Abstract
Glucocorticoid receptor (GR) agonist dexamethasone (Dex) induces hepatic steatosis and enhances constitutive androstane receptor (CAR) expression in the liver. CAR is known to worsen hepatic injury in nonalcoholic hepatic steatosis. Because transcription coactivator MED1/PPARBP gene is required for GR- and CAR-mediated transcriptional activation, we hypothesized that disruption of MED1/PPARBP gene in liver cells would result in the attenuation of Dex-induced hepatic steatosis. Here we show that liver-specific disruption of MED1 gene (MED1ΔLiv) improves Dex-induced steatotic phenotype in the liver. In wild-type mice Dex induced severe hepatic steatosis and caused reduction in medium- and short-chain acyl-CoA dehydrogenases that are responsible for mitochondrial β-oxidation. In contrast, Dex did not induce hepatic steatosis in mice conditionally null for hepatic MED1, as it failed to inhibit fatty acid oxidation enzymes in the liver. MED1ΔLiv livers had lower levels of GR-regulated CAR mRNA compared to wild-type mouse livers. Microarray gene expression profiling showed that absence of MED1 affects the expression of the GR-regulated genes responsible for energy metabolism in the liver. These results establish that absence of MED1 in the liver diminishes Dex-induced hepatic steatosis by altering the GR- and CAR-dependent gene functions.
Key words: Mediator complex subunit 1 (MED1), PPARBP, Hepatic steatosis, Dexamethasone, Glucocorticoid receptor, Constitutive androstane receptor
INTRODUCTION
Nuclear receptors regulate a diverse array of biological processes, including development, differentiation, and neoplasia, as well as energy and xenobiotic metabolism (29). These receptors bind, as homo- or heterodimers, to specific response elements in target gene promoters to regulate gene expression (29). The binding of ligands to nuclear receptors initially influences the recruitment of coactivator complexes, such as members of p160 family, which exhibit histone acetyltransferase activity to facilitate chromatin remodeling (19). Subsequent docking of other cofactors, or preformed multisubunit protein complexes, including mediator complex, facilitates interaction of liganded receptors with RNA polymerase II and the general basal transcription machinery (14).
Mediator subunit 1 (MED1), which was originally identified as peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP/PPARBP) (37), and subsequently identified as thyroid hormone receptor-associated protein 220 (TRAP220) (35), and vitamin D receptor interacting protein 205 (DRIP205) in TRAP and DRIP transcriptional complexes (28), appears to be a pivotal cofactor for the maintenance of mediator complex integrity (14). Gene knockout studies in the mouse have established that disruption of MED1/PPARBP gene is embryonic lethal, implying that MED1 may be widely involved in the functioning of many transcription factors (6,38). To study the function of MED1/PPARBP gene in adult tissues, we generated mice for conditional gene disruption using the Cre-loxP strategy (8). Evidence obtained to date from conditional disruption of this gene in the liver has established that MED1 is essential for PPARα and constitutive androstane receptor (CAR)-regulated gene expression in the liver (8,9).
Because CAR expression is glucorcorticoid receptor (GR) dependent and that a functional glucocorticoid response element (GRE) is present in the CAR gene promoter (24), it is envisaged that MED1 deficiency in vivo may affect the transcription of GR target genes in response to GR ligand dexamethasone (Dex) (24). To examine this aspect, we decided to study the role of MED1 in Dex-induced hepatic steatosis (16,20). Dex, a synthetic compound of glucocorticoid class of steroid hormones, used widely as an anti-inflammatory and immunosuppressant agent, is known to enhance the expression of CAR in the liver (24). In humans, as well as in rodents, treatment with Dex causes hepatic steatosis, by mechanisms that are not well understood (16). Dex-activated GR is transported to the nucleus where it binds as homodimer to GRE sequences in target gene promoters (16). Dex increases the levels of CAR, pregnane X receptor (PXR) and retinoid X receptor (RXR) mRNAs and proteins, to potentiate xenobiotic-mediated induction of CYP2B6, CYP2C8/9, and CYP3A4 (24). In the present investigation, we used MED1 liver conditional null (MED1ΔLiv) mice to assess the development of hepatic steatosis caused by Dex treatment. The studies show that absence of MED1/PPARPB gene in liver markedly diminishes hepatic steatotic response in liver following Dex administration, implying that this coactivator is essential for GR and CAR functions.
MATERIALS AND METHODS
Animals and Treatment
Generation of mice carrying liver-specific MED1 ablation (MED1ΔLiv) has been described previously (8). Mice used in these studies were age matched (∼5-week-old males; 5–8 mice for each time point) and maintained on a 12-h light/dark cycle. Dex, dissolved in corn oil, was administered IP at a dose of 50 mg/kg body weight for 1, 2, or 3 days. Mice injected with an equal volume of corn oil served as controls (27). All animal studies were approved by the Northwestern University Institutional Animal Care and Use Committee.
Livers were fixed in 10% formalin or 4% paraformaldehyde, and 4-μm-thick paraffin sections were cut and stained with hematoxylin and eosin. Additional sections were stained immunohistochemically with antibodies against MED1 [TRAP220 (C-19); Lot 20, Santa Cruz Biotechnology] as described elsewhere (8,17). Frozen sections of formalin-fixed liver (5 μm thick) were stained with Oil red O and counterstained with Giemsa.
Northern and Immunoblotting Procedures
Total RNA was isolated from liver using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. For Northern blotting, total RNA was glyoxylated, separated on 0.8% agarose gel, transferred to nylon membrane, and hybridized with 32P-labeled cDNA probes (8). For immunoblotting, total liver proteins were subjected to 10 % SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibodies as previously described (8). Protein concentration was determined by using a protein assay kit (Bio-Rad).
Real-Time PCR Analysis
For quantitative analysis, reverse transcription was performed on 2 μg of total RNA in a reaction volume of 20 μl using Superscript III First Strand Synthesis System for RT-PCR (Invitrogen). Quantitative real-time PCR (qPCR) was carried out in triplicates using primer pairs (Table 1) and normalized with 18S ribosomal RNA. PCR was composed of 1 μl (100 pmol) of sense and antisense primers and 10 μl of 2× SYBR Green Supermix (Applied Biosystems) to make a final volume of 20 μl and performed by using the ABI 7300 (Applied Biosystems).
TABLE 1.
REAL-TIME POLYMERASE CHAIN REACTION PRIMERS
| ID | Target | Forward | Reverse |
|---|---|---|---|
| 1 | Constitutive androstane receptor (Car) | GCCATGGCCCTCTTCTCCC | TCAGCCAGCAGGCCCATCAG |
| 2 | Cyp2b10 | GAACTGCGGAAATCCCAGGGAG | TCCAGCAGGCGCAAGAACTGAC |
| 3 | Cyp3A11 | CTGCATTCCTTGGCCACTCACC | TGACTGCATCCCGTGGCACAAC |
| 4 | IGFBP2 | TGCACCCGCCACGAGCAC | GGGCCATCAGGTGGAAGCTGTC |
| 5 | Acyl-CoA synthetase 2 | CGCTTGTGGAGCATTGTGGAC | GGTCAGCATATGGCCACCTG |
| 6 | Saa1 | TGTTCACGAGGCTTTCCAAGG | CACTGCGGCCATGTCTGTTG |
| 7 | Glucokinase | TGGGCTTCACCTTCTCCTTCC | AGCCGGTGCCCACAATCATG |
| 8 | ATP-binding cassette C3 | GGGTGAGATCGTCATTGATGG | CTCCAAGTCAATGGCAGCAGTG |
| 9 | Tissue inhibitor of metalloproteinase 4 | TGGTGCAGAGGGAGAGCCTG | TCGGTACCAGCTGCAGATGC |
| 10 | Glucose-6-phosphatase | CGCTATCTCCAAGTGAATTACC | CAAAGAGATGATGCAGGACC |
| 11 | Thyroid hormone responsive SPOT14 | ATCCCAAGAACTGCCTGCTGAC | TTTCAGCAGCGTTCTCAG |
| 12 | Solute carrier family 2 | CCATCTTCATGTCGGTGGGAC | CGTAAGGCCCAAGGAAGTCC |
| 13 | Acyl-CoA thioesterase 1 | GGGCATCACAGCTGCTGTGG | CGCTCTTCCAGTTGTGGTCG |
| 14 | p21 | TGGCCTTGTCGCTGTCTTGCAC | GGGCTCCCGTGGGCACTTC |
| 15 | p55 | AGATCCGGGATCAGCACCTTG | CTGCAACCACAGAACAAGTG |
Microarray Hybridizations and Data Analysis
Total RNA isolated from wild-type and MED1ΔLiv mouse livers, following 3-day Dex treatment, was used for reverse transcription and second-strand synthesis. The cDNA product was then used for preparing biotin-labeled cRNA, which was purified, fragmented, and hybridized to 430 2.0 arrays (Affymetrix). After hybridization, bound cRNA was fluorescently labeled using R-phycoerythrin streptavidin (Molecular Probes), and the fluorescence was intensified by the antibody amplification method as previously described (17,34).
RESULTS
MED1 Deficiency Diminishes Dex-Induced Hepatic Steatosis
Dex, given at 50 mg/kg body weight daily for 3 days, caused ∼60% increase in liver weight in MED1+/+ mice compared to MED1ΔLiv mice (data not shown). In wild-type (MED1+/+) mice, macrovesicular fatty change was evident in liver lobules with 1-day Dex treatment, which became prominent at 3 days (Fig. 1A–D). In contrast, in MED1ΔLiv livers, Dex treatment caused no perceptible increase in hepatic steatosis at day 1, and only a minimal increase at 3 days (Fig. 1E–H). Oil red O staining of liver sections obtained from MED1+/+ mice treated with Dex for 3 days confirmed hepatic lipid accumulation (Fig. 1D, H). Immunohistochemical staining with anti-MED1 antibodies revealed that all hepatocyte nuclei in wild-type mouse liver were stained positively for MED1 (Fig. 1C), but no nuclear staining was noted in MED1ΔLiv livers (Fig. 1G). Due to large fat droplet accumulation in 3-day Dex treatment in wild-type hepatocytes, MED1-stained nuclei, which were pushed peripherally, gave these cells a typical signet ring appearance (Fig. 1C).
Figure 1.
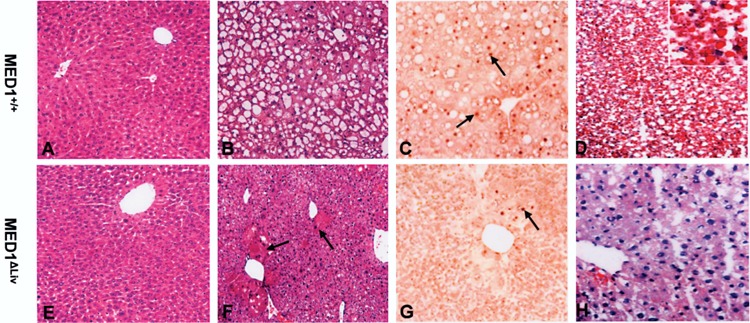
Histological analysis of liver from wild-type (MED1+/+) and MED1ΔLiv mice given Dex (50 mg/kg body weight, IP) or corn oil as vehicle once daily for 3 days. The liver sections of mice treated for 0 (A, E), and 3 days (B, F) were stained with hematoxylin and eosin. Liver sections from 3-day Dex-treated mice were processed for immunohistochemical localization of MED1 (C, G). Dex-treated MED1+/+ mouse liver reveals prominent macrovesicular fatty change (B) and shows MED1 nuclear staining (C). Note the peripheral location of MED1-positive nucleus (arrows) due to displacement by large fat vacuole (C). Fatty change is minimal in MED1ΔLiv mouse liver even at 3-day Dex treatment. An occasional MED1-positive nucleus is seen (G, arrow) due to escape from Cre-mediated deletion. Oil red O (D, H) staining of liver sections obtained from MED1+/+ (D) and MED1ΔLiv (H) mice treated with Dex for 3 days. Hepatic steatosis seen in Dex-treated MED1+/+ mice with hematoxylin and eosin staining (B) is confirmed by Oil red O staining (D). In the livers of 3-day Dex-treated liver conditional knockout mouse an occasional large hepatocyte that escaped Cre-mediated deletion is present (F, arrows), which reveal MED1-positive nucleus in MED1ΔLiv mouse liver (G, arrow).
MED1 Deficiency Prevents Dex-Induced Reductions in Fatty Acid Oxidation Enzymes
Glucocorticoids inhibit mitochondrial fatty acid β-oxidation enzymes and hepatic lipid secretion (16). Dex has been shown to inhibit mitochondrial matrix located long-, medium-, and short-chain dehydrogenases (LCAD, MCAD, and SCAD, respectively) and this inhibition results in the impairment of mitochondrial fatty acid β-oxidation contributing to hepatic steatosis (16). The mechanism by which Dex, a ligand for nuclear receptor GR, inhibits these enzymes is unclear. It is possible that Dex could interfere with the functions of certain transcriptional cofactors and affect nuclear receptor function (12). We have investigated the role of coactivator MED1 in Dex-mediated inhibition of fatty acid metabolizing enzymes in the liver (16). Constitutive basal levels of expression of LCAD, MCAD, and SCAD that are involved in mitochondrial β-oxidation system appeared similar in MED1+/+ and MED1ΔLiv livers (Fig. 2A, B). Likewise, the levels of peroxisomal β-oxidation enzymes acyl-CoA oxidase-1 (ACOX1), enoyl-CoA hydratase/L-3-hydroxyacyl-CoA dehydrogenase (L-PBE), and 3-ketoacyl-CoA thiolase (PTL), and microsomal fatty acid oxidation enzyme CYP4A1 were also similar in both MED1+/+ and MED1/PBPΔLiv livers (Fig. 2A, B). We show that the hepatic levels of mitochondrial, peroxisomal, and microsomal fatty acid oxidation enzymes LCAD, MCAD, SCAD, ACOX1, L-PBE, PTL, and CYP4A1 were inhibited by treatment with Dex for 3 days in wild-type mouse liver (Fig. 2). In MED1ΔLiv mice, Dex treatment failed to inhibit the levels of these proteins in liver (Fig. 2A, B). Reduction in hepatic carnitine octanoyltransferase (COT) protein content was more marked in Dex-treated wild-type mouse liver compared to MED1ΔLiv mouse livers (Fig. 2A, B). COT is involved in converting products of peroxisomal β-oxidation as substrates for mitochondrial β-oxidation (21). No reduction in electron transfer flavoprotein (ETF) and catalase (CTL) content was observed in wild-type and MED1ΔLiv mice treated for 3 days with Dex (Fig. 2).
Figure 2.
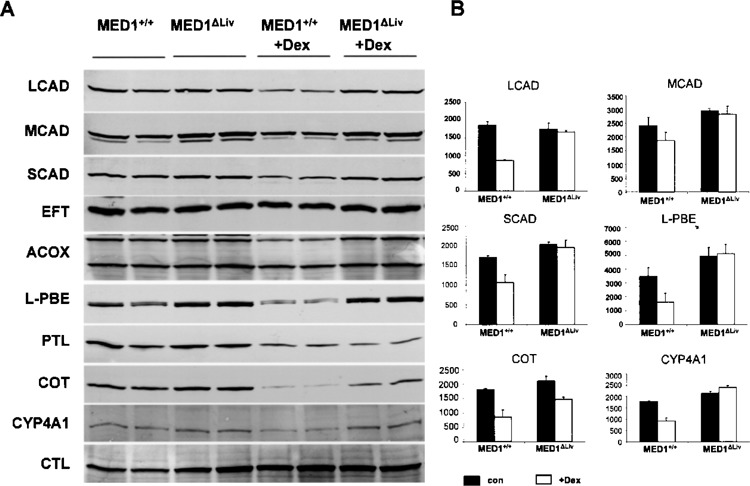
(A) Immunoblot analysis of liver proteins for changes in some enzymes responsible for fatty acid oxidation. LCAD, MCAD, and SCAD represent mitochondrial β-oxidation system enzymes, while ACOX, L-PBE, and PTL are members of peroxisomal β-oxidation pathway. CYP4A1 is a microsomal fatty acid β-oxidation system enzyme. Also included are EFT, COT, and CTL. (B) The histogram is the densitometric analysis of the Western blot signals. Black bars refer to wild-type (con) and white bars to Dex treatment (Dex) in MED1+/+ and MED1ΔLiv mice. All data are presented as the mean ± SD of three independent measurements.
Reduction in CAR mRNA Level in MED1-Deficient Liver
Because nuclear receptor CAR is known to play a role in the pathogenesis of nonalcoholic steatohepatitis (33), it appeared necessary to ascertain hepatic CAR mRNA expression level in MED1ΔLiv mice in response to GR ligand Dex (Fig. 3A). Disruption of MED1/PPARBP gene in liver resulted in a marked reduction in CAR mRNA expression but did not alter GR mRNA levels. Basal GR mRNA levels in liver were similar in wild-type and MED1ΔLiv mice and these levels did not differ significantly after Dex treatment (Fig. 3). Real-time PCR data obtained from mice treated with Dex for 1, 2, or 3 days showed that Dex reduced CAR level slightly but transiently in wild-type liver after 2-day treatment but these levels recovered after 3-day Dex treatment. Dex administration did not affect the already low CAR mRNA levels in MED1ΔLiv mouse livers (Fig. 3B).
Figure 3.
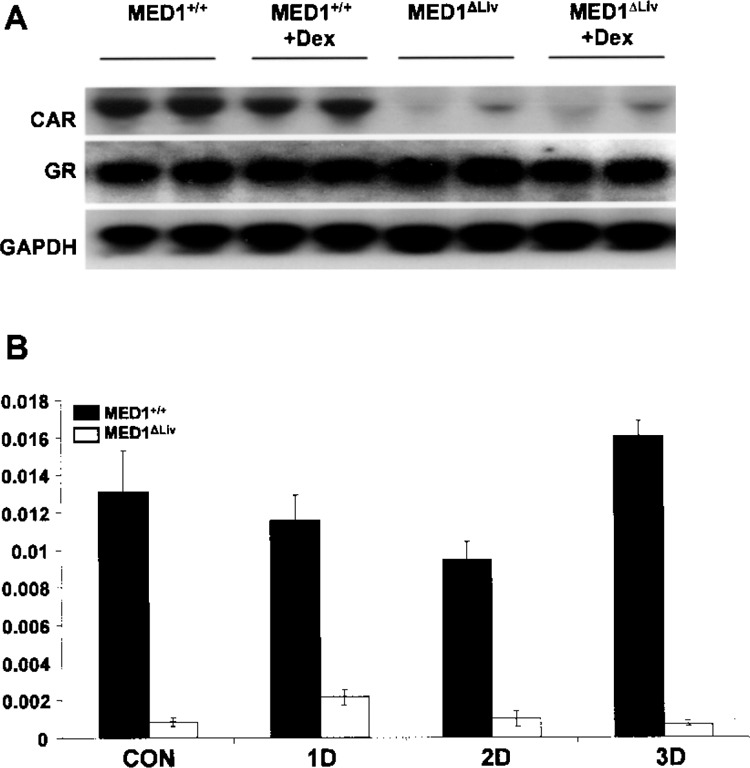
(A) Northern blot analysis for CAR and GR mRNA levels in MED1+/+ and MED1ΔLiv mouse livers without and with Dex treatment for 3 days. GAPDH is used as RNA loading control. (B) Quantitative real-time PCR analysis of CAR mRNA level in MED1+/+ and MED1ΔLiv mouse livers following treatment with Dex for 0 (con), 1 (1D), 2 (2D), and 3 (3D) days. Dark bars refer to wild-type (MED1+/+) and white bars represent MED1ΔLiv mice. All data are presented as the mean ± SD of three independent experiments.
Gene Expression Changes in MED1 Null Mice Following Dex Administration
To investigate further the role of MED1 in GR-regulated gene expression in the liver, we performed cDNA microarray analysis using liver RNA isolated from 3-day Dex-treated wild-type and MED1ΔLiv mice. Biotin-labeled RNA probes from two groups were hybridized to Affymetrix 430 2.0 microarray chips containing 45,000 genes. Following Dex treatment, several genes were upregulated fourfold or higher in wild-type mouse liver when compared to MED1ΔLiv and these fall into diverse functional categories (Table 2). A majority of genes induced by Dex in wild-type mouse liver is involved in lipid and glucose metabolism (1,7,10,23,25). Genes involved in lipid metabolism that were increased greater than sixfold include hydroxysteroid dehydrogenase-3β, bile acid-coenzyme A, fatty acid binding protein 1, elongation of very long chain fatty acids, ATP-binding cassette subfamily 3, hydroxysteroid 11β-dehydrogenase 1 (11pHSD1) (13), lipocalin 13, and thyroid hormone responsive SPOT14 (2). Acyl-CoA synthetase, phos-phoenoylpyruvate carboxykinase-1 (PEPCK1), hepatic lipase, glucose-6-phosphatase (G6Pase), and solute carrier family 27 fatty acid transporter, which also play a role in lipid and glucose metabolism, were also upregulated by Dex in wild-type mouse livers compared to livers lacking MED1. The expression of complement components 8 and 9, serum amyloid A1, A2, and kallikrein B, and others related to inflammatory and acute response processes was also sixfold or greater in MED1+/+ livers compared to MED1ΔLiv mouse livers treated with Dex. A significant correlation has been reported between the expression of inflammatory genes and liver triglyceride content (30). In wild-type mouse liver Dex treatment also caused an increase in serine peptidase inhibitor clade A4, several members of cytochrome P450 family, aldehyde oxidase, deiodinase iodothyronine type 1, tissue inhibitor of metalloproteinase 4 (TIMP4), and others. Several genes, such as acyl-CoA thioesterase 1, cell death-inducing DFFA-like effector C (>15-fold), cyclin-dependent kinase inhibitor 1A (p21), cyclin D1, phosphotidylinositol 3-kinase, and nucleic acid binding protein 1 (NABP1) (11), were upregulated in MED1ΔLiv mouse liver following Dex treatment when compared to Dex-treated wild-type mouse (Table 3).
TABLE 2.
GENES UPREGULATED FOURFOLD OR GREATER IN DEX-TREATED WILD-TYPE (MED1+/+) LIVER THAN IN MED1ΔLiv LIVER
| GenBank Accession | Fold Induction | Gene |
|---|---|---|
| Lipid metabolism genes | ||
| NM_008295.1 | 233.0 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta |
| NM_007519 | 22.7 | Bile acid-Coenzyme A: amino acid N-acyltransferase |
| NM_017399 | 16.8 | Fatty acid binding protein 1 (Fabp1), liver |
| BC016468 | 11.0 | Elongation of very long chain fatty acids 3 |
| AK006128.1 | 10.7 | ATP-binding cassette, sub-family C3 |
| NM_008288.1 | 10.0 | Hydroxysteroid 11-beta dehydrogenase l(HP-HSDl) |
| BC019882.1 | 9.9 | 3-Ketoacyl-CoA thiolase B |
| NM_030611.1 | 9.7 | Aldo-keto reductase family 1, member C6 |
| BC027556 | 7.8 | Lipocalin 13 |
| NM_009381 | 6.7 | Thyroid hormone responsive SPOT14 homolog (Rattus) |
| M63244 | 6.5 | Aminolevulinic acid synthase 2, erythroid |
| NM_013821.1 | 5.9 | Hydroxysteroid dehydrogenase-6, delta<5>-3-beta |
| NM_019811.1 | 5.8 | Acyl-CoA synthetase short-chain family member 2 |
| NM_019878.1 | 5.4 | Sulfotransferase family 1B, member 1 |
| BC026757.1 | 5.3 | Hydroxysteroid dehydrogenase-2, delta<5>-3-beta |
| AV257512 | 5.2 | Insulin induced gene 2 |
| BC021836.1 | 5.1 | Hydroxysteroid (17-beta) dehydrogenase 9 |
| NM_008156.1 | 4.9 | Glycosylphosphatidylinositol-specific phospholipase Dl |
| NM_008280.1 | 4.9 | Lipase, hepatic |
| NM_019811.1 | 4.5 | Acyl-CoA synthetase short-chain family member 2 |
| BB004104 | 4.4 | Cytochrome P450, family 51 |
| NM_133943.1 | 4.3 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 7 |
| NM_009512.1 | 4.1 | Solute carrier family 27 (fatty acid transporter), member 5 |
| Cell cycle, cell growth, and apoptosis genes | ||
| NM_007634 | 28.9 | Cyclin F |
| NM_008566 | 20.3 | Cell division cycle 46 (S. cerevisiae) |
| NM_013808.1 | 14.0 | Cysteine and glycine-rich protein 3 |
| BC002031.1 | 11.9 | BH3 interacting domain death agonist |
| NM_022032.1 | 10.1 | TP53 apoptosis effector |
| NM_016719.1 | 9.0 | Growth factor receptor bound protein 14 |
| AV369812 | 8.7 | Epidermal growth factor receptor |
| AK013765.1 | 6.6 | Endothelial cell growth factor 1 |
| AA796766 | 6.4 | Metallothionein 2 |
| AK011784.1 | 6.0 | Insulin-like growth factor binding protein 2 |
| NM_053176.1 | 5.8 | Histidine-rich glycoprotein |
| AI256288 | 4.9 | E2F transcription factor 8 |
| AF275367.1 | 4.6 | Epidermal growth factor receptor |
| AV252933 | 4.3 | Growth arrest and DNA-damage-inducible 45 alpha |
| NM_011871.1 | 4.2 | Protein kinase, interferon inducible double stranded RNA dependent activator |
| BC008997.1 | 4.0 | Annexin A7 |
| Glucose regulation genes and glycosylation | ||
| BC011139.1 | 17.0 | Glucokinase |
| BC021766.1 | 7.5 | C-type lectin domain family 2, member h |
| NM_031197.1 | 6.5 | Solute carrier family 2 (facilitated glucose transporter) |
| NM_133748.1 | 6.4 | Insulin induced gene 2 |
| AI265463 | 5.4 | Phosphoenolpyruvate carboxykinase 1, cytosolic(PCK) |
| NM_028803.1 | 4.9 | Glucan (1,4-alpha-), branching enzyme |
| AJ429133.1 | 4.9 | Asparagine-linked glycosylation 12 homolog (yeast) |
| BB667395 | 4.7 | Dehydrogenase E1 and transketolase domain containing 1 |
| NM_008061 | 4.2 | Glucose-6-phosphate |
| Signal transduction genes | ||
| BB319311 | 18.9 | Protein kinase, cGMP-dependent, type I |
| AW491150 | 11.2 | MAP/microtubule affinity-regulating kinase 1 |
| BB667397 | 10.7 | Protein kinase C, nu |
| AV216412 | 8.0 | Eukaryotic translation initiation factor 4E binding protein 1 |
| NM_032400.1 | 7.4 | Succinate receptor 1 |
| BG075165 | 6.8 | Insulin-like growth factor 1 |
| NM_016847.1 | 6.4 | Arginine vasopressin receptor 1A |
| NM_009647.1 | 5.7 | Adenylate kinase 3 alpha-like 1 |
| BI156474 | 4.9 | Phosphatidylinositol 4-kinase type 2 beta |
| AK004874.1 | 4.2 | Rap guanine nucleotide exchange factor (GEF) 4 |
| Transcription/translation regulation genes | ||
| NM_011082.1 | 23.6 | Polymeric immunoglobulin receptor |
| C80642 | 21.5 | Ankyrin repeat and IBR domain containing 1 |
| BB458460 | 21.3 | Coiled-coil-helix-coiled-coil-helix domain |
| BCO10807.1 | 8.7 | Transcription elongation factor A (SII), 3 |
| BM239828 | 8.0 | Interferon inducible GTPase 1 |
| NM_009349.1 | 6.9 | Indolethylamine N-methyltransferase |
| AI461691 | 6.2 | Heat shock 70kDa protein 4 like |
| BF018652 | 5.5 | SoxLZ/Sox6 leucine zipper binding protein in testis |
| BB183854 | 4.8 | B-cell leukemia/lymphoma 6 |
| BB305306 | 4.7 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 47 |
| BQ177743 | 4.7 | Wolf-Hirschhorn syndrome candidate 1 (human) |
| BG092043 | 4.6 | Insulin-like growth factor 2, binding protein 3 |
| D90176.1 | 4.5 | Nuclear factor I/A |
| BB284697 | 4.1 | Zinc finger protein 161 |
| Extracellular matrix/cell structure, receptor, adhesion, and chaperone genes | ||
| AV241307 | 65.4 | Myomesin 2 |
| NM_009255.1 | 24.5 | Serine (or cysteine) peptidase inhibitor, clade E |
| BCO 15252.1 | 21.2 | Claudin 2 |
| AI447325 | 15.0 | Rho GTPase activating protein 26 |
| AKO17358.1 | 12.0 | Intergral membrane protein 1 |
| NM_008645.1 | 11.9 | Murinoglobulin 1 |
| NM_019410.1 | 9.0 | Profilin 2 |
| AV227581 | 8.2 | Claudin 1 |
| NM_012050.1 | 5.8 | Osteomodulin |
| AI461691 | 5.8 | Heat shock 70-kDa protein 4 like |
| BG065575 | 5.7 | Poliovirus receptor-related 1 |
| NM_010746.1 | 5.2 | Natural cytotoxicity triggering receptor 1 |
| AK019164.1 | 4.9 | Multiple PDZ domain protein 1 |
| NM_133903.1 | 4.2 | Spondin 2, extracellular matrix protein |
| Inflammation/immune-related genes | ||
| BC027748.1 | 98.6 | Complement component 8 |
| NM_013485.1 | 24.2 | Complement component 9 |
| NM_009117 | 17.8 | Serum amyloid A 1 |
| NM_011314.1 | 16.4 | Serum amyloid A 2 |
| BC026555.1 | 13.8 | Kallikrein B, plasma 1 |
| BB794642 | 13.0 | Melanoma antigen, 80 kDa |
| BC024380.1 | 12.0 | Defensin beta 1 |
| NM_017370.1 | 8.5 | Haptoglobin |
| NM_007576.1 | 7.9 | Complement component 4 binding protein |
| BI328146 | 7.3 | Retinoic acid receptor responder (tazarotene induced) |
| NM_016704.1 | 6.0 | Complement component 6 |
| BC022123.1 | 4.9 | Complement component 1, s subcomponent |
| BB667823 | 4.8 | Ring finger protein 125 |
| BE628912 | 4.7 | Orosomucoid 1 |
| Metabolic and other genes | ||
| BF383739 | 143.3 | Serine (or cysteine) peptidase inhibitor, clade A, 4 |
| BC025936.1 | 134.8 | Cytochrome P450, family 4, subfamily a, polypeptide 12 |
| NM_023617.1 | 96.7 | Aldehyde oxidase 3 |
| NM_007825.1 | 79.3 | Tissue inhibitor of metalloproteinase 4 |
| BB328405 | 71.6 | Cytochrome P450, family 7, subfamily b, polypeptide 1 |
| NM_007860.1 | 59.2 | Deiodinase, iodothyronine, type I |
| BC010973.1 | 46.3 | Cytochrome P450, family 8, subfamily b1 |
| AK011413.1 | 39.4 | Major urinary protein 1 |
| BC026598.1 | 25.4 | Solute carrier family 22, member 7 |
| AI327006 | 23.5 | Cytochrome P450, family 4, subfamily a14 |
| NM_134144.1 | 22.7 | Cytochrome P450, family 2, subfamily C50 |
| NM_008030 | 20.0 | Flavin containing monooxygenase 3 |
| AK003671.1 | 19.0 | Carbonic anhydrase 3 |
| NM_021456.1 | 16.4 | Carboxylesterase 1 |
| NM_009993.1 | 12.2 | Cytochrome P450, family 1, subfamily a2 |
| NM_023135.1 | 12.0 | Sulfotransferase family 1E, member 1 |
| AA571276 | 11.5 | Liver-expressed antimicrobial peptide 2 |
| NM_009669.1 | 10.1 | Amylase 2, pancreatic |
| NM_017473.1 | 10.0 | Retinol dehydrogenase 7 |
| L07645.1 | 9.6 | Histidine ammonia lyase |
| NM_053215.1 | 9.3 | UDP-glucuronosyltransferase 2 family, polypeptide B37 |
| BC021378.1 | 9.2 | NADPH oxidase 4 |
| J03953.1 | 8.6 | Glutathione S-transferase, mu 3 |
| BC025819.1 | 8.5 | Cytochrome P450, family 2, subfamily C44 |
| BB293163 | 8.1 | Mitochondrial ribosomal protein L30 |
| AF128849.1 | 8.0 | Cytochrome P450, family 2, subfamily b10 |
| M63244.1 | 7.9 | Aminolevulinic acid synthase 2, erythroid |
| AB021226 | 7.7 | Matrix metallopeptidase 24 |
| NM_007809.1 | 7.6 | Cytochrome P450, family 17, subfamily a1 |
| BC012682.1 | 7.3 | Hydroxysteroid (17-beta) dehydrogenase 2 |
| NM_011579.1 | 6.9 | T-cell specific GTPase |
| AK013765.1 | 6.6 | Endothelial cell growth factor 1 |
| NM_007817.1 | 6.5 | Cytochrome P450, family 2, subfamily F2 |
| AB039380.1 | 6.2 | Cytochrome P450, family 3, subfamily A44 |
| NM_010403.1 | 5.5 | Hydroxyacid oxidase 1, liver |
| BF783609 | 5.7 | Cytochrome P450, family 2, subfamily j, polypeptide 5 |
| NM_017396.1 | 4.8 | Cytochrome P450, family 3, subfamily a, polypeptide 41 |
| BB139766 | 4.8 | Cytochrome P450, family 2, subfamily r, polypeptide 1 |
| AI172943 | 4.5 | Glutathione S-transferase, alpha 3 |
| AV078914 | 4.3 | Hydroxyacyl-Coenzyme A dehydrogenase type II |
| BC025940.1 | 4.3 | UDP glycosyltransferases 3 family, polypeptide A1 |
| NM_009676.1 | 4.2 | Aldehyde oxidase 1 |
| NM_011996.1 | 4.2 | Alcohol dehydrogenase 4 (class I), pi polypeptide |
| Transport and storage genes | ||
| M16359.1 | 215.3 | Major urinary protein III (MUP III) |
| AB031813.1 | 95.3 | Solute carrier organic anion transporter family 1 |
| NM_007474.1 | 23.5 | Aquaporin 8 |
| BB553107 | 9.6 | Solute carrier organic anion transporter family, 2b1 |
| BC021154.1 | 8.7 | Solute carrier family 10 (sodium/bile acid cotransporter family), member 1 |
| NM_009205.1 | 6.2 | Solute carrier family 3, member 1 |
| AA276202 | 6.0 | Solute carrier family 23 (nucleobase transporters) |
| NM_007752.1 | 5.0 | Ceruloplasmin |
| AW208574 | 4.2 | Lipopolysaccharide binding protein |
| NM_030687.1 | 4.0 | Solute carrier organic anion transporter family, member 1a4 |
| Miscellaneous | ||
| BB795733 | 165.9 | UDP-Gal:betaGlcNAc beta 1,3-galactosyltransferase |
| NM_026822.1 | 5.9 | Small proline rich-like 5 |
| AK004030.1 | 4.9 | Synaptogyrin 2 |
| NM_029562.1 | 4.9 | Cytochrome P450, family 2, subfamily d, polypeptide 26 |
| NM_134069.1 | 4.6 | Solute carrier family 17 (sodium phosphate), member 3 |
| BC024104.1 | 4.6 | Protein Z, vitamin K-dependent plasma glycoprotein |
| BC027200.1 | 4.5 | UDP glucuronosyltransferase 2 family, polypeptide B1 |
| NM_029550.1 | 4.3 | Kidney expressed gene 1 |
| NM_029796.1 | 4.2 | Leucine-rich alpha-2-glycoprotein 1 |
| AI876438 | 4.1 | Ectonucleotide pyrophosphatase/phosphodiesterase 3 |
| AU016334 | 4.0 | Heterogeneous nuclear ribonucleoprotein A3 |
| NM_012059.2 | 4.0 | SH3 domain protein D19 |
TABLE 3.
GENES UPREGULATED FOURFOLD OR GREATER IN DEX-TREATED MED1ΔLiv LIVER COMPARED TO WILD-TYPE (MED1+/+) LIVER
| GenBank Accession | Fold Induction | Gene |
|---|---|---|
| Lipid metabolism genes | ||
| NM_012006.1 | 10.8 | Acyl-CoA thioesterase 1 (Acotl) |
| NM_134188.1 | 7.5 | Acyl-CoA thioesterase 2 (Acot2) |
| AU022584 | 7.2 | SMC6 structural maintenance of chromosomes 6-like 1 |
| Cell cycle, cell growth, and apoptosis genes | ||
| BB221402 | 15.4 | Cell death-inducing DFFA-like effector c |
| NM_007669.1 | 9.6 | Cyclin-dependent kinase inhibitor 1A (P21) |
| BB538325 | 8.1 | Cyclin Dl |
| U52193.1 | 7.7 | Phosphatidylinositol 3-kinase |
| BE951810 | 7.0 | Neurofibromatosis 2 |
| BB814564 | 6.1 | Transformation related protein 53 binding protein 2 |
| NM_011361.1 | 5.6 | Serum/glucocorticoid regulated kinase |
| AV227314 | 4.8 | Cysteine rich transmembrane BMP regulator 1 (chordin like) |
| AI451482 | 4.6 | Cyclin-dependent kinase 7 |
| BG065754 | 4.2 | Cyclin Gl |
| BB398886 | 4.2 | PRKC, apoptosis, WT1, regulator |
| BI328541 | 4.0 | Kinesin family member 5B |
| Glucose regulation genes | ||
| AV272221 | 7.7 | Nuclear receptor interacting protein 1 |
| AU040643 | 7.3 | Asparaginase like 1 |
| NMJ008062.1 | 4.1 | Glucose-6-phosphate dehydrogenase X-linked |
| Signal transduction genes | ||
| BB034265 | 13.7 | Regulator of G-protein signaling 2 |
| AA414954 | 13.2 | Phosphatidylinositol 3 kinase, regulatory subunit, polypeptide 3 (p55) |
| NM_013749.1 | 13.0 | Tumor necrosis factor receptor superfamily, member 12a |
| AA590970 | 9.6 | Huntingtin interacting protein 1 related |
| M68515.1 | 8.4 | Ephrin type-A receptor 3 |
| BG070037 | 8.4 | Neuronal PAS domain protein 2 |
| NM_139059.1 | 6.8 | Casein kinase 1, delta |
| BC003906.1 | 5.7 | Tumor necrosis factor, alpha-induced protein 1 (endothelial) |
| AI451642 | 5.5 | B-cell scaffold protein with ankyrin repeats 1 |
| AK005325.1 | 4.9 | A kinase (PRKA) anchor protein 10 |
| BC023022.1 | 4.8 | Calcium binding atopy-related autoantigen 1 |
| BF136544 | 4.8 | Fibrinogen-like protein 2 |
| AV025452 | 4.2 | G protein-coupled receptor 135 |
| D17444.1 | 4.1 | Leukemia inhibitory factor receptor |
| Transcription genes | ||
| AI506429 | 19.8 | RIKEN cDNA 5830411E10 gene |
| NM_025705.1 | 17.9 | Discoidin, CUB and LCCL domain containing 1 |
| AV305197 | 15.8 | Striatin, calmodulin binding protein 3 (Strn3) |
| NM_008539.1 | 13.4 | MAD homolog 1 (Drosophila) |
| AI414736 | 12.1 | Zinc finger, MYND domain containing 12 |
| AV227804 | 10.0 | U3 small nucleolar ribonucleoprotein, homolog (yeast) |
| BC028550.1 | 9.9 | Histone 1, H4h |
| BB333374 | 9.5 | Zinc finger protein 533 |
| NM_013550.1 | 8.7 | Histone 1, H3a |
| NM_133971.1 | 8.5 | Ankyrin repeat domain 10 |
| BM235074 | 8.0 | Interleukin enhancer binding factor 2 |
| BM245170 | 7.5 | Fos-like antigen 2 |
| AV012790 | 7.3 | Splicing factor, arginine/serine-rich 12 |
| NMJ009637.1 | 6.6 | AE binding protein 2 |
| AV312048 | 6.3 | Splicing factor, arginine/serine-rich 2, interacting protein |
| NMJ023672 | 6.1 | Single-stranded DNA binding protein 3 |
| NMJ025299.1 | 5.9 | Thioredoxin-like 4 |
| NM_016859.1 | 5.6 | Bystin-like |
| BQ177107 | 5.3 | Potassium channel tetramerisation domain containing 13 |
| NMJ009281.2 | 5.2 | Zinc finger protein 143 |
| AK018187.1 | 4.8 | Zinc finger protein 518 |
| BI690586 | 4.5 | Thyroid hormone receptor associated protein 1 |
| BC016565.1 | 4.5 | Peptidyl prolyl isomerase H |
| BB234363 | 4.4 | Transmembrane protein 69 |
| BC024613.1 | 4.4 | Transmembrane protein 37 |
| BQ179556 | 4.1 | Mitochondrial ribosomal protein L17 |
| AW985925 | 4.0 | Transmembrane protein 23 |
| W77144 | 4.0 | Inhibitor of DNA binding 4 |
| Extracellular matrix/cell structure genes | ||
| BB667295 | 13.5 | RIKEN cDNA 2310044D20 gene |
| NM_007598.1 | 6.7 | CAP, adenylate cyclase-associated protein 1 |
| NM_020568.1 | 5.5 | Plasma membrane associated protein, S3-12 |
| AV071536 | 4.6 | Integrin alpha 1 |
| NM_023662.1 | 4.5 | Pericentriolar material 1 |
| Inflammation-related genes | ||
| NM_009846.1 | 11.9 | CD24a antigen |
| NM_007572.1 | 5.6 | Complement component 1, q subcomponent, alpha polypeptide |
| Metabolic and other genes | ||
| AF192558.2 | 22.0 | Mitochondrial carrier homolog 1 (C. elegans) |
| N28171 | 13.5 | DNA segment, Chr 16, Brigham & Women’s Genetics 1494 expressed |
| BC027445.1 | 9.7 | Protein tyrosine phosphatase 4a3 |
| AW763765 | 9.2 | Heat shock protein 1A |
| AV023312 | 7.2 | ADP-ribosylation factor 2 |
| NM_011884.1 | 6.3 | RNA guanylyltransferase and 5’-phosphatase |
| AV313469 | 5.6 | Zinc finger, CSL-type containing 3 |
| NM_021486.2 | 5.5 | Beta-carotene 15,15’-monooxygenase |
| BM222742 | 5.5 | PDZ and LIM domain 5 |
| BB667459 | 5.0 | Vmyotubularin related protein 12 |
| NM_133705.1 | 4.9 | Pyrroline-5-carboxylate reductase family, member 2 |
| BG067251 | 4.9 | O-sialoglycoprotein endopeptidase-like 1 |
| AV317107 | 4.6 | SUMO/sentrin specific peptidase 2 |
| BC024135.1 | 4.6 | Coenzyme Q6 homolog (yeast) |
| AV023994 | 4.3 | Cathepsin L |
| NM_007423.1 | 4.2 | Alpha fetoprotein |
| AA792094 | 4.1 | Glutamate oxaloacetate transaminase 1, soluble |
| Transport and storage genes | ||
| NM_011075.1 | 17.7 | ATP-binding cassette, sub-family B (MDR/TAP), member 1B |
| NM_025960.1 | 10.0 | Trafficking protein particle complex 6A |
| AV343478 | 8.3 | ATPase, Ca++ transporting, plasma membrane 2 |
| AY061807.1 | 6.7 | Calmodulin-like 4 |
| NM_025409 | 6.2 | Immediate early response 3 interacting protein 1 |
| BB497312 | 6.1 | Solute carrier family 13, member 3 |
| BM250411 | 5.9 | Solute carrier family 39 (zinc transporter), member 10 |
| NM_054055 | 4.8 | Solute carrier family 13, member 3 |
| BE686616 | 4.0 | Mitochondrial translational release factor 1-like |
| Miscellaneous | ||
| AI507307 | 12.9 | Suprabasin |
| BB104669 | 12.2 | Kinesin family member 2C |
| BG067897 | 12.0 | Trinucleotide repeat containing 6b (Tnrc6b), transcript variant 1 |
| C77631 | 10.7 | Expressed sequence |
| BB021163 | 10.0 | EST |
| BC008229.1 | 9.6 | RIKEN cDNA 1500011J06 gene |
| BB102308 | 9.4 | Expressed sequence AW228944 |
| BC028777.1 | 9.3 | RIKEN cDNA 1600002H07 gene |
| BC011230.1 | 9.2 | RIKEN cDNA 2510015F01 gene |
| AK017143.1 | 9.2 | RIKEN cDNA 5031425E22 gene |
| BM936291 | 8.9 | RIKEN cDNA 2310047C04 gene |
| BB546429 | 8.8 | EST |
| AV145060 | 8.7 | Phosphofurin acidic cluster sorting protein 2 |
| BB344827 | 8.4 | PRP4 pre-mRNA processing factor 4 homolog B (yeast) |
| AI510297 | 8.4 | RIKEN cDNA 2700007P21 gene |
| BM230508 | 8.2 | RIKEN cDNA A030007D23 gene |
| NM_030697.1 | 8.2 | Ankyrin repeat domain 47 |
| BM932452 | 8.2 | Short coiled-coil protein |
| BG071865 | 6.7 | Serine/arginine repetitive matrix 1 |
| AV369290 | 6.7 | TBC1 domain family, member 5 |
| AK017464.1 | 6.6 | Sestrin 3 |
| BC018474.1 | 6.1 | EPM2A (laforin) interacting protein 1 |
| AF265663.1 | 5.9 | MLX interacting protein |
| BG069557 | 5.7 | PRP3 pre-mRNA processing factor 3 homolog (yeast) |
| AV218922 | 5.7 | RIKEN cDNA 2610002J02 gene |
| BB667703 | 5.6 | RIKEN cDNA 2410127E18 gene |
| BF225441 | 5.4 | RIKEN cDNA 2210010L05 gene |
| BI453712 | 5.2 | Hematological and neurological expressed sequence 1 |
| BI737178 | 5.2 | RIKEN cDNA 2610005L07 gene |
| BE945468 | 5.1 | RIKEN cDNA 9530057J20 gene |
| BB375402 | 5.1 | cDNA sequence BC027663 |
| BC027371.1 | 4.7 | RIKEN cDNA 5830411E10 gene |
| NM_024452.1 | 4.6 | Leucine zipper protein 1 |
| BE457727 | 4.6 | RIKEN cDNA 5830411E10 gene |
| AK006222.1 | 4.6 | RIKEN cDNA 1700021P22 gene |
| BE980579 | 4.5 | A disintegrin and metallopeptidase domain 17 |
| BC021385.1 | 4.3 | RIKEN cDNA 9030617O03 gene |
| AW558420 | 4.2 | DNA segment, Chr 6, ERATO Doi 253, expressed |
| BF147713 | 4.0 | Leucine rich repeat containing 39 |
| BB752934 | 4.0 | RIKEN cDNA 3300001M20 gene |
Validation of Gene Expression of Selected Genes With Quantitative PCR Analyses
From the highly expressed genes identified by microarray, using 3-day Dex-treated wild-type and MED1ΔLiv mouse livers we selected several genes for validation by quantitative PCR analysis (Figs. 4 and 5). We used RNA from mice treated for 1, 2, and 3 days. The predicted high levels of expression of genes in wild-type mouse livers were confirmed by qPCR analysis. These include insulin-like growth factor binding protein 2 (IGFBP2) (22,36), glucokinase, G6Pase, thyroid hormone responsive SPOT14 (2), tissue inhibitor of metalloproteinase 4, Saa1, solute carrier family 2 (glucose transporter), member 2, ATP-binding cassette, C3 (abcc3), and others (Fig. 4). We also validated the predicted increases in the expression of genes identified by microarray in Dex-treated MED1ΔLiv mouse livers (Figs. 4, 5). These included acyl-CoA thioesterase 1, cyclin-dependent kinase inhibitor 1A (p21), and phosphatidylinositol 3 kinase (p55) (Figs. 4, 5).
Figure 4.
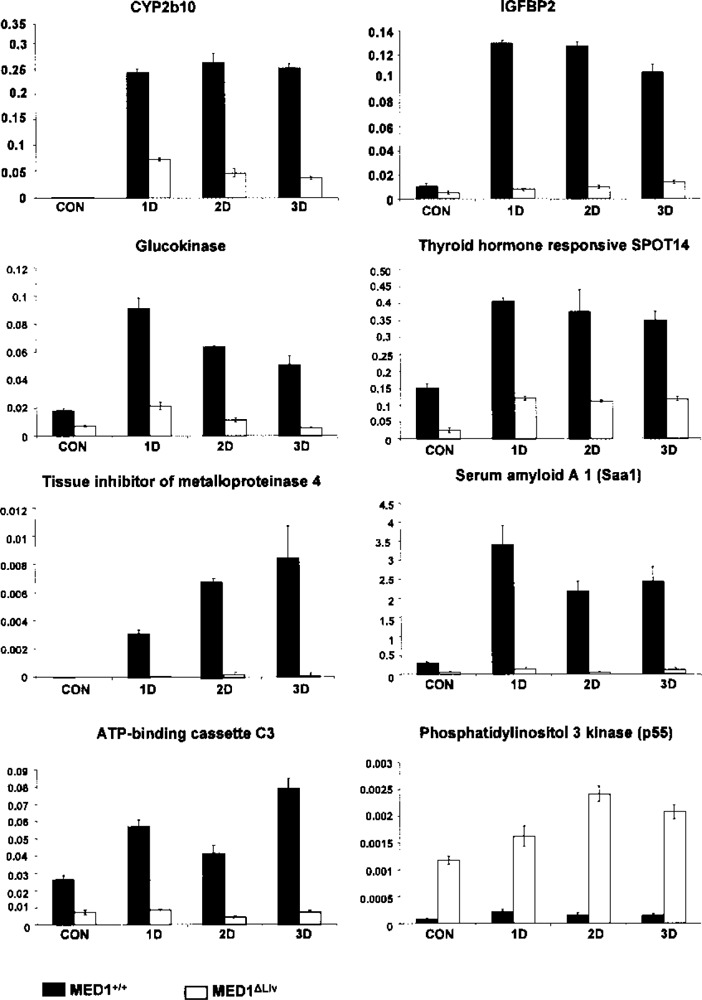
Comparative expression of genes in liver selected from microarray profile data in the livers of MED1+/+ and MED1ΔLiv mice after 1, 2, and 3 days of treatment with Dex. Increases in CYP2b10, IGFBP2, glucokinase, thyroid responsive SPOT14, tissue inhibitor of metalloproteinase 4, Saa1, and ATP-binding cassette C3 are seen in Dex-treated wild-type (black bars) compared to MED1ΔLiv mice (white bars). The microarray predicted increase in phophatidylinositol 3 kinase (p55) in Dex-treated MED1ΔLiv mouse liver is confirmed by quantitative PCR. The specific amplification of genes was normalized with 18S RNA signal and the arbitrary values are shown. All data are presented as the mean ± SD of three independent experiments.
Figure 5.
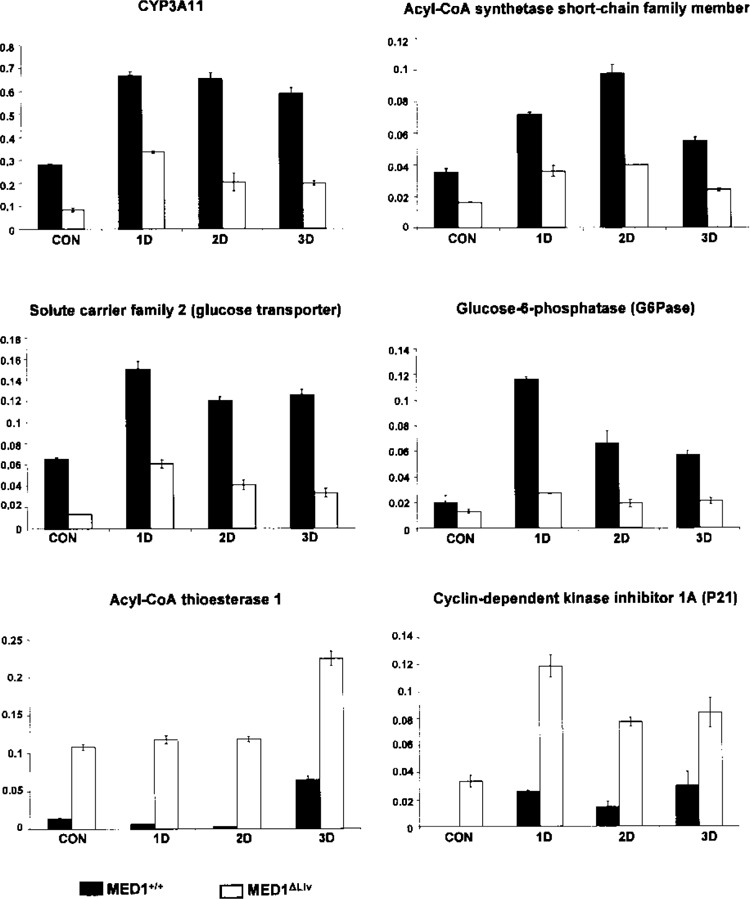
Further validation of microarray findings by quantitative PCR of hepatic RNA of MED1+/+ and MED1ΔLiv mice after 1, 2, and 3 days of treatment with Dex. Increases in CYP3A11, acyl-CoA synthetase, glucose transporter, glucose-6-phosphatase (G6pc) are seen in Dex-treated wild-type (black bars) compared to MED1ΔLiv mice (white bars). On the other hand, increases in acyl-CoA thioesterase 1 and cyclin-dependent kinase inhibitor (p21) are observed in Dex-treated MED1ΔLiv mouse liver. The specific amplification of genes was normalized with 18S RNA signal and the arbitrary values are shown. Data are shown as the mean ± SD of three independent experiments.
DISCUSSION
Nonalcoholic fatty liver disease (NAFLD) results from an excessive accumulation of TGs in hepatic parenchymal cells emanating from a variety of pathophysiological perturbations. These include metabolic diseases such as obesity, type 2 diabetes, and conditions associated with chronic increases in glucocorticoid levels resulting in sustained activation of the GR, a transcription factor (31). The GR is present in the cytosol, which, upon glucocorticoid binding, translocates into the nucleus to serve as a transcriptional regulator of distinct sets of target genes to elicit a plethora of glucocorticoid responses (10,12). Like other transcription factors, GR recruits several cofactors, including MED1, a pivotal subunit of mediator complex, for optimal transcriptional activation (3,4,8,9). Whole-body MED1 knockout mice are not viable, demonstrating the critical importance of this coactivator for proper functioning of many genes for survival (6,38). In this study, we used conditional gene disruption approach to show that the absence of MED1 in mouse liver abrogates GR agonist Dex-induced hepatic steatosis. In the liver, GR is a critical regulator of lipid and glucose homeostasis (15,26,32). Multiple genes are either repressed or activated during Dex-mediated GR activation, which determines overall energy homeostasis (10,26,32). In particular, Dex-induced fatty liver development is attributed to both increased TG synthesis in the liver, due in part to the induction of acetyl-CoA-carboxylase and fatty acid synthase, the key lipogenic enzymes (15,32), and to decrease in mitochondrial fatty acid β-oxidation by molecular mechanisms that remain largely elusive (16).
Dex activation of the GR has been shown to decrease hepatic expression of the cAMP-inducible transcriptional repressor hairy enhancer of split1 (Hes1) and this reduction appears to be a common feature of hepatic steatosis (15). Liver-specific knockdown of the GR expression has been shown to improve steatotic phenotype due to Hes1 overexpression, which represses or downregulates PPARα and its downstream regulator FSP27 and limits liver lipid accumulation (15,18,34). Our microarray data revealed approximately twofold increase in Hes1 mRNA level in liver of MED1ΔLiv mice when compared to wild-type mouse liver following Dex treatment. Thus, the effect of MED1 deficiency in liver appears similar to that observed with GR knockdown in liver. Increases in Hes1 level in GR and MED1-deficient livers could repress PPARα and its downstream lipogenic genes, resulting in the reduction of hepatic steatosis induced by Dex.
The results of this study with MED1ΔLiv mice suggest that MED1 is required for the repression as well as activation of GR-regulated genes in the liver. For example, absence of MED1 reverses Dex-mediated repression of fatty acid oxidation. As a consequence, energy burning remains unaffected in Dex-treated MED1ΔLiv mice, thus abrogating the development of hepatic steatosis. Dex and other glucocorticoids induce lipogenic enzymes such as fatty acid synthase, acetyl-CoA carboxylase, and 11pHSD1 in the liver, which add to hepatic lipid burden (32). These lipogenic genes were not induced in MED1ΔLiv liver, suggesting that MED1 is needed for GR-mediated transcriptional activation of these genes. Glucocorticoids enhance hepatic gluconeogenic capacity by upregulating PEPCK transcript levels but, as seen in the present studies, PEPCK is not induced in the liver in the absence of MED1. PEPCK gene promoter has GR recognition elements and receptor occupation of these GREs results in the recruitment of cofactors such as PGC-1, SRC-1, and CBP/p300 for optimal transcriptional activation (32). The failure of the induction of lipogenic and gluconeogenic genes in MED1-deficient livers suggests that MED1 is critical for GR transcriptional activity. MED1 has been shown to interact with GR in a ligand-dependent manner (3,4). These studies also show that the absence of MED1 in the liver affects the induction of many genes involved in drug metabolism, possibly by influencing the transcriptional functions of nuclear receptors GR, CAR, PXR, and others (10,12,25). Using MED1 liver conditional null mice we establish that MED1 is required for in vivo function of GR. Our earlier work has also shown the pivotal role of MED1 in PPARα and CAR function in liver (5,8,9,17). It should be noted that PXR and CAR expression is glucocorticoid dependent (25) and a functional GRE has been identified in the CAR gene promoter (24). Previously, work from our laboratory established that MED1 is critical for CAR function in the liver and the data presented here show that CAR levels are markedly reduced in liver in the absence of MED1, clearly establishing that MED1 is need for GR-CAR signal transmission. CAR, like GR, is a cytoplasmic receptor, which when activated by a ligand translocates to the nucleus and that translocation is MED1 dependent (5,9). Whether GR translocation to the nucleus in the liver is MED1 dependent remains to be established. These studies clearly establish the importance of using tissue-specific deletion of MED1 to study the role of this coregulator in gene expression.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Grants GM23750 (J.K.R.), CA104578 (J.K.R.), and DK083163 (J.K.R.).
REFERENCES
- 1. Aluru N.; Vijayan M. M. Hepatic transcriptiome response to glucocorticoid receptor activation in rainbow trout. Physiol. Genomics 31:483–491; 2007. [DOI] [PubMed] [Google Scholar]
- 2. Cambell M. C.; Anderson G. W.; Mariash C. N. Human Spot 14 glucose and thyroid hormone response: Characterization and thyoid hormone response element identification. Endocrinology 144:5242–5248; 2003. [DOI] [PubMed] [Google Scholar]
- 3. Chen W.; Rogatsky I.; Garabedian M. J. MED14 and MED1 differentially regulate target-specific gene activation by glucocorticoid receptor. Mol. Endocrinol. 20:560–572; 2006. [DOI] [PubMed] [Google Scholar]
- 4. Chen W.; Roeder R. G. The mediator subunit MED1/ TRAP220 is required for optimal glucocorticoid receptor-mediated transcription activation. Nucleic Acids Res. 35:6161–6169; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo D.; Sarkar J.; Ahmed M. R.; Viswakarma N.; Jia Y.; Yu S.; Rao M. S.; Reddy J. K. Peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP) but not PPAR-interacting protein (PRIP) is required for nuclear translocation of constitutive androstane receptor in mouse liver. Biochem. Biophys. Res. Commun. 347:485–495; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Ito M.; Yuan C. X.; Okano H. J.; Darnell R. B.; Roeder R. G. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol. Cell 5:683–693; 2000. [DOI] [PubMed] [Google Scholar]
- 7. James C. G.; Ulici V.; Tuckermann J.; Underhill T. M.; Beier F. Expression profiling of dexamethasone-treated primary chondrocytes identifies targets of glucocorticoid signaling in endochondral bone development. BMC Genomics 8:205; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia Y.; Qi C.; Kashireddi P.; Surapureddi S.; Zhu Y. J.; Rao M. S.; Le Roith D.; Chambon P.; Gonzalez F. J.; Reddy J. K. Transcription coactivator PBP, the peroxisome proliferators-activated receptor (PPAR)-binding protein, is required for PPARα-regulated gene expression in liver. J. Biol. Chem. 279:24427–24434; 2004. [DOI] [PubMed] [Google Scholar]
- 9. Jia Y.; Guo G. L.; Surapureddi S.; Sarkar J.; Qi C.; Guo D.; Xia J.; Kashireddi P.; Yu S.; Cho Y. W.; Rao M. S.; Kemper B.; Ge K.; Gonzalez F. J.; Reddy J. K. Transcription coactivator peroxisome proliferators-activated receptor binding protein/mediator 1 deficiency abrogates acetaminophen hepatotoxicity. Proc. Natl. Acad. Sci. USA 102:12531–12536; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. John S.; Johnson T. A.; Sung M-H.; Biddie S. C.; Trump S.; Koch-Paiz C. A.; Davis S. R.; Walker R.; Meltzer P. S.; Hager G. L. Kinetic complexity of the global response to glucocorticoid receptor action. Endocrinology 150:1766–1774; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang H. S.; Beak J. Y.; Kim Y-S.; Petrovich R. M.; Collins J. B.; Grissom S. F.; Jetten A. M. NABP1, a novel ROR-regulated gene encoding a single-stranded nucleic-acid-binding protein. Biochem. J. 397:89–99; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kassel O.; Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspects. Mol. Cell. Endorinol. 275:13–29; 2007. [DOI] [PubMed] [Google Scholar]
- 13. Kimura H.; Li X.; Torii K.; Okada T.; Kamiyama K.; Mikami D.; Takahashi N.; Yoshida H. Dexamethasone enhances basal and TNF-α-stimulated production of PAI-1 via the glucocorticoid receptor regardless of 11β-hydroxysteroid dehydrogenase 2 status in human proximal renal tubular cells. Nephrol. Dial. Transplant. (in press; Doi:10.1093/ndt/gfn756). [DOI] [PubMed] [Google Scholar]
- 14. Kornberg R. D. Mediator and the mechanisms of transcriptional activation. Trends Biochem. Sci. 30:235–239; 2005. [DOI] [PubMed] [Google Scholar]
- 15. Lemke U.; Krones-Herzig A.; Diaz M. B.; Narvekar P.; Zeigler A.; Vegiopoulos A.; Cato A. C.; Bohl S.; Klingmuller U.; Screaton R. A.; Decker K. M.; Kersten S.; Herzig S. The glucocorticoid receptor controls hepatic dyslipidemia through Hes 1. Cell Metab. 8:212–223; 2008. [DOI] [PubMed] [Google Scholar]
- 16. Letteron P.; Brahimi-Bourouina N.; Robin M-A.; Moreau A.; Feldman G.; Pessayre D. Glucocorticoids inhibit mitochondrial matrix acyl-CoA dehydrogenases and fatty acid β-oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 272:G1141–G1150; 1997. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto K.; Yu S.; Jia Y.; Ahmed M. R.; Viswakarma N.; Sarkar J.; Kashireddy P. V.; Rao M. S.; Karpus W.; Gonzalez F. J.; Reddy J. K. Critical role for transcription coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein/TRAP220 in liver regeneration and PPARα ligand-induced liver tumor development. J. Biol. Chem. 282:17053–17060; 2007. [DOI] [PubMed] [Google Scholar]
- 18. Matsusue K.; Kusakabe T.; Noguchi T.; Takiguchi S.; Suzuki T.; Yamano S.; Gonzalez F. J. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 7:302–311; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKenna N. J.; O’Malley B. W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474; 2002. [DOI] [PubMed] [Google Scholar]
- 20. Micuda S.; Fuksa L.; Mundlova L.; Osterreicher J.; Mokry J.; Cermanova J.; Brcakova E.; Staud F.; Pokorna P.; Martinkova J. Morphological and functional changes in P-glycoprotein during dexamethasone-induced hepatomegaly. Clin. Exp. Pharmacol. Physiol. 34:296–303; 2007. [DOI] [PubMed] [Google Scholar]
- 21. Miyazawa S.; Ozasa H.; Osumi T.; Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J. Biochem. 94:529–542; 1983. [DOI] [PubMed] [Google Scholar]
- 22. Novosyadlyy R.; Lelbach A.; Sheikh N.; Tron K.; Pannem R.; Ramadori G.; Scharf J.-G. Temporal and spatial expression of IGF-1 and IGFBP-1 during acute-phase response induced by localized inflammation in rats. Growth Hormone IGF Res. 19:51–60; 2009. [DOI] [PubMed] [Google Scholar]
- 23. Nyirenda M. J.; Lindsay R. S.; Kenyon C. J.; Burchell A.; Seckel J. R. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenoylpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J. Clin. Invest. 101:2174–2181; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pascussi J-M.; Coniat M. B. L.; Maurel P.; Vilarem M-J. Transcriptional analysis of the orphan nuclear receptor constitutive androstane receptor (NR113) gene promoter: Identification of a distal glucocorticoid response element. Mol. Endocrinol. 17:42–55; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Pascussi J-M.; Gerbal-Chaloin S.; Duret C.; Daujat-Chavanieu M.; Vilarem M-J.; Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: Crosstalk and consequences. Annu. Rev. Pharmacol. Toxicol. 48:1–32; 2008. [DOI] [PubMed] [Google Scholar]
- 26. Paterson J. M.; Holmes M. C.; Kenyon C. J.; Carter R.; Mullins J. J.; Seckl J. R. Liver-selective transgene rescue of hypothalamic-pituitary-adrenal axis dysfunction in 11-β-hydroxysteroid dehydrogenase type 1 deficient mice. Endocrinology 148:961–966; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qatanani M.; Wei P.; Moore D. D. Alterations in the distribution and orexigenic effects of dexamethasone in CAR-null mice. Pharmacol. Biochem. Behav. 78:285–291; 2004. [DOI] [PubMed] [Google Scholar]
- 28. Rachez C.; Lemon B. D.; Suldan Z.; Bromleigh V.; Gamble M.; Naar A. M.; Erdjument-Bromage H.; Tempst P.; Freedman L. P. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398:824–828; 1999. [DOI] [PubMed] [Google Scholar]
- 29. Sonoda J.; Pei L.; Evans R. M. Nuclear receptors: Decoding metabolic disease. FEBS Lett. 582:2–9; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stienstra R.; Mandard S.; Patsouris D.; Maass C.; Kersten S.; Muller M. Peroxisome proliferator-activated receptor protects against obesity-induced hepatic inflammation. Endocrinology 148:2753–2763; 2007. [DOI] [PubMed] [Google Scholar]
- 31. Targher G.; Bertolini L.; Rodella S.; Zoppini G.; Zenari L.; Falezza G. Associations between liver histology and cortisol secretion in subjects with nonalcoholic fatty liver disease. Clin. Endocrinol. (Oxf.) 64:337–341; 2006. [DOI] [PubMed] [Google Scholar]
- 32. Vegiopoulos A.; Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 275:43–61; 2007. [DOI] [PubMed] [Google Scholar]
- 33. Yamazaki Y.; Kakizaki S.; Horiguchi N.; Sohara N.; Sato K.; Takagi H.; Mori M.; Negishi M. The role of the nuclear receptor constitutive androstane receptor in the pathogenesis of non-alcoholic steatohepatitis. Gut 56:565–574; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu S.; Matsusue K.; Kashireddy P.; Cao W-Q.; Yeldandi V.; Yeldandi A. V.; Rao M. S.; Gonzalez F. J.; Reddy J. K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) over-expression. J. Biol. Chem. 278:498–505; 2003. [DOI] [PubMed] [Google Scholar]
- 35. Yuan C. X.; Ito M.; Fondell J. D.; Fu Z. Y.; Roeder R. G. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc. Natl. Acad. Sci. USA 95:7939–7944; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou J.; Li W.; Kamei H.; Duan C. Duplication of the IGFBP-2 gene in teleost fish: Protein structure and functionality conservation and gene expression divergence. PLoS One 3:e3926; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu Y.; Qi C.; Jain S.; Rao M. S.; Reddy J. K. Isolation and characterization of PBP, a protein that interacts with peroxisome proliferator-activated receptor. J. Biol. Chem. 272:25500–25506; 1997. [DOI] [PubMed] [Google Scholar]
- 38. Zhu Y.; Qi C.; Jia Y.; Nye J. S.; Rao M. S.; Reddy J. K. Deletion of PBP/PPARBP, the gene for nuclear receptor coactivator peroxisome proliferator-activated receptor-binding protein, results in embryonic lethality. J. Biol. Chem. 275:14779–14782; 2000. [DOI] [PubMed] [Google Scholar]


