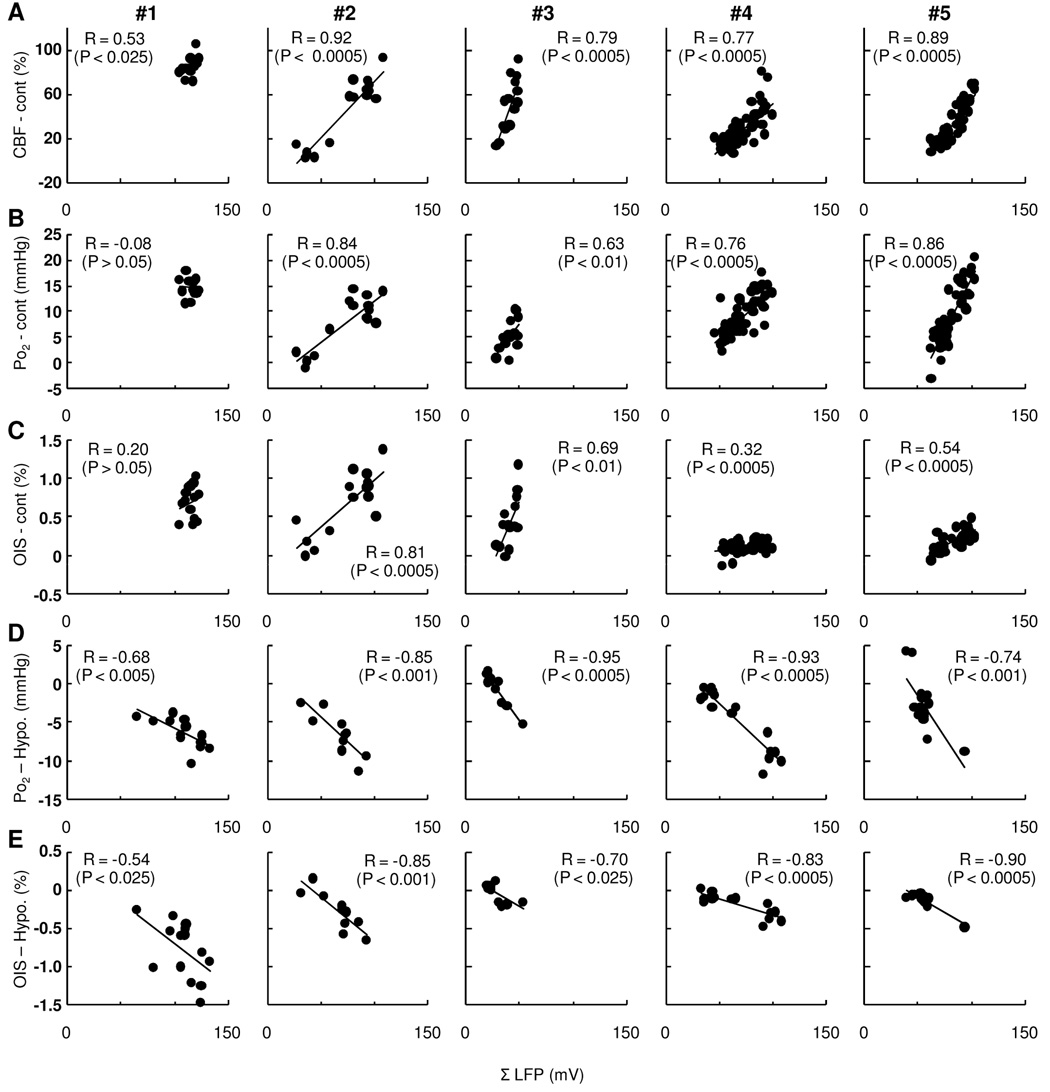
In single-trial basis, the response magnitude of CBF, Po2, and OIS also varied under either control or hypotension conditions (Fig. 4). The correlation analysis performed between the measured ΣLFP and CBF data on a trial-by-trial basis showed that the trial-basis variations were strongly correlated in all subjects under control conditions (t = 2.14 to 12.91, R = 0.53 to 0.92, p < 0.05 to 0.0001, Fig. 5A). In contrast, a lower correlation was found between ΣLFP and Po2 (t = −0.71 to 9.02 to R = −0.08 to 0.86, p < 0.10 to 0.0001, Fig. 5B) and between ΣLFP and OIS under control conditions (t = 0.70 to 4.93, R = 0.20 to 0.81, p < 0.50 to 0.005 Fig. 5C). Under hypotension conditions, a strong linear correlation between ΣLFP and Po2 was found (t = −2.23 to −10.39, R = −0.68 to −0.95, p < 0.05 to 0.0001, Fig. 5D). Similarly, the OIS data showed reasonably good correlation with ΣLFP (t = −2.23 to −7.34, R = −0.54 to −0.90, p < 0.05 to 0.0001, Fig. 5E). These results indicate that the variability in the CBF response and apparent changes in CMRO2 were well correlated with the trial-by-trial variations in ΣLFP.
