Abstract
Background
Despite the widely reported inverse associations with insulin resistance and adiposity, adiponectin has been associated with both increased and decreased risk of cardiovascular disease. We examined whether adiponectin is associated with total and cardiovascular mortality in a large population of older adults.
Methods
We analyzed data from 3,075 well-functioning adults ages 69–79 years. Total adiponectin concentrations were measured at baseline and body composition was measured with abdominal and thigh CT scans. Mortality data was obtained over 6.6±1.6 years. We used Cox proportional hazards models adjusting for covariates in stages to examine the association between adiponectin and total and cardiovascular mortality.
Results
There were 679 deaths and 38% were from cardiovascular disease. Unadjusted levels of adiponectin were not associated with total or cardiovascular mortality. However, after adjusting for sex and race, adiponectin was associated with an increased risk of both total (HR 1.26, 95% CI 1.15–1.37, per SD) and cardiovascular mortality (HR 1.35, 95% CI 1.17–1.56, per SD). Further adjustment for study site, smoking, hypertension, diabetes, prevalent heart disease, HDL, LDL, cystatin C, fasting insulin, triglycerides, BMI, visceral fat, thigh intermuscular fat, and thigh muscle area did not attenuate this association. This association between adiponectin and increased mortality risk did not vary by sex, race, BMI, diabetes, smoking, or weight loss.
Conclusions
Higher levels of adiponectin were associated with increased risk of total and cardiovascular mortality in this study of well-functioning community-dwelling older persons. This paradoxical association needs further study of underlying factors that might explain these results including differential association for high molecular weight adiponectin.
INTRODUCTION
Adipocytokines, hormones and proteins secreted by adipose tissue, may link obesity with morbidity and mortality. Adiponectin, the most abundant circulating serum adipocytokine, has been recognized as a biomarker of insulin resistance and the metabolic syndrome 1. Lower concentrations of circulating adiponectin are associated with higher body mass index (BMI), reduced insulin sensitivity, and less favorable plasma lipid profiles 1. Adiponectin is inversely correlated with computed tomography (CT) measures of intra-abdominal fat 1 and visceral adipocytes from obese patients secrete less adiponectin in vitro2. Adiponectin also