Abstract
Objective:
After intravenous (i.v.) injection of lipopolysaccharide (LPS) macrophages release nitric oxide (NO) due to the expression of the inducible NO synthase (iNOS). After LPS NO is abundantly produced also in the cardiovascular system and may contribute to the development of hypotension and shock. Since the immune response, the synthesis of NO and the regulation of blood pressure (BP) differ between males and females, in the present study the effect of LPS on BP, renal function, the plasma and urinary concentration of the metabolites of NO as well as the splenic and aortic expression of the iNOS gene were compared between male and female rats.
Methods:
BP and renal function were measured in anesthetized rats following the i.v. injection of LPS (E. coli, 4 mg/kg). The and (metabolites of NO=NOx) concentration was measured by the Griess reaction. The iNOS gene expression was studied by RT-PCR.
Results:
Four hours after LPS, BP of males (n=9) was reduced by 63±12 mmHg versus 10±4 in females (n=7, P<0.005). Aminoguanidine, a selective inhibitor of iNOS, prevented the reduction of BP in males. The plasma concentration of NOx (PNOx, μM) was lower in hypotensive males (128±20) than in normotensive females (235±29, P<0.005). Males also exhibited lower urinary NOx excretion (UNOxV) after LPS (P<0.001 vs. females). Prior castration of males provided protection against hypotension (fall of BP: −4±4 mmHg, n=6, P<0.02 versus males) and resulted in higher PNOx as well as UNOxV (both P<0.001 versus males and not different from females). Prior ovariectomy (n=5) had no influence on the hemodynamic and NOx response to LPS. Male rats displayed enhanced aortic iNOS/beta-actin ratio relative to females after LPS (n=3 in each group, P<0.05).
Conclusions
(1) Male gender may sensitize to LPS-induced shock and (2) sensitivity of males to endotoxin is associated with an attenuated, not exaggerated total rate of NO synthesis.
Keywords: Endotoxins, gender, Nitric oxide, Shock
1. Introduction
Sepsis and endotoxic shock are major medical complications with a high mortality rate, caused by lipopolysaccharide (LPS), a component of Gram-negative bacteria [1]. LPS has many effects, some of which are expressed indirectly, through mediators such as tumor necrosis factor, interleukin-1 and 6, interferon, thromboxane A2, platelet activating factor, superoxide anion and the potent vasodilator, nitric oxide (NO) [2]. Of these, NO apparently plays a primary role in the development of circulatory shock which occurs during endotoxemia. Endotoxic shock in man [2-5], and administration of LPS in experimental animals [6-17] is associated with greatly enhanced synthesis of NO by a newly induced NO synthase (NOS), or inducible NOS (iNOS). In man the severity of symptoms including hypotension correlates with the plasma and urinary concentration of and , the stable oxidation products of NO [5]. The NOx concentration of body fluids is determined by several sources, among them activated macrophages ingesting LPS. A clinically important cardiovascular overproduction of NO is evidenced by the successful prevention of circulatory shock during endotoxemia by the pharmacological prevention of iNOS expression with glucocorticoids [10] or posttranscriptional inhibition with aminoguanidine [11]. The importance of iNOS in endotoxin shock is reflected by iNOS knock-out mice which are resistant to LPS-induced hypotension [12]. In man, nonselective NOS inhibitors are currently the only agents which restore vascular sensitivity to infused norepinephrine during septic shock [4].
Estrogens can be protective against death after endotoxin [18], and different forms of major tissue injury [19]. In response to LPS, interleukin-1 production is much greater in monocytes of female vs. male mice [20], demonstrating an enhanced responsiveness of the macrophage system (the first line of defence) during endotoxemia [2,21]. Chemotaxis and phagocytic function of macrophages are also higher in female than male mice [6]. On the other hand, androgens suppress immunity [22-26]. Sepsis may have a higher incidence and worse outcome in men vs. women [27] and in male vs. female mice [24]. Of note, most rat studies published on LPS-induced shock have been performed on males [8-12,15,17,18,26,28].
These observations suggest that females may enjoy some protection against endotoxin and soft tissue trauma-induced circulatory shock. The present studies were conducted to test this hypothesis in the LPS-induced model of shock in the rat, and were specifically designed to investigate the relationship between NO production, gender and susceptibility to hypotension.
2. Methods
This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised in 1996).
In the first series, studies were conducted on Sprague–Dawley rats (25 males and 19 females aged 3–5 months) obtained from Harlan Sprague–Dawley, Inc. (Indianapolis). Rats were shipped to the West Virginia University Medical School Animal Care Facility at least 7 days prior to study and were allowed ad libitum access to drinking water and a low-nitrate diet (AIN 76C semipurified diet, ICN Pharmaceuticals, Inc., Costa Mesa, to CA; NOx content 125 μmol/kg) for at least 4 days prior acute study.
On the day of study, general anesthesia was induced (Inactin, 120 mg/kg i.p.), rats were put on a temperature-controlled table and prepared as described earlier for the measurement of blood pressure (BP) and renal function [29,30]. Briefly, the trachea, the left jugular vein, the left femoral vein and artery were cannulated. The surgery was clean but not sterile. The arterial catheter contained sterile physiological saline with 50 U/ml heparin and was connected to a pressure transducer and a polygraph (ICT-2H Demograph, Gilson, Middletown, WI) for the measurement of BP, heart rate (HR) and the collection of blood samples. Tritiated inulin (2–5 μCi/ml) and para-amino-hippuric acid (PAH, 1%) in 0.9% NaCl was infused at a rate of 5 μl/min/100 g body weight through the jugular vein, with an initial bolus of 0.4 ml. Since no precautions were taken to maintain euvolemia, these animals were moderately volume contracted during the experiment. After a 30-min stabilization period baseline BP and HR were recorded and then either vehicle or Escherichia coli lipopolysaccharide (LPS serotype 055:B5, Difco, Detroit, MI, 0.4 mg/100 g) was injected over 2 min into the femoral vein. A continual series of urine collections were made from the time of administration of LPS or vehicle (0 min) for the next 260 min. Urine 1 from 0 to 100 min, urine 2 from 101 to 120, urine 3 from 121 to 180 min, urine 4 from 181 to 200 min, urine 5 from 201 to 240 min and urine 6 from 241 to 260 min after vehicle/LPS. The urine flow-rate (V) and the excretion (UNOxV) was measured on all samples while BP and HR were continuously recorded. Three 20-min clearance periods were performed, at ~2, 3 and 4 h after LPS (urine collections 2, 4 and 6), in which measurements were made of renal plasma flow (RPF), glomerular filtration rate (GFR), the urinary excretion of sodium (UNaV), hematocrit (hct) and plasma protein concentration (Ppr), as described earlier [29,30]. After centrifugation of blood, plasma was separated for analysis and blood cells were resuspended in sterile 0.9% NaCl and restored to the rat. At the end of the last clearance period (260 min after LPS or vehicle) a terminal blood sample (~6 ml) was collected into EDTA and the plasma saved for NOx measurement. Plasma and urine samples were stored at −70°C until assay. Seminal vesicles and right uterine horns were harvested for the measurement of wet weight.
The following groups of rats were studied: For time controls (TC), six intact males (bw: 382±23 g, mean±S.E.) and seven intact females (bw: 265±5 g) received vehicle (40 μl 0.9% NaCl/100 g bw); nine intact males (bw: 398±10 g) and seven intact females (bw: 249±4 g) received LPS (0.4 mg/100 g bw); six male rats were given LPS 7–10 days after castration (CAST, bw: 394±12 g) and five females were given LPS 7–10 days after ovariectomy (OVEC, bw: 278±8 g). Castrations were under general anesthesia (Brevital, Eli Lilly and Co., Indianapolis; 50 mg/kg i.p.) using full sterile technique. An additional group of four male rats (bw: 370±10 g) were studied only for BP and were pretreated with aminoguanidine (AG), 25 mg/100 g bw gavage, 18 and 2 h prior to injection of LPS.
The second series of experiments was performed on 4–5 months old Wistar rats (nine males, bw: 487±15 g and nine females bw: 301±14 g) from the inbred colony of the Semmelweis University, Budapest, Hungary. Prior to study rats were provided ad libitum access to drinking water and rat chow (Gödöllõ, Hungary). On the day of study, six males and six females were anesthetized and prepared for the measurement of BP and administration of vehicle or LPS, as described above. Three males and three females received LPS and three rats of each sex received the vehicle. BP was measured for 4 h and then a terminal blood sample was collected and the spleen and the aorta were harvested for RNA isolation. A blood sample and the aorta were also collected from three intact male and three intact female rats. Tissues were harvested in 500 μl of cold lysis solution (4 M guanidine isothiocyanate [GITC], 25 mM sodium citrate [pH 7.0], 0.1 M beta-mercaptoethanol, 0.5% sarcosyl [all from Sigma, St. Louis, MO]), frozen in liquid nitrogen and stored on −80°C.
2.1. Analytical techniques
Urine volume (collected under mineral oil) was measured gravimetrically and the urine was analysed for tritiated inulin activity and PAH concentration. Arterial blood samples were analysed for hct, Ppr, tritiated inulin activity, and PAH concentration. Tritiated inulin activity was measured in 10-μl samples of urine and plasma in a scintillation counter. PAH concentration was measured colorimetrically, as described previously [29,30]. Plasma protein concentration was measured on a refractometer. These measurements allow calculation of inulin clearance which equals GFR, and PAH clearance, which when factored for renal PAH excretion (=0.85) gives renal plasma flow (RPF). Renal vascular resistance (RVR) and UNaV were calculated as described elsewhere [29,30]. Urine and filtered plasma samples were analyzed for NOx concentration, by the Griess assay as described by us previously [29,30]. UNOxV was expressed as an average excretion over the 4-h collection period, because of the unreliability of spot urinary NOx excretion as an indicator of NO production [29].
2.2. Isolation of RNA
RNA was extracted according to the method of Chomczinsky et al. [31]. Briefly, frozen tissues were mixed with 4 ml GITC buffer and acid phenol–chloroform (pH 4, Roth GmbH, Karlsruhe, Germany) and homogenized. The samples were centrifuged at 1500×g for 10 min at 20°C. The supernatant was collected and treated with an equal volume of isopropanol. The mixture was centrifuged and the RNA was washed with Rneasy using the total RNA Isolations Kit (Qiagen GmbH, Hilden, Germany) and stored at −80°C until further processing. RNA concentration was measured spectrophotometrically.
2.3. Reverse transcription (RT)
RNA was amplified by RT with an Oligo(dT)12–18 primer (Gibco/BRL, Life Technologies GmbH, Eggenstein, Germany). One μg of total RNA was added to 0.5 μg of primer. A reaction mixture containing 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 5 mM MgCl2 and 5 mM dithiotreitol (Gibco), adenosine triphosphate, thymidine triphosphate, guanosine triphosphate, cytosine triphosphate, 2 mM each, (Boehringer GmbH, Mannheim, Germany), and 0.5 μl of 40 U/μl recombinant ribonuclease inhibitor (Promega Deutschland GmbH, Mannheim, Germany) and 0.5 μl of 200 U/μl M-MLV reverse transcriptase (Gibco)) were added and the first chain reaction allowed to proceed (42°C, 1 h). The reaction was halted by heating to 95°C for 5 min followed by cooling on ice.
2.4. Amplification of specific complementary DNA (cDNA)
Specific cDNA products corresponding to mRNA for rat iNOS (5′-GTGTTCACCAGGAGATGTTG-3′ and 5′-CTCCTGCCCACTGAGTTCGTC-3′, 576 bp) [32] and beta-actin (5′-CTATCGGAATGAGCGGTTC-3′ and 5′-CTTAGGAGTTGGGGGTGGCT-3′, 762 bp) [33] were amplified using the polymerase chain reaction (PCR). One μl from the RT reaction was taken for PCR, which was performed in PCR buffer (750 mM Tris–HCl, pH 9.0, 200 mM (NH4)2SO4, 0.1% (w/v) Tween, 20 mM MgCl2, (Dianova, Hamburg, Germany), to which 0.2 mM of deoxy nucleoside triphosphates were added. The mixture contained 1 μM of both primers (Eurogentec, Sevaing, Belgium), and 2.5 U Thermus aquaticus (Taq) DNA polymerase (Dianova). A Perkin-Elmer Thermal Cycler (Model 2400, Norwalk, CT) was used for amplification with the following sequence profile: initial denaturation at 94°C for 3 min followed by 30 cycles (beta-actin) and 40 cycles (iNOS) of three temperature PCR (denaturing: 94°C for 30 s; annealing: 50°C [beta-actin] and 55°C [iNOS] for 30 s; and extension, 72°C for 30 s) ending with a final extension at 72°C for 7 min and cooling to 4°C.
2.5. Gel electrophoresis
The amplified PCR product was identified by electrophoresis of 10-μl aliquots on 1.5% agarose gel stained with 0.5 μg/ml of ethidium bromide. The sample products were visualized by UV transillumination and the gel was photographed. Specific products were identified by size in relation to a known 1 kb oligonucleotide DNA ladder (Gibco/BRL) run with each gel. iNOS cDNA was semiquantitated by densitometric comparison with beta-actin (internal control) from the same sample after the positive image was digitized by video for computerized densitometry. The results are given as the ratio of intensity of iNOS and beta-actin mRNA.
2.6. Statistics
Data are expressed as means±S.E. The statistical analysis of data was performed by ANOVA of repeated measures with the post-hoc Student's–Newman–Kuel test using SPSS for Windows. Statistical significance was noted when P<0.05.
3. Results
3.1. Time-control (TC) rats
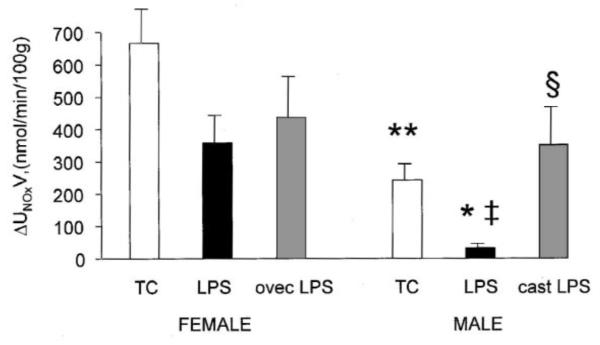
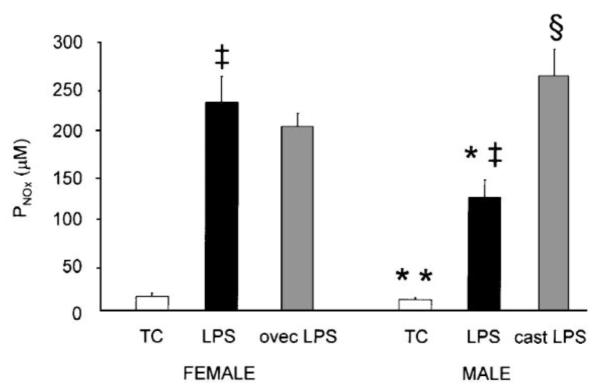
During the 260 min of observation, baseline BP of TC male (114±4 mmHg) and female (113±4) rats fell ~10 mmHg (ns), HR, hct and Ppr were stable (Table 1, Fig. 1). GFR, and UNaV were also constant over 2–4 h although RVR declined with time in both sexes and urine flow (V) fell in males (Table 2). The 260 min UNOxV was higher in females (667±107 nmol/min/100 g) vs. males (243±36, P<0.01, Fig. 2), whereas the average V was similar between the sexes. There was no difference in the plasma concentration of NOx (PNOx) between the sexes (16.6±3.2 μM in females and 12.8±1.8 in males, ns., Fig. 3).
Table 1.
Effect of LPS and gonadectomy+LPS on heart rate (HR), hematocrit (hct) and plasma protein concentration (Ppr ) in SD rats (mean±S.E.)
| Group | Hours after LPS/saline |
HR (bpm) |
Hct (%) |
Ppr (g %) |
|---|---|---|---|---|
| Male time- control (n=6) |
0 | 360±9 | – | – |
| 2 | 372±14 | 45±1 | 6.1±0.1 | |
| 3 | 377±10 | 44±1 | 6.2±0.1 | |
| 4 | 387±12 | 44±1 | 6.1±0.1 | |
| Male LPS (n=9) |
0 | 371±8 | – | – |
| 2 | 453±12** | 55±1** | 5.0±0.2** | |
| 3 | 453±23** | 51±1* | 4.7±0.2** | |
| 4a | 435±25(6)* | 49±1(6) | 4.5±0.3(6)** | |
| Cast LPS (n=6) |
0 | 327±9*** | – | – |
| 2 | 395±11*** | 48±0 | 5.4±0.1 | |
| 3 | 415±9 | 45±0 | 5.1±0.04 | |
| 4 | 435±6 | 44±1 | 5.2±0.1 | |
| Female time-cont. (n=7) |
0 | 372±11 | – | – |
| 2 | 372±11 | 45±1 | 6.1±0.1 | |
| 3 | 377±10 | 44±1 | 6.2±0.1 | |
| 4 | 387±12 | 44±1 | 6.1±0.1 | |
| Female LPS (n=7) |
0 | 364±13 | – | – |
| 2 | 398±9 | 49±1* | 5.0±0.2** | |
| 3 | 438±5** | 46±1 | 4.6±0.2** | |
| 4 | 450±10* | 45±1 | 4.7±0.2** | |
| Ovec+LPS (n=5) |
0 | 339±12 | – | – |
| 2 | 402±7 | 45±1 | 5.0±0.2 | |
| 3 | 396±10 | 41±1 | 4.6±0.1 | |
| 4 | 423±12 | 39±1 | 4.6±0.1 |
Three of nine LPS-treated males died between the 3rd and 4th hour.
P<0.002–0.05,
P<0.001 vs. time-cont;
P<0.05 vs. LPS-treated intact males.
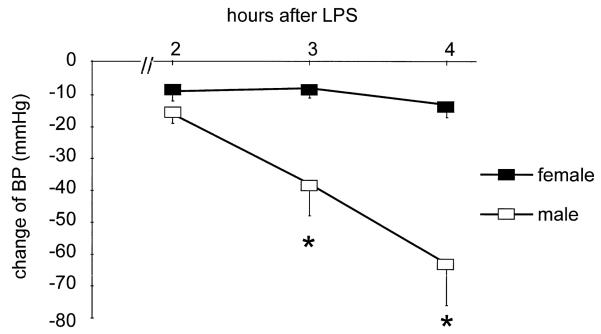
Fig. 1.
Change of mean arterial blood pressure (BP) in anesthetized female (n=7) and male (n=9) rats after the bolus i.v. injection of LPS (4 mg/kg) (mean ± S.E.). The baseline BP of females and males was 101±4 and 114 ±4 mmHg, respectively. * P<0.01 vs. female.
Table 2.
Effect of LPS and gonadectomy+LPS on renal function in SD rats (mean±S.E.)
| Group | Hours after LPS/ vehicle |
RPF (ml/min/ 100 g bw) |
RVR (mmHg/ml/ min/100 g bw) |
GFR (ml/min/ 100 g bw) |
UNaV (mmol/min/ 100 g bw) |
V (μl/min/ 100 g bw) |
Average urine flow through 260 min after LPS/vehicle (μl/min/100 g bw) |
|---|---|---|---|---|---|---|---|
| Male time- control (n=6) |
2 | 4.01±0.27 | 30.0±2.3 | 0.59±0.03 | 0.62±0.2 | 11±2 | 9±0.8 |
| 3 | 4.03±0.10 | 26.9±1.4 | 0.58±0.01 | 0.48±0.10 | 6±0.4 | ||
| 4 | 4.19±0.36 | 26.1±2.1 | 0.53±0.04 | 0.47±0.08 | 5±0.5 | ||
| Male LPS (n=9) |
2 | 3.15±0.33 | 31.8±2.8† | 0.29±0.04†‡ | 0.03±0.01†‡ | 3±0.3†‡ | 3±0.6†‡ |
| 3 | 2.86±0.90 | 71.9±22.3 | 0.31±0.08†‡ | 0.09±0.04†‡ | 7±3 | ||
| 4 (n=6)a | 3.56±1.08 | 47.9±18.5 | 0.26±0.06†‡ | 0.10±0.04†‡ | 6±2 | ||
| Cast+LPS (n=6) |
2 | 4.81±0.44 | 22.3±1.9 | 0.39±0.04 | 0.06±0.03 | 3±0.4 | 3±0.4 |
| 3 | 3.05±0.42 | 37.7±5.4 | 0.35±0.05 | 0.15±0.03 | 4±0.3 | ||
| 4 | 2.45±0.33 | 44.5±4.7 | 0.25±0.03 | 0.11±0.03 | 3±0.3 | ||
| Female time- control (n=7) |
2 | 4.29±0.38 | 28.3±1.9 | 0.69±0.06 | 1.22±0.27 | 12±2 | 12±2 |
| 3 | 5.03±0.51 | 23.2±2.1 | 0.7±0.05 | 1.55±0.34 | 13±3 | ||
| 4 | 5.37±0.45 | 20.6±1.9 | 0.73±0.07 | 1.31±0.19 | 9.5±2 | ||
| Female LPS (n=7) |
2 | 5.77±0.73 | 16.6±2.2‡ | 0.62±0.06 | 0.5±0.28 | 8±2 | 8±2 |
| 3 | 4.78±0.29 | 18.6±1.4 | 0.55±0.01 | 1.84±0.91 | 15±6 | ||
| 4 | 3.42±0.38‡ | 24.1±5.3 | 0.43±0.06 | 1.16±0.68 | 9±4 | ||
| Ovec+LPS (n=5) |
2 | 4.67±1.25 | 28.9±7.1* | 0.47±0.09 | 0.06±0.01 | 4±1 | 5±0.6 |
| 3 | 3.52±0.43 | 28.6±2.8 | 0.39±0.02 | 0.88±0.24 | 9±1 | ||
| 4 | 3.32±0.54 | 33.9±7.1 | 0.29±0.02 | 0.51±0.1 | 6±1 |
Three of nine LPS-treated males died between the 3rd and 4th hour.
P<0.05 vs. female;
P<0.05 vs. time-cont;
P<0.05 vs. LPS-treated intact females.
Fig. 2.
Average urinary excretion of , the stable metabolites of NO, in time-control (TC), LPS-treated and gonadectomized+LPS-treated male and female rats (mean±S.E.). LPS, lipopolysaccharide treatment; cast, castration; ovec, ovariectomy. * P<0.05, ** P<0.001 vs. female; ‡ P<0.001 vs. TC; § P<0.001 vs. intact male.
Fig. 3.
Plasma concentration of at 4 h after treatment in time-control (TC), LPS-treated and gonadectomized+LPS-treated male and female rats (mean±S.E.). LPS, lipopolysaccharide treatment; cast, castration ovec, ovariectomy. * P<0.02 vs. female; ‡ P<0.001 vs. TC; § P<0.001 vs. intact male.
3.2. The effect of LPS
The administration of LPS caused circulatory shock in male but not female rats (Fig. 1, Table 1). BP fell dramatically in males at 3 and 4 h after LPS and 3/9 died between 3 and 4 h. None of the LPS-treated females died, and their BP profile was similar to the TC females. Tachycardia, increased hct at 2 h and decreased Ppr were seen in both sexes in response to LPS (Table 1). Males developed a pronounced decline in GFR, whereas in females GFR was only slightly reduced at 4 h (Table 2). UNaV was very low in males with LPS and less affected in females. Because of the variability of the RVR response, the progressive increase in males given LPS was not statistically significant whereas females showed an early fall in RVR vs. TC. RVR was higher at 2 and 4 h post-LPS in males vs. females (Table 2). In males, LPS caused a large fall in UNOxV and increased PNOx vs. TC whereas in females, UNOxV was non significantly reduced and a large rise occurred in PNOx vs. TC (Figs. 2 and 3). The average value of V during 4 h of collection was ~50% less in males than females, while UNOxV was ~90% lower in males vs. females given LPS (Table 2, Fig. 2). Despite the low UNOxV and declines in GFR, the PNOx was lower in LPS-treated males, than females (P<0.05, Fig. 3), indicating that the overall synthesis of NO induced by LPS was less in male than female rats. Pretreatment with the iNOS inhibitor AG completely prevented the development of hypotension in male rats. BP was 107±5 mmHg before and 108±10 mmHg at 240 min after LPS administration.
3.3. Effect of gonadectomy
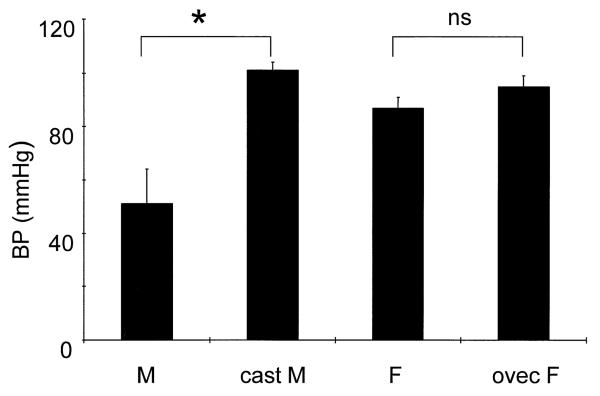
Seven to ten days after gonadectomy males had atrophied seminal vesicles (wet weight: 0.11±0.02 g vs. 0.37±0.04 g of intact males, P<0.01). Ovariectomized females had atrophied uteri (left horn wet weight: 0.09±0.02 g vs. 0.55±0.06 g of intact females, P<0.01). Castrated males were completely resistant to the hypotensive effect of LPS (Table 1, Fig. 4), but tachycardia, increased hct, decreased Ppr and deterioration of renal function still occurred (Tables 1 and 2). Both the UNOxV and the PNOx were higher than in intact males after LPS and indistinguishable from LPS-treated intact or ovec females (Figs. 2 and 3). Ovariectomized females remained resistant to LPS-induced falls in BP and GFR as did intact females. Thus, the male sensitivity to LPS-induced shock and the concomitant, relatively lower rate of overall NO production is apparently due to the presence of the testes (androgens) and not the absence of ovaries (estrogens).
Fig. 4.
Castration (cast M) performed 7–10 days prior to the administration of bacterial lipopolysaccharide prevents hypotension seen in intact males (M). Prior ovariectomy (ovec F) has no influence on the blood pressure effect of LPS. * P<0.02 vs. intact male; ns: not significant vs. intact female (F).
3.4. Effects of LPS on BP and tissue iNOS gene expression
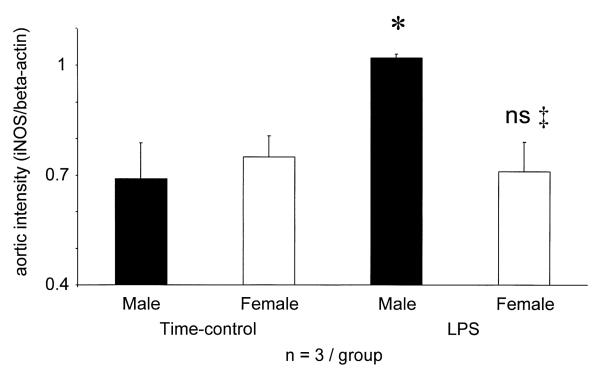
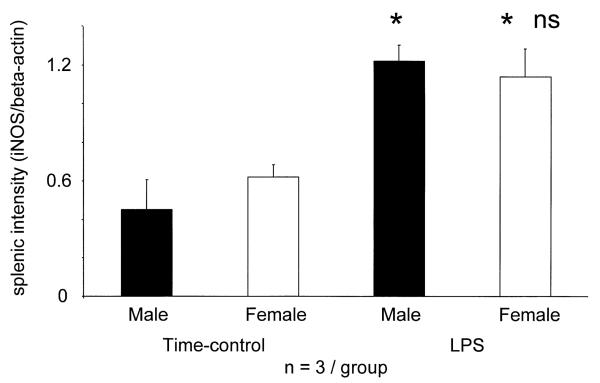
The aorta of intact, Wistar male (n=3) and female (n=3) rats, not subjected to prior surgery, contained beta-actin but no detectable iNOS gene (not shown). In contrast, the aorta of TC males and females (subjected to surgery and given vehicle) showed similar iNOS gene expression (Fig. 5). TC males had slightly lower BP at 4 h, females showed no change (P<0.05 males vs. females, Table 3). No rats given LPS had died, but BP of males fell by 48±8 mmHg, whereas in females only 10±10 mmHg (P<0.002 vs. males) (Table 3). In the aorta of males, but not females, LPS enhanced the induction of the iNOS gene above that seen in TC (Fig. 5). Intact, unprepared rats expressed beta-actin, but no iNOS gene in the spleen (not shown), whereas spleens of TC males and females had similar iNOS/beta-actin ratios. LPS treatment increased the splenic iNOS gene expression similarly in both sexes (Fig. 6).
Fig. 5.
Aortic iNOS gene expression (iNOS/beta-actin ratio) of surgically prepared and vehicle-treated time-control (TC) and surgically prepared and LPS treated male and female rats. iNOS mRNA was not detectable in completely intact, unprepared male (n=3) and female (n=3) rats. * P<0.05 vs. time-control male, or female; ns: not significant vs. LPS male.
Table 3.
Effect of lipopolysaccharide (LPS) and vehicle (NaCl) on the course of mean arterial blood pressure (BP) in Wistar rats (mean±S.E.)a
| Male |
Female |
|||
|---|---|---|---|---|
| LPS (n=3) |
Vehicle (n=3) |
LPS (n=3) |
Vehicle (n=3) |
|
| Baseline BP (mmHg) |
122±8 | 120±6 | 118±7 | 106±3 |
| BP 4 h after LPS/NaCl |
77±8†‡ | 106±10 | 108±8 | 108±10 |
For meaning of symbols see legend to Table 1.
Fig. 6.
Splenic iNOS gene expression (iNOS/beta-actin mRNA ratio) of surgically prepared and vehicle-treated time-control (TC) and surgically prepared and LPS-treated male and female rats. iNOS mRNA was not detectable in completely intact, unprepared male (n=3) and female (n=3) rats. * P<0.05 vs. time-control male; ‡ P<0.05 vs. LPS-treated males.
4. Discussion
The new observations of this study are: (1) In response to low level immune stimulation as a result of non-sterile acute surgical preparation, female rats make more total NO (reflected by UNOx V and plasma NOx) than males; (2) Females produce more NO also during the first 4 h after LPS but are resistant to the development of endotoxin shock; (3) Prior castration of the male, but not ovariectomy of the female eradicates the sexual dimorphism of the BP and NO response to LPS, suggesting that androgens are responsible for the greater tendency of the male to develop endotoxic shock.
There is considerable evidence that basal NO synthesis is higher in normal females than males. Constitutively generated NO from endothelial NOS (eNOS) and neuronal NOS (nNOS) is upregulated by estrogen (or its receptor) [34], both via transcription and by acute, non genomic stimulation of NO production [35-37]. The isolated aorta of females from several species show increased eNOS levels/activity [35,38,39], as does the renal medulla in the rat [40]. This enhancement of constitutive NOS activity in females, may contribute to the higher UNOx V seen in anesthetized TC females vs. males in the present study. We have previously shown, however, that much of this increased NO in female rats originates from an AG-inhibitable iNOS source, presumably stimulated by acute, non sterile surgery [30]. Indeed, in the present study, RT-PCR documents the induction of the iNOS gene in the spleen and the aorta, with similar levels seen in TC males and females. Based on the slight fall in BP, with time, in both male and female TC rats, the vascular expression of the iNOS gene may have some functional impact. The present study confirms our earlier observation [30] on the surgery-induced increase of NO of iNOS origin. Together, these findings suggest that NOx levels in plasma and urine from acutely prepared rats of both genders reflect a substantial iNOS component which has relatively little hemodynamic effect. Thus, acute UNOx V and plasma NOx cannot give a useful index of cardiovascular NO in this preparation.
We observed sex differences in total body NO synthesis both under baseline conditions and following the administration of LPS, which leads to widespread iNOS activation. Three to four hours after LPS, male rats develop shock, although females, who have much higher body fluid NOx concentrations, are resistant to hypotension. This is another indication for the abundant release of NO from sites not directly involved in BP regulation, at least in females. After LPS, a major source of NOx is the reticuloendothelial system (RES) [41] of the spleen, liver and lung [9,10,13], since these are the organs where LPS is mainly taken up 1–4 h after i.v. injection [21,42]. Macrophages in these organs are more responsive to various stimuli in females than males [24,43] and according to Ikejama et al. [15] estrogen potentiates the LPS-triggered induction of iNOS in rat liver. Similarly, LPS is reported to induce greater iNOS expression in the renal medulla of female vs. male rats [47]. Of note, iNOS induction by LPS is not necessarily proportional in various organs [17]. For example, in the immunologically stimulated rat aorta 17β-estradiol can inhibit NO production [44]. A female gender-related shift of LPS-induced iNOS expression from the cardiovascular system to the RES may explain the combination of high UNOx V and PNOx with normal BP in females and lower UNOx V and PNOx with low BP in males. Perhaps males have a less active hepatic and macrophage clearance of LPS and hence the cardiovascular toxicity of LPS is enhanced by prolonged exposure to LPS. Indeed, in the aorta, the gene encoding iNOS is further induced by LPS only in males. Male sex steroids can depress macrophage immune function [22] and testosterone receptor blockade can improve cardiovascular function after major trauma in male rats [23].
Castration of the male provides complete protection against iNOS-induced hypotension and yet at the same time increases overall NO production, as indicated by UNOx V and PNOx, to the range of LPS-treated females. Castration of male rodents is known to strengthen both the humoral and cellular immune system [45], while attenuating the febrile response to LPS [46]. More activated macrophages of castrated males will take up LPS more rapidly, immediately triggering the induction of iNOS and meanwhile reducing the cardiovascular exposure to LPS. Therefore, in castrated males, cardiovascular induction of iNOS, at least within 4 h of LPS, would become negligible while overall NO generation from activated macrophages would be increased; exactly consistent with our functional observations. Ovariectomy of mature female rodents does not significantly alter the immune response [45], also consistent with the present data where ovec rats show similar increases in NO generation to intact females. It is important to point out, however, that the effects of exogenously administered estrogen on the LPS response may be time dependent [18] since by 12 h after LPS administration total NO production (indicated by lower plasma NOx) is reduced, in intact and estrogen restored rats vs. ovec rats [47].
Protection against the toxicity of LPS in females extends to lack of hypotension and relative protection of renal function but the capillary leak, reflected by higher hct and to lower Ppr is of similar severity in both sexes. In addition to NO, LPS causes the accumulation of many other toxic mediators, and one candidate for the capillary leak may be increased NO from constitutive sources. An early increase in vascular NO from constitutive sources precedes iNOS induction [48] and eNOS gene upregulation is more abundant than increased iNOS in LPS-stimulated rat liver [49]. Thus, cardiovascular iNOS, in this model of endotoxemia, may be critical in mediating hypotension, but play a minimal role in the generation of capillary leak, as published by Hock et al. [50]. Based on these observations we suggest that male gender causes sensitivity to LPS-induced hypotension, possibly by selectively enhancing the cardiovascular expression of iNOS. Additionally, males may have a less active macrophage clearance of LPS and its cardiovascular toxicity may be enhanced by a consequently prolonged exposure.
As we have indicated previously, interpretation of UNOx V and PNOx data must be circumspect, especially when urine is collected for short periods [29]. However, with a 4-h urine collection, controlled low NOx intake and knowledge of PNOx as well as GFR, we suggest that together, these indices provide a qualitative indication of the total NO production. What is quite clear from this study as well as from our earlier work, is that these indices can not be assumed to measure ‘hemodynamically active’ NO since when NO production increases, much of the extra NO originates from a large pool which is ‘hemodynamically inactive’ [29,30,51]. In fact, even 24-h urine NOx, obtained under conditions of dietary control do not always reflect ‘hemodynamically active’ NO [51].
In summary, male gender confers a susceptibility to hypotensive shock in response to LPS but paradoxically, results in a lower total stimulation of NO. The site of NO generation is likely to be critical in terms of the hemodynamic response and the vascular synthesis of iNOS is enhanced in males but not females challenged with LPS, in agreement with the functional data.
Acknowledgements
These studies were funded by NIH grant #HL 31933 (to CB), OTKA 025422, the Hungarian Kidney Foundation, the Research Funds of the Department of Pathophysiology of Semmelweis University Budapest (to GL), the Deutsche Akademische Austauschdienst (to UH) and the Oertel Stiftung (to AS and VM). The technical assistance of Glenn Keunzig, Kevin Engels, Lennie Sam-sell and Daisy Kertai are gratefully acknowledged.
References
- 1.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) J Am Med Assoc. 1992;268:3452–3455. [PubMed] [Google Scholar]
- 2.Hewett JA, Roth RA. Hepatic and extrahepatic pathobiology of bacterial lipopolysaccharides. Pharmacol Rev. 1993;45:381–411. [PubMed] [Google Scholar]
- 3.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 4.Nava E, Palmer RMJ, Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991;338:1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- 5.Evans TE, Carpenter H, Kindermann H, Cohen J. Evidence of increased nitric oxide production in patients with sepsis syndrome. Circ Shock. 1993;41:77–81. [PubMed] [Google Scholar]
- 6.Ferrandes MD, La Fuente MD. Effects of age, sex and physical exercise on the phagocytic process of murine peritoneal macrophages. Acta Physiol Scand. 1999;166:47–53. doi: 10.1046/j.1365-201x.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- 7.Heneka MC, Lischmann PA, Osswald H. Polymerized hemoglobin restores cardiovascular and kidney function in endotoxin-induced shock in the rat. J Clin Invest. 1997;99:47–54. doi: 10.1172/JCI119132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Julou-Schaeffer G, Gray GA, Fleming I, Schott G, Parratt JR, Stoclet JC. Loss of vascular responsiveness induced by endotoxin involves l-arginine pathway. Am J Physiol. 1990;259:H1038–1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- 9.Knowles RG, Merrett M, Salter M, Moncada S. Differential induction of brain, lung, and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990;270:833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles RG, Salter M, Brooks SL, Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990;172:1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths MJD, Messent M, MacAllister RJ, Evans TJ. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993;110:963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Adcock IM, Old RW, Barnes PJ, Evans TW. Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochem Biophys Res Commun. 1993;196:1208–1213. doi: 10.1006/bbrc.1993.2380. [DOI] [PubMed] [Google Scholar]
- 14.Kanno K, Hirata Y, Imai T, Marumo F. Induction of nitric oxide synthase gene by interleukin in vascular smooth muscle cells. Hypertension. 1993;22:34–39. doi: 10.1161/01.hyp.22.1.34. [DOI] [PubMed] [Google Scholar]
- 15.Ikejima K, Enomoto N, Thurman RG, Brenner DA. Estrogen increases induction of nitric oxide synthase in the liver due to endotoxin. (Abstract) Gastroenterology. 1997;112:1288. [Google Scholar]
- 16.Forfia PR, Zhang X, Ochoa F, Ochoa M, Xu X, Bernstein R, Sehgal PB, Ferreri NR, Hintze TH. Relationship between plasma NOx and cardiac and vascular dysfunction after LPS injection in anesthetized dogs. Am J Physiol. 1998;274:H193–201. doi: 10.1152/ajpheart.1998.274.1.H193. [DOI] [PubMed] [Google Scholar]
- 17.Del Castillo D, Agarwal A, Jaimes EA, Raij L. Glomerular and vascular tissues do not down-regulate nitric oxide synthesis during protracted endotoxemia. Kidney Int. 1997;52:460–467. doi: 10.1038/ki.1997.353. [DOI] [PubMed] [Google Scholar]
- 18.Nolan JP. Protective action of oestrogen against the lethal effect of endotoxin in the rat. Nature. 1967;213:201–202. doi: 10.1038/213201a0. [DOI] [PubMed] [Google Scholar]
- 19.Altura BM. Sex and estrogen in protection against circulatory stress reactions. Am J Physiol. 1976;231:842–847. doi: 10.1152/ajplegacy.1976.231.3.842. [DOI] [PubMed] [Google Scholar]
- 20.Dominiczak AF, McIntyre M, Rees D, Hamilton CA, Reid JL. Estrogen effects on nitric oxide release (letter) Hypertension. 1997;29:1357. [PubMed] [Google Scholar]
- 21.Praaning-van Dalen D, Brouwer A, Knook DL. Clearance capacity of rat liver Kupffer, endothelial, and parenchymal cells. Gastroenterology. 1981;81:1036–1044. [PubMed] [Google Scholar]
- 22.Wichman MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Mechanism of immunosuppression in males following trauma–hemorrhage. Arch Surg. 1996;131:1186–1192. doi: 10.1001/archsurg.1996.01430230068012. [DOI] [PubMed] [Google Scholar]
- 23.Wichmann MW, Ayala A, Chaudry IH. Male sex steroids are responsible for depressing macrophage immune function after trauma–hemorrhage. Am J Physiol. 1997;273:C1335–1340. doi: 10.1152/ajpcell.1997.273.4.C1335. [DOI] [PubMed] [Google Scholar]
- 24.Zellweger R, Wichmann MW, Ayala A, Stein S, deMaso C, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 26.Remmers DE, Wang P, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma–hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–2925. doi: 10.1152/ajpheart.1997.273.6.H2919. [DOI] [PubMed] [Google Scholar]
- 27.McGowen JE, Barnes MW, Finland N. Bacteriemia at Boston City Hospital: occurrence and mortality during 12 selected years (1935–72) with special reference to hospital-acquired cases. J Infect Dis. 1975;132:316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- 28.Baker CH, Truitt-Sutton E. Arteriolar endothelium-dependent vasodilation occurs during endotoxin shock. Am J Physiol. 1993;264:H1118–1123. doi: 10.1152/ajpheart.1993.264.4.H1118. [DOI] [PubMed] [Google Scholar]
- 29.Sütõ T, Losonczy Gy, Qui C, et al. Acute changes in urinary excretion of nitrite+nitrate do not necessarily predict renal vascular NO production. Kidney Int. 1995;48:1272–1277. doi: 10.1038/ki.1995.411. [DOI] [PubMed] [Google Scholar]
- 30.Losonczy Gy, Bloch JF, Samsell L, Schoenl M, Venuto R, Baylis C. Impact of surgery on nitric oxide in rats: evidence for activation of nitric oxide synthase. Kidney Int. 1997;51:1943–1949. doi: 10.1038/ki.1997.265. [DOI] [PubMed] [Google Scholar]
- 31.Chomczinsky P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate–phenol–chloroform extraction. Ann Biochem. 1987;162:156–161. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Nadeau KC, Azuma H, Tilney NL. Sequential cytokine dynamics in chronic rejection of rat renal allographts: Roles for cytokines RANTES and MCP-1. Proc Natl Acad Sci USA. 1995;92:8729–8733. doi: 10.1073/pnas.92.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegling A, Lehmann M, Platzer C, Emmrich F, Volk HD. A novel, multispecific competitor fragment for quantitative PCR analysis of cytokine gene expression in rats. J Immunol Methods. 1994;177:23–28. doi: 10.1016/0022-1759(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 34.Rubányi G, Freay AD, Kauser K, et al. vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. J Clin Invest. 1997;99:2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide syntheses by sex hormones. Proc Natl Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol. 1997;273:L119–126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 37.Hiyashi T, Ishikawa T, Yamada K, et al. Biphasic effect of estrogen on neuronal constitutive nitric oxide synthase via Ca2+-calmodulin dependent mechanism. Biochem Biophys Res Commun. 1994;203:1013–1019. doi: 10.1006/bbrc.1994.2283. [DOI] [PubMed] [Google Scholar]
- 38.Kauser K, Rubányi GM. Gender difference in bioassayable endothelium-derived nitric oxide from isolated rat aortae. Am J Physiol. 1994;267:H2311–2317. doi: 10.1152/ajpheart.1994.267.6.H2311. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci USA. 1992;89:11259–11263. doi: 10.1073/pnas.89.23.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neugarten J, Ding Q, Friedman A, Lei J, Silbiger S. Sex hormones and renal nitric oxide synthases. J Am Soc Nephrol. 1997;8:1240–1246. doi: 10.1681/ASN.V881240. [DOI] [PubMed] [Google Scholar]
- 41.Stuehr DJ, Marletta MA. Mammalian nitrite biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uragoh K, Sueishi K, Nakamura T, Iwanaga S. A novel immuno-histochemical method for in vivo detection of endotoxin using horshoe crab factor C. J Histochem Cytochem. 1988;36:1275–1283. doi: 10.1177/36.10.3047230. [DOI] [PubMed] [Google Scholar]
- 43.Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem. 1989;34:241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- 44.Kauser K, Sonnenberg D, Henrichmann F, Rubányi GM. 17β-estradiol inhibits IL-1b-induced suppression of contractile reactivity in isolated rat thoracic aorta (abstract) Circulation. 1994;90(2):I577. [Google Scholar]
- 45.Schuurs AHWM, Verheul HAM. Effects of gender and sex steroids on the immune response [general review] J Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 46.Murakami N, Ono T. Sex-related differences in fever development of rats. Am J Physiol. 1987;252:R284–289. doi: 10.1152/ajpregu.1987.252.2.R284. [DOI] [PubMed] [Google Scholar]
- 47.Kauser K, Sonnenberg D, Tse J, Rubanyi GM. 17β-Estradiol attenuates endotoxin-induced excessive nitric oxide production in ovariectomized rats in vivo. Am J Physiol. 1997;273:H506–H509. doi: 10.1152/ajpheart.1997.273.1.H506. [DOI] [PubMed] [Google Scholar]
- 48.Szabó C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide mediated hyporeactivity to noradrenaline preceedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993;108:786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bucher M, Ittner KP, Zimmermann M, Wolf K, Hobbhahn J, Kurtz A. Nitric oxide synthase isoform III gene expression in rat liver is upregulated by lipopolysaccharide and lipoteichoic acid. FEBS Lett. 1997;412:511–514. doi: 10.1016/s0014-5793(97)00835-1. [DOI] [PubMed] [Google Scholar]
- 50.Hock CE, Yin K, Yue G, Wong PYK. Effects of inhibition of nitric oxide synthase by aminoguanidine in acute endotoxemia. Am J Physiol. 1997;272:H843–850. doi: 10.1152/ajpheart.1997.272.2.H843. [DOI] [PubMed] [Google Scholar]
- 51.Baylis C, Vallance P. Brenner BM, editor. Editorial review: measurement of nitrite and nitrate (NOx) levels in plasma and urine; what does this measure tell us about the activity of the endogenous nitric oxide. Current opinion in nephrology and hypertension. 1998;7:1–4. doi: 10.1097/00041552-199801000-00010. [DOI] [PubMed] [Google Scholar]