Figure 4. Trial-by-trial variations in ΣLFP, CBF, Po2, and OIS.
Single trial-basis variations were compared across the initial five trials obtained under control (left) and hypotension (right) conditions from one representative animal. Each color line represents the normalized signal changes in response to ΣLFP shown in top panel. The signal changes in CBF, Po, and OIS were normalized by respective baseline level (3-sec prestimulus). For better comparison, each time-course data were shown after averaging successive 15 samples (3-sec time periods) in each trial.
Figure 5. Trial-by-trial basis correlations to ΣLFP in individual subjects (#1 – #5).
Activity-induced CBF (A), Po2 (B), and OIS (C) compared with ΣLFP simultaneously measured in control experiments (A–C). A strong linear correlation between CBF and ΣLFP was observed in all subjects (R = 0.53 to 0.92). In contrast, a lower correlation was found between Po2 and ΣLFP (R = −0.08 to 0.86) as well as between OIS and ΣLFP (R = 0.20 to 0.81). In the hypotension experiments (D–E), a strong linear correlation was consistently observed between Po2 (D) and ΣLFP (R = −0.68 to −0.95) as well as OIS (E) and ΣLFP (R = −0.54 to −0.90).
A paired analysis between the R-score of the ΣLFP vs. OIS under control and hypotension conditions (Rmean = 0.54, pmean < 0.11 and Rmean = −0.76, pmean < 0.15, respectively) determined that the correlation of the data under hypotension conditions was significantly improved (p < 0.05). The ΣLFP vs. Po2 under control and hypotension conditions (Rmean = 0.60, pmean < 0.10 and Rmean = −0.77, pmean < 0.01, respectively) revealed similar results but with lower significance (p < 0.10). These analyses indicate that CMRO2 changes are more directly correlated with single trial-basis ΣLFP compared to oxygen-sensitive signals under normal conditions (BOLD). In terms of the neural correlation with CBF vs. CMRO2, no significant differences were observed between the R-scores of the ΣLFP vs. CBF under control conditions (Rmean = 0.78, pmean < 0.01) and the ΣLFP vs. OIS under hypotension conditions, as well as between ΣLFP vs. CBF under control conditions and the ΣLFP vs. Po2 under hypotension conditions.
Discussion
The present study showed that the variability in the trial-by-trial CBF responses is well correlated with the variability in the trial-by-trial ΣLFP responses (Fig. 5A). In addition, trial-by-trial variations in Po2 and OIS under hypotension conditions strongly depended on the trial-by-trial ΣLFP variation (Figs. 5D–5E), indicating that the CMRO2 responses are also strongly correlated with the ΣLFP. It was evident that the Po2 and OIS changes under normal conditions closely depended on the ΣLFP (Figs. 5B–5C). Since OIS represents deoxyhemoglobin-weighted signal changes, our findings support the notion that trial-by-trial variability in BOLD fMRI also represents the variability in neural responses in agreement with similar studies that have simultaneously measured EEG and fMRI. Further, our results indicate that the trial-by-trial variability in the neural activity can be determined by either neuro-vascular or neuro-metabolic measurements.
The trial-by-trial variation in ΣLFP can explain 63%, 45% and 35% of the total variance in the LDF, pO2 and OIS measurements, respectively, under control conditions. Similarly, variations in pO2 and OIS under hypotension conditions can be significantly explained by ΣLFP variations (61 and 60%, respectively). Another contributing source is the fluctuation of the baseline physiology (e.g. CBF). We observed slightly larger trial-by-trial variations in the hypotension experiments as compared to the control experiments; baseline variations in blood pressure (CV = 0.09 vs. 0.10), LDF (CV = 0.07 vs. 0.23), Po2 (CV = 0.14 vs. 0.29) and OIS (CV = 0.012 vs. 0.014), in control vs. hypotension experiments, respectively. The larger baseline variation under the hypotension condition is mainly due to the time-dependent vasodilator effects that produce slow drifts in the CBF baseline over the recording periods. Including a pre-stimulation LDF baseline value in the regression analysis slightly improved the trial-wise explained variations in LDF, pO2 and OIS under control conditions to 70%, 51% and 47%, respectively. In summary, our data showed that the trial-by-trial variability in ΣLFP contributed over 50% to the variability in the amplitude of CBF (~63%) and CMRO2 (~60%).
Under hypotension conditions, LFP activities were preserved even without CBF responses (Fig. 2B). This finding shows that the neural activity-induced CBF response is not required to maintain activation-induced neural functions as performed in this study. We also observed that the tissue Po2 under hypotension conditions never reached below hypoxic levels, which could potentially cause the depression of electrical neural activity (Foster et al., 2005). In preliminary experiments, preserved LFP activities were observed for 1-min long stimulation periods under hypotension conditions (data not shown). Additionally, neural activity-induced CBF responses were observed to peak earlier than the apparent CMRO2 changes (Fig. 3D), suggesting that a tight control of the tissue Po2 level is not likely to be a principal factor regulating CBF during functional activation under normal conditions. These findings support the notion that the quick response of CBF induced shortly after the onset of neural activation is not necessary to overcome the deficiency of oxygen during normal neural functions (Masamoto et al., 2007b).
Since the change in Po2 and OIS under hypotension conditions is directly related to the relative change in CMRO2, the impact of the physiologic changes in the CMRO2 and CBF on each modality was determined. The ratio of the CMRO2 to CBF impacts on the detected signal change was calculated to be 0.31 ± 0.09 and 0.40 ±0.23 (N = 5) with respect to the Po2 and OIS data, respectively, assuming that the CMRO2 response was consistent under control and hypotension experiments (not normalized by ΣLFP). The ratio of the signal changes induced by CMRO2 to CBF was calculated in previous experiments performed by our group in cats (Nagaoka et al., 2006) and similar results with respect to the present OIS data were obtained for BOLD fMRI data (~0.41). Other groups have reported the CMRO2/CBF coupling ratio induced by neural activity to be between 0.3 and 0.6 in anesthetized rats using optical imaging (Sheth et al., 2004) and fMRI (Mandeville et al., 1999; Liu et al., 2004). The discrepancies in the reported values can be due to methodological variations and/or differences in physiologic controls.
Conclusions
The present study showed that variability in the activation-induced CMRO2 and CBF changes can be used as a reliable index of trial-by-trial variability in neural activity. Although the amplitude of deoxyhemoglobin-weighted OIS, analogous to BOLD fMRI, was also observed to be sensitive to the variability in neural activity in individual trials, our results suggest that exclusive CBF or CMRO2-sensitive imaging methods would be more sensitive to the underlying neural activities.
Acknowledgments
We thank Hiro Fukuda and Michelle Tasker for their helpful comments. This study was supported by the National Institutes of Health (EB003375, NS044589).
References
- Antognini JF, Wang XW, Carstens E. Quantitative and qualitative effects of isoflurane on movement occurring after noxious stimulation. Anesthesiology. 1999;91:1064–1071. doi: 10.1097/00000542-199910000-00027. [DOI] [PubMed] [Google Scholar]
- Auer L. The action of sodium nitroprusside on the pial vessels. Acta Neurochir (Wien) 1978;43:297–306. doi: 10.1007/BF01587964. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler M. A graphical approach for evaluating effective connectivity in neural systems. Philos Trans R Soc Lond B Biol Sci. 2005;360:953–967. doi: 10.1098/rstb.2005.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Beaver CJ, Turner DA. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neuroscience. 2005;132:645–657. doi: 10.1016/j.neuroscience.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Wang P, Moon CH, Tanifuji M, Kim SG. Spatial specificity of the enhanced dip inherently induced by prolonged oxygen consumption in cat visual cortex: implication for columnar resolution functional MRI. Neuroimage. 2006;30:70–87. doi: 10.1016/j.neuroimage.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Hall RD, Lindholm EP. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 1974;66:23–38. [Google Scholar]
- Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kennerley AJ, Berwick J, Martindale J, Johnston D, Papadakis N, Mayhew JE. Concurrent fMRI and optical measures for the investigation of the hemodynamic response function. Magn Reson Med. 2005;54:354–365. doi: 10.1002/mrm.20511. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Villringer A, Dirnagl U. Characterization of CBF response to somatosensory stimulation: model and influence of anesthetics. Am J Physiol. 1993;264:H1223–H1228. doi: 10.1152/ajpheart.1993.264.4.H1223. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007a;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kershaw J, Ureshi M, Takizawa N, Kobayashi H, Tanishita K, Kanno I. Apparent diffusion time of oxygen from blood to tissue in rat cerebral cortex: implication for tissue oxygen dynamics during brain functions. J Appl Physiol. 2007b;103:1352–1358. doi: 10.1152/japplphysiol.01433.2006. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Zhao F, Wang P, Harel N, Kennan RP, Ogawa S, Kim SG. Increases in oxygen consumption without cerebral blood volume change during visual stimulation under hypotension condition. J Cereb Blood Flow Metab. 2006;26:1043–1051. doi: 10.1038/sj.jcbfm.9600251. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Santori EM, Toga AW. Mapping functional activity in rodent cortex using optical intrinsic signals. Cereb Cortex. 1994;4:195–204. doi: 10.1093/cercor/4.2.195. [DOI] [PubMed] [Google Scholar]
- Nemoto M, Sheth S, Guiou M, Pouratian N, Chen JW, Toga AW. Functional signal- and paradigm-dependent linear relationships between synaptic activity and hemodynamic responses in rat somatosensory cortex. J Neurosci. 2004;24:3850–3861. doi: 10.1523/JNEUROSCI.4870-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, Rich GF, Uncles DR, Daugherty MO, Frank DU. Sites of vasodilation by inhaled nitric oxide vs. sodium nitroprusside in endothelin-constricted isolated rat lungs. J Appl Physiol. 1994;77:51–57. doi: 10.1152/jappl.1994.77.1.51. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Tegeler C, Strother SC, Anderson JR, Kim SG. Reproducibility of BOLD-based functional MRI obtained at 4 T. Hum Brain Mapp. 1999;7:267–283. doi: 10.1002/(SICI)1097-0193(1999)7:4<267::AID-HBM5>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel D, van Gelderen P, Immisch I, Pfeiffer C, Hallett M. The variability of serial fMRI data: correlation between a visual and a motor task. Neuroreport. 2000;11:3843–3847. doi: 10.1097/00001756-200011270-00048. [DOI] [PubMed] [Google Scholar]