Abstract
Objective
The objective of this preliminary study was to document general somatic and wound nitric oxide (NO) levels during and after hyperbaric oxygen therapy (HBOT).
Design
The study evaluated 6 chronic wound patients that responded favorably to HBOT treatment (20 treatments; 2.0 atmosphere absolute [ATA] × 90 minutes). Successful HBOT was associated with increased wound granulation tissue formation and significantly improved wound closure. Wound fluid and fasting plasma samples were obtained for measurement of nitrate and nitrite (NOx), the stable oxidation products of NO; plasma L-arginine (L-Arg); and asymmetric dimethylarginine (ADMA). NOx measurements were obtained before treatment (baseline), after 10 and 20 treatments, and at 1 and 4 weeks after therapy.
Results
Wound fluid NOx levels tended to increase during treatments, were significantly elevated at 1 and 4 weeks after therapy, and correlated with reductions in wound area. Plasma L-Arg and ADMA were unchanged during and after HBOT.
Conclusion
This preliminary study documents a significant increase in local wound NO levels (by NOx measurements) after successful HBOT and suggests that this mechanism may be an important factor in promoting enhanced wound healing and wound closure associated with this therapy.
Hyperbaric oxygen therapy (HBOT) accelerates the healing of chronic wounds and is now primarily used as an adjunctive therapy in managing selected problem wound healing.1–4 Hyperoxia during HBOT is an important mediating factor for wound collagen deposition and cross-linking,5 neutrophil-dependent microbial killing,6 and neovascularization.7 Other HBOT-mediated processes in wound healing include the inhibition of integrin-mediated intravascular leukocyte adhesions and platelet aggregation,8 enhancement of cutaneous microvascular homeostasis after ischemia-reperfusion syndrome,9 increased oxygen capacitance and survival of ischemic flaps,10 and upregulation of platelet-derived growth factor (PDGF) receptor mRNA.11
Experimental normobaric oxygen activates gene expression in human dermal fibroblasts, supporting the concept of oxygen as a possible signal transducer.12 An additional signaling system modulated by oxygen availability is provided by the naturally occurring oxygen-free radicals (oxidants), or reactive oxygen species (ROS), found in the wound environment. ROS have been observed to increase cellular vascular endothelial growth factor (VEGF) expression and signal transduction that is considered to promote an overall enhancement of the wound healing process.13 Further, accelerated wound healing during HBOT may be mediated by the combined effects of hyperoxia and the increased local (wound) production of nitric oxide (NO), an important cellular signal for tissue repair.14,15
Ongoing experimental and clinical wound healing studies have established NO as a critical mediator of normal tissue repair.16 Angiogenesis, granulation tissue formation, epidermal migration, collagen deposition, and microvascular homeostasis are significant processes critical to normal wound repair that are regulated by NO production and bioactivity.17–20 Functional recovery of cutaneous vascular beds after ischemia-reperfusion injury21 and increased local random tissue flap survival22 have also been linked to increased vascular endothelial NOS activity in experimental models. Optimal NO activity is required for the full expression and receptor upregulation of VEGF and PDGF.17,23 A deficiency in NO bioactivity is associated with diabetes-impaired wound healing.24 Successful recombinant topical PDGF-BB (becaplermin) therapy for chronic lower-extremity ulcer (LEU) patients with diabetes may also depend on optimal NO production for successful wound treatment.25
Although HBOT and NO may mediate comparable mechanisms of cutaneous tissue repair, direct evidence of a relationship between enhanced wound healing, HBOT, and increased NO production has not been demonstrated. The purpose of this preliminary study was to document general somatic and local wound NO production during and after HBOT. This was accomplished by serially measuring NOx (nitrate and nitrite, which are the stable oxidation products of NO) in the fasting plasma and wound fluid of 6 patients receiving adjunctive HBOT who demonstrated enhanced healing of their chronic wounds with this therapy.
Materials and Methods
Six adult patients with chronic, nonhealing wounds requiring adjunctive HBOT were randomly selected for this study. Three patients had a history of diabetes mellitus (Type I [n = 1, subject 5, Table 1] and Type II [n = 2, subjects 1 and 6, Table 1]) and were receiving treatment for neuropathic LEUs at Wagner stage III or higher. The other 3 patients had no history of diabetes and were scheduled to receive HBOT for (n = 1 each) local flap failure after reconstruction of an injured Achilles tendon (subject 3, Table 1); failed local skin flap closure for abdominal wound reconstruction (subject 4, Table 1); and failed split-thickness skin graft reconstruction after treatment of a lower extremity venous ulcerations (VSU) (subject 2, Table 1). All 6 patients were documented with normal renal function without evidence of microalbuminuria. All 6 patients exhibited a favorable response to HBOT during and after treatment. This favorable response was associated with progressive wound granulation tissue deposition, enhanced wound epidermal migration, and progressive wound closure. These enhancements of individual wound healing were substantially increased after HBOT compared with the progression or absence of wound improvement documented for 1 month before HBOT. All treated wounds were free of signs or symptoms of infection. Patients received no additional interactive therapies (such as tissue-engineered skin substitutes or growth factors) during the study or within 90 days of the start of HBOT. The Centers for Medicare and Medicaid Services (CMS) guidelines were followed for patient selection and treatment. For Medicare guidelines regarding HBO therapy, refer to the CMS Internet online manual at: http://www.cms.hhs.gov. Investigational Review Board (IRB) approval and informed patient consent were obtained before performing these observations. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected by approval of the Retreat Hospital Human Research Review Committee.
Table 1. Hbot Study Subject Data.
| HBOT Study Subjects | |
|---|---|
| n | 6 |
| Age (years) | 58.15 (±2.98) |
| Male/Female | 2/4 |
| Diabetes Mellitus | Type I – 1/6 |
| Type II – 2/6 | |
| Weight (kg) | 90.54 (±10.90) |
| BMI (kg/m2) | 31.82 (±4.26) |
| Wound Type and Area (cm2) | 1) Left leg VU; 5.6 cm2; Type II DM. |
| 2) Bilateral ankle VU; 25.0 cm2; 12.0 cm2. | |
| 3) Left lower extremity ulcer; 7.8 cm2. | |
| 4) Abdominal wound; 2.0 cm2. | |
| 5) Left plantar hallux ulcer; 0.36 cm2; Type I DM. | |
| 6) Left ankle VU; 11.0 cm2; Type II DM. |
BMI = body mass index; VU = venous ulcer; DM = diabetes mellitus.
Patients received HBOT, using a Sechrist 3200 monoplace chamber (Sechrist Industries, Anaheim, CA). All patients received 20 daily treatment sessions of HBOT with 100% oxygen for 2.0 ATA for 90 minutes. For patient preparation and education, standard nursing protocols of the HCA Retreat Hospital Wound Healing Center's Hyperbaric Medicine Program were used.
Before HBOT, fasting baseline hematology and chemistries, including complete blood count (CBC) and complete metabolic profile (CMP), were obtained. The patients' anterior-posterior and lateral chest radiographs and electrocardiograms were also evaluated. Baseline measurements of fasting plasma and wound fluid NOx, plasma L-Arg, and plasma ADMA were obtained before HBOT. During the study, fasting blood and wound fluid samples were obtained after 10 and 20 HBOT treatments and at 1 week and 4 weeks after HBOT. For wound fluid NOx determinations, nitrate-free filter paper was placed against the wound for 24 hours. All specimens were stored at −70°C before analysis. All laboratory personnel were blinded as to the identity of specimens prepared for assay. Wound measurements used to determine wound area for statistical analysis were performed by the nursing staff of the Retreat Hospital Wound Healing Center using direct measuring (averaging) methods.
The NOx level of plasma and wound fluid was measured using the Griess assay after conversion of NO3 to NO2 with the NO3 reductase enzyme as described previously.26 Wound fluid and plasma was first filtered through Millipore Ultrafree-MC 10,000 molecular weight limit centrifugation filters.
Plasma L-Arg was measured by reverse-phase high-pressure liquid chromatography (HPLC) with precolumn derivatization and fluorescent detection, using a modification of the AccQ Tag system for amino acid analysis (Waters, Milford, MA) as described previously.27 For measurement of the endogenous NOS inhibitor ADMA, the reverse-phase HPLC with AccQ Tag method of Anderstam et al28 was used with minor modifications by the authors. Details of these assays have been reported by the authors previously.27
Statistical analyses of wound area measurements, fasting plasma and wound fluid NOx, plasma arginine, plasma ADMA, and the arginine/ADMA ratios from baseline determinations and interval measurements during and after HBOT were by multivariate analysis of variance (MANOVA) or by 1-way analysis of variance followed by Dunn's post-hoc test or by paired t tests, as appropriate. The level of significance was taken as P < .05, and results are expressed as mean ± standard error (SE).
Results
Wound Area Measurements
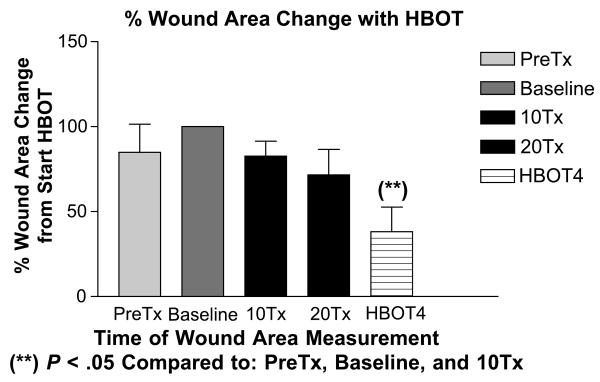
During the 4 weeks before the start of HBOT, mean wound areas tended to increase, although this was not statistically significant. After the start of HBOT, wound areas stabilized and then demonstrated a progressive reduction in area as compared to baseline values. In all cases, increased granulation tissue formation appeared at or after HBOT 8/20. Fully granulated wounds preceded wound edge (epidermal) migration. At 1 month after HBOT, the mean treated wound areas were decreased ∼ 60% and were significantly smaller than the pretreatment wound area, baseline wound area, and the wound area reduction observed after 10 treatments (Figure 1). At 1 month after HBOT, 4 patients had achieved complete wound closure (Table 1; patients 1, 3, 4, and 5), and by 3 months, patient 2 (Table 1) had achieved closure. Patient 6 displayed a fully granulated wound with 80% closure at 3 months but ultimately required endoscopic ligation of a marginal venous perforator at the ulcer site to achieve closure at 4 months after HBOT. Complete wound closure was defined as skin re-epithelialization without drainage or dressing requirements confirmed at 2 consecutive study visits 2 or more weeks apart.
Figure 1. Percentage of Wound area Change with Hbot.
Illustration of changes in mean wound area (percentage) observed with HBOT study subjects 1 month before HBOT (PreTx), at baseline, after 10 treatments (10Tx), after 20 treatment (20Tx), and at 4 weeks after the completion of HBOT (HBOT4).
**P < .05 for wound area reduction as compared to PreTx and HBOT values.
Plasma measures of NO system
Fasting plasma NOx levels did not differ throughout the study, with a baseline value of 43 ± 18 μmol/L, 51 ± 23 μmol/L at 1 week and 31 ± 7 μmol/L at 1 month after HBOT. There were no differences in plasma arginine levels before, during, or after HBOT. Plasma ADMA was also similar throughout the study (Table 2). The functionally important measure of the ratio of plasma arginine to ADMA was also similar throughout the study.
Table 2. Arginine, ADMA, and Arg/ADMA Ratios for Study Subjects Following HBOT.
| HBOT Baseline (n = 6) | HBOT 4 (n = 6) | |
|---|---|---|
| Arginine | 92 ± 6 | 94 ± 20 |
| ADMA | 0.36 ± 0.05 | 0.42 ± 0.04 |
| Arg/ADMA | 277 ± 37 | 231 ± 26 |
This table lists values of plasma arginine and ADMA (μM/L ± SE) and Arg/ADMA ratios for subjects at baseline and 4 weeks after HBOT (HBOT4). Arg = arginine; ADMA = asymmetric dimethylarginine.
Wound fluid NOx
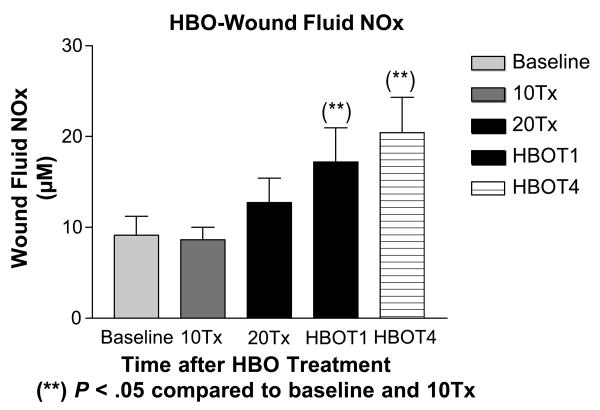
At baseline, the wound fluid NOx level was 9 ± 2 μmol/L. Wound fluid NOx levels increased during HBOT, and significant increases in wound fluid NOx were documented at 1 week (17 ± 4 μmol/L) and 4 weeks (20 ± 4 μmol/L) after HBOT, as compared to baseline measurement (Figure 2).
Figure 2. HBO-Wound Fluid NOx.
Illustration of changes in mean wound fluid NOx (μM/L ± SE) values in study subjects at baseline, after 10 treatments (10Tx), after 20 treatments (20Tx), at 1 week after HBOT completion (HBOT1), and at 4 weeks after HBOT completion (HBOT4).
**P < .05 as compared to baseline and 10Tx values.
Discussion
The main finding in this study is that clinically successful HBOT leading to the healing of refractory wounds is associated with elevations in wound fluid NO production, as determined by local NOx measurements. No differences in plasma NOx, L-Arg, or ADMA levels were detected, suggesting that the increased NO production is a local, rather than systemic, event. These findings support a role of HBOT-mediated, increased local wound NO production as a factor in promoting increased granulation tissue deposition and accelerated epidermal migration and wound closure (Figure 3).
Figure 3.
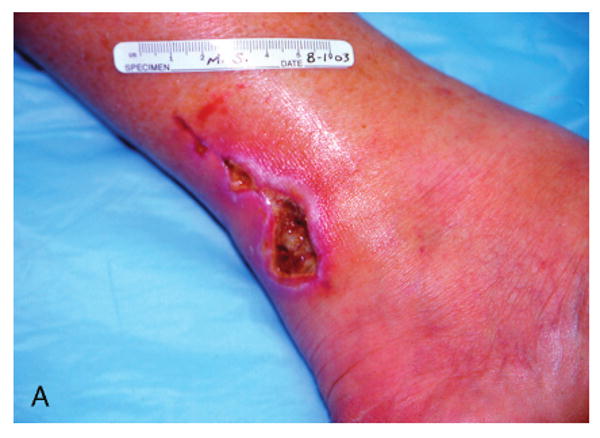
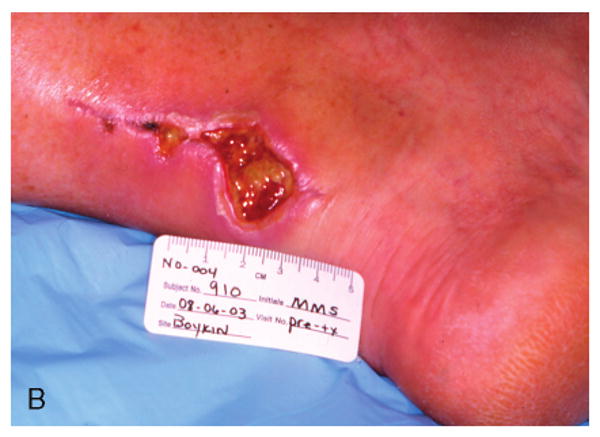
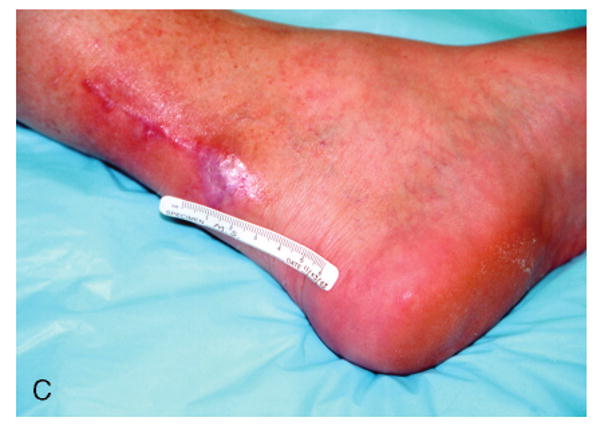
Patient #3 (Table 1) at 6 weeks following failed attempts at recovery of local flaps used for reconstruction of ruptured left Achilles tendon.
Patient #3 after wound bed preparation for hyperbaric oxygen therapy (HBOT) for treatment of failed flaps used with left Achilles tendon reconstruction.
Patient #3 demonstrating complete closure of chronic wound of posterior left lower leg Achilles incision and local flaps 2 months following completion of HBOT.
NOx measurements have been used as surrogate markers for determining NO production in many situations, including experimental and clinical wound healing research.16,24,25,29 NOx determinations have been proven to be highly sensitive in documenting declines in NO production associated with impaired wound healing and enhanced NO delivery associated with improved chronic wound recovery. Impaired wound healing caused by diabetes,24 protein-calorie malnutrition,30 cutaneous irradiation,31 steroid therapy,32 and metabolic inhibition of NO synthesis33 was associated with reductions in wound fluid NOx levels. Reduced wound fluid NOx during impaired wound closure was, in turn, associated with decreased collagen accumulation,34 wound tensile strength, type I and III collagen gene expression,35 VEGF expression, granulation tissue formation, and wound microvascular perfusion.36 Conversely, in studies of enhanced NO-mediated wound repair, increasing NOx measurements correlated with the successful reversal of impaired healing by interventional therapy with L-Arg supplementation37 or adenoviral, inducible NO synthase (iNOS) replacement in iNOS knockout mice.38
In the present study, HBOT was the intervention, and a progressive increase in wound fluid NOx occurred when HBOT was started; the increase was statistically significant at 1 and 4 weeks after HBOT. Increased wound fluid NOx correlated with significant reductions in wound areas following HBOT. The likely source of increased NO production is either endothelial NO synthase (eNOS) or iNOS isoform because human skin, keratinocytes, fibroblasts, and endothelial cells can produce both isoforms, and the wound macrophage and keratinocyte possess the iNOS.34
The enhanced elaboration of NO in the chronic wound is likely mediated by HBOT-upregulated activation of inflammatory cellular processes and growth factors. During the inflammatory phase of wound healing, monocytes infiltrate the wound in response to specific chemoattractants (ie, fragments of extracellular-matrix proteins, transforming growth factor-β, and monocyte chemoattractant protein-1) and become activated macrophages that release growth factors, such as PDGF and VEGF, which initiate the formation of granulation tissue.4 The macrophage is the major cellular source of wound NO, which is provided by the iNOS.34 PDGF also stimulates the chemotaxis of NO laden polymorphonuclear leukocytes and macrophages as well as the proliferation of smooth muscle and endothelial cells.39 Hyperoxia during HBOT may enhance the release of macrophage-derived growth factor, which stimulates fibroblast mitosis and the deposition of collagen, fibronectin, and glycosaminoglycans.40 Also, during HBOT, PDGF-BB receptor mRNA activity is upregulated, providing a robust synergy for inflammatory cell recruitment, increased wound NO production, and endothelial cell proliferation.11
Circulating bone marrow derived stem and progenitor cells may also contribute to increased wound NO production during HBOT, which increases stem cell and endothelial progenitor cell (EPC) mobilization from bone marrow by stimulating NO synthesis.41 These cells may differentiate into mature endothelial cells, contributing to re-endothelialization, neovascularization, and nitric oxide production. Circulating EPCs are observed homing to sites of injury, where cellular adhesion and insertion into the monolayer of mature endothelial cells occurs. The EPCs then differentiate into mature vascular endothelial cells capable of producing NO and sustaining wound neoangiogenesis.42 Circulating EPCs play a role in the repair of injured vessels, ischemic or damaged tissue,43 and diabetic skin wounds.44 Although knowledge of HBOT-induced stem cell and EPC mobilization is noteworthy, the overall contribution of EPC-mediated NO production likely represents a small fraction of the increased wound NO production; a greater contribution is made by inflammatory cellular processes.
Wound area reduction after HBOT was associated with accelerated epidermal migration and decreased wound areas. In experimental studies, increased endogenous NO production is a prerequisite for epidermal cell migration and may act to switch the epithelial cell from the stationary to the locomoting phenotype.19 This effect of NO is essential for the spatial and temporal coordination of locomoting epithelial cells during wound closure. The activity of iNOS was higher at the edges of epithelial wounds than the activity of eNOS, which showed no temporal or spatial changes associated with the wounds. Inhibition of iNOS reduced the rate of spontaneous epithelial migration and migration induced by epidermal growth factor, insulin-like growth factor-I, hepatocyte growth factor, and fibroblast growth factor.19
The demonstration of significant elevations of wound fluid NOx in chronic wound patients responding to HBOT suggests that supernormal wound NO production may be a priority for normal tissue repair processes and, perhaps, wound recovery. Experimental models of normal wound healing also demonstrate supernormal elevations of wound fluid NOx production during the course of healing.18,34,36,45 Plasma NOx values remained constant during this observation period, whereas wound fluid NOx increased steadily after wounding. These studies established supernormal elevations of wound fluid NOx, produced primarily by wound macrophages after injury, as a constant feature that may be correlated with successful wound repair.
HBOT is also associated with increased oxidative stress that could result in NO inactivation and formation of the cytotoxic peroxynitrite.46 However, evidence indicates that the transcription factor nuclear factor-κB that is activated by inflammatory cytokines and leads to iNOS activation also upregulates the antioxidant enzyme extracellular superoxide dismutase in dermal fibroblasts and other cells.47 Combined increases in extracellular superoxide dismutase and iNOS activity should protect NO bioactivity and avoid the formation of peroxynitrite anion during inflammation. Clearly, maintaining the balance between increasing free oxygen radicals and the antioxidant defenses versus increasing NO production is critical in the impact of HBOT on wound healing.
A potential concern with chronic HBOT is that increased oxidative stress might lead to increased levels of the endogenous NOS inhibitor, ADMA, because protein methylation is increased and ADMA breakdown is inhibited by oxidative stress.48 Increased ADMA has recently emerged as a major cardiovascular risk factor.48 The finding in the present study that chronic HBOT does not alter plasma ADMA levels or the ratio of L-Arg to ADMA is reassuring and suggests that activation of compensatory antioxidant systems during successful wound healing can overcome the adverse effects of increasing reactive oxygen species.
Conclusions
In summary, this preliminary study reports significantly increased local wound NO levels (by NOx measurements) after HBOT in patients successfully responding to it. In contrast, HBOT has no effect on systemic NO production or on ADMA and L-Arg levels, suggesting no systemic impact of HBOT on the NO system. These data suggest that enhanced local NO-mediated processes may provide an important mechanism for the improved wound healing and closure observed in patients responding to HBOT. Additional studies of NO-related mechanisms are needed with larger patient groups, including patients who do not respond favorably to HBOT, and more diverse wound pathology to expand these observations. Further research is also indicated for the development of clinical wound diagnostics for HBOT, using NOx or other biological surrogates of NO bioactivity as tools for patient selection and HBOT protocol development.
Acknowledgments
The authors thank Ms Lennie J. Samsell for technical assistance in the performance of laboratory measurements for these studies and also acknowledge the assistance of the nursing staff of the HCA/Retreat Hospital Wound Healing Center for patient management and data collection performed in this study. This study was funded in part by the National Institutes of Health (NIH), Bethesda, MD (NIH Grants R01-DK56843 and DK45517 [CB]).
Footnotes
This investigation was presented in part at the Wound Healing Society 14th Annual Meeting & Exhibition, May 23-26, 2004, Atlanta, GA.
References
- 1.Hammarlund C, Sundberg T. Hyperbaric oxygen reduced size of chronic leg ulcers: a randomized double-blind study. Plastic Reconstr Surg. 1994;93:829–34. [PubMed] [Google Scholar]
- 2.Kessler L, Bilbault P, Ortega F, et al. Hyperbaric oxygenation accelerates the healing rate of non-ischemic chronic diabetic foot ulcers. Diabetes Care. 2003;26:2378–82. doi: 10.2337/diacare.26.8.2378. [DOI] [PubMed] [Google Scholar]
- 3.Zamboni WA, Browder LK, Martinez J. Hyperbaric oxygen and wound healing. Clin Plast Surg. 2003;30:67–75. doi: 10.1016/s0094-1298(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 4.Boykin JV. Hyperbaric oxygen therapy: a physiological approach to selected problem wound healing. WOUNDS. 1996;8(6):183–98. [Google Scholar]
- 5.Silver IA. Local and systemic factors which affect the proliferation of fibroblasts. In: Kulonen E, Pikkarainen J, editors. Biology of Fibroblasts. New York, NY: Academic Press; 1973. pp. 507–20. [Google Scholar]
- 6.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of 2 parts) N Engl J Med. 1978;298:659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 7.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis: effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–70. [PubMed] [Google Scholar]
- 8.Thom SR. Functional inhibition of leukocyte B2 integrins by hyperbaric oxygen in carbon monoxide-mediated brain injury in rats. Toxicol Appl Pharmacol. 1993;123:248–56. doi: 10.1006/taap.1993.1243. [DOI] [PubMed] [Google Scholar]
- 9.Zamboni WA, Roth AC, Russell RC, Smoot EC. The effect of hyperbaric oxygen on reperfusion of ischemic axial skin flaps: a laser Doppler analysis. Ann Plast Surg. 1992;28:339–41. doi: 10.1097/00000637-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui A, Davidson JD, Mustoe TA. Ischemic tissue oxygen capacitance after hyperbaric oxygen therapy: a new physiologic concept. Plast Reconstr Surg. 1997;99:148–55. doi: 10.1097/00006534-199701000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Bonomo SR, Davidson JD, Yu Y, Xia Y, Lin X, Mustoe TA. Hyperbaric oxygen as a signal transducer: upregulation of platelet derived growth factor-beta receptor in the presence of HBO2 and PDGF. Undersea Hyperb Med. 1998;25:211–6. [PubMed] [Google Scholar]
- 12.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186:259–63. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 13.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;6(277):33284–90. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- 14.Boykin JV., Jr The nitric oxide connection: hyperbaric oxygen therapy, becaplermin, and diabetic ulcer management. Adv Skin Wound Care. 2000;13(4 Pt 1):169–74. [PubMed] [Google Scholar]
- 15.Boykin JV., Jr Re: Oxygen in wound healing: more than a nutrient. Wound Repair Regen. 2001;9:391–2. doi: 10.1046/j.1524-475x.2001.00391.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwentker A, Billiar TR. Nitric oxide and wound repair. Surg Clin North Am. 2003;83:521–30. doi: 10.1016/S0039-6109(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 17.Fukumura D, Gohongi R, Kadambi A, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;27(98):2604–9. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollock JS, Webb W, Callaway D, Sathyanarayana, O'Brien W, Howdieshell TR. Nitric oxide synthase isoform expression in a porcine model of granulation tissue formation. Surgery. 2001;129:341–50. doi: 10.1067/msy.2001.111700. [DOI] [PubMed] [Google Scholar]
- 19.Noiri E, Peresleni T, Srivastava N, et al. Nitric oxide is necessary for a switch from stationary to locomoting phenotype in epithelial cells. Am J Physiol. 1996;270(3 Pt 1):C794–802. doi: 10.1152/ajpcell.1996.270.3.C794. [DOI] [PubMed] [Google Scholar]
- 20.Schaffer MR, Tantry U, Thornton FJ, Barbul A. Inhibition of nitric oxide synthesis in wounds: pharmacology and effect on accumulation of collagen in wounds in mice. Eur J Surg. 1999;165:262–7. doi: 10.1080/110241599750007153. [DOI] [PubMed] [Google Scholar]
- 21.Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischemia-reperfusion. Cardiovasc Res. 1996;32:743–51. [PubMed] [Google Scholar]
- 22.Um SC, Suzuki S, Toyokuni S, et al. Involvement of nitric oxide in survival of random pattern skin flap. Plast Reconstr Surg. 1998;101:785–92. doi: 10.1097/00006534-199803000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Dhaunsi GS, Ozand PT. Nitric oxide promotes mitogen-induced DNA synthesis in human dermal fibroblasts through cGMP. Clin Exp Pharmacol Physiol. 2004;31(12):46–9. doi: 10.1111/j.1440-1681.2004.03948.x. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer MR, Tantry U, Efron PA, Ahrendt GM, Thornton FJ, Barbul A. Diabetes-impaired healing and reduced nitric oxide synthesis: a possible pathophysiologic correlation. Surgery. 1997;121:513–9. doi: 10.1016/s0039-6060(97)90105-7. [DOI] [PubMed] [Google Scholar]
- 25.Boykin JV, Kalns JE, Shawler LG, Sommer VL, Crossland MC. Diabetes-impaired wound healing predicted by urinary nitrate assay: a preliminary, retrospective study. WOUNDS. 1999;11(3):62–9. [Google Scholar]
- 26.Suto T, Losonczy G, Qiu C, et al. Acute changes in urinary excretion of nitrite + nitrate (UNOXV) do not necessarily predict renal vascular NO production. Kidney Int. 1995;48:1272–7. doi: 10.1038/ki.1995.411. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C. Nitric oxide production is low in patients with end stage renal disease patients on peritoneal dialysis. Am J Physiol. 1999;276(5 Pt 2):F794–7. doi: 10.1152/ajprenal.1999.276.5.F794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderstam B, Katzarski K, Bergstrom J. Serum levels of NG, NG-dimethyl-L arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol. 1997;8:1437–42. doi: 10.1681/ASN.V891437. [DOI] [PubMed] [Google Scholar]
- 29.Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine –What does this measure tell us about the activity of the endogenous nitric oxide system? Curr Opin Nephrol Hypertens. 1998;7:59–62. doi: 10.1097/00041552-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Schaffer MR, Tantry U, Ahrendt GM, Wasserkrug HL, Barbul A. Acute protein-calorie malnutrition impairs wound healing: a possible role of decreased wound nitric oxide synthesis. J Am Coll Surg. 1997;184:37–43. [PubMed] [Google Scholar]
- 31.Schaffer M, Weimer W, Wider S, et al. Differential expression of inflammatory mediators in radiation-impaired wound healing. J Surg Res. 2002;107:93–100. [PubMed] [Google Scholar]
- 32.Ulland AE, Shearer JD, Coulter C, Caldwell MD. Altered wound arginine metabolism by corticosterone and retinoic acid. J Surg Res. 1997;70:84–8. doi: 10.1006/jsre.1997.5099. [DOI] [PubMed] [Google Scholar]
- 33.Schaffer MR, Tantry U, Thornton FJ, Barbul A. Inhibition of nitric oxide synthesis in wounds: pharmacology and effect on accumulation of collagen in wounds in mice. Eur J Surg. 1999;165:262–7. doi: 10.1080/110241599750007153. [DOI] [PubMed] [Google Scholar]
- 34.Schafer MR, Tantry U, van Wesep RA, Barbul A. Nitric oxide metabolism in wounds. J Surg Res. 1997;71:25–31. doi: 10.1006/jsre.1997.5137. [DOI] [PubMed] [Google Scholar]
- 35.Witte MB, Kiyama R, Barbul A. Nitric oxide enhances experimental wound healing in diabetes. Br J Surg. 2002;89:1594–601. doi: 10.1046/j.1365-2168.2002.02263.x. [DOI] [PubMed] [Google Scholar]
- 36.Howdieshell TR, Webb WL, Sathyanarayana, McNeil PL. Inhibition of inducible nitric oxide synthase results in reductions in wound vascular endothelial growth factor expression, granulation tissue formation, and local perfusion. Surgery. 2003;133:528–37. doi: 10.1067/msy.2003.128. [DOI] [PubMed] [Google Scholar]
- 37.Shi HP, Efron DT, Most D, Tantry US, Barbul A. Supplemental dietary arginine enhances wound healing in normal but not inducible nitric oxide synthase knockout mice. Surgery. 2000;128:374–8. doi: 10.1067/msy.2000.107372. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki K, Edington HD, McClosky C, et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest. 1998;101:967–71. doi: 10.1172/JCI2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Gramates P, Deuel TF. Transforming growth factor beta reverses the glucocorticoid-induced wound healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci USA. 1998;86:2229–33. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt TK, Pai MP. Effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972;135:561–7. [PubMed] [Google Scholar]
- 41.Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290(4):H1378–86. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 42.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8(4):498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–6. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 44.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–77. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 45.Lee RH, Efron D, Tantry U, Barbul A. Nitric oxide in the healing wound: a time-course study. J Surg Res. 2001;101:104–8. doi: 10.1006/jsre.2001.6261. [DOI] [PubMed] [Google Scholar]
- 46.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–50. [PubMed] [Google Scholar]
- 47.Brady TC, Chang LY, Day BJ, Crapo JD. Extracellular superoxide dismutase is upregulated with inducible nitric oxide synthase after NF-κB activation. Am J Physiol. 1997;273(5 Pt 1):L1002–6. doi: 10.1152/ajplung.1997.273.5.L1002. [DOI] [PubMed] [Google Scholar]
- 48.Baylis C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol. 2006;2:209–20. doi: 10.1038/ncpneph0143. [DOI] [PMC free article] [PubMed] [Google Scholar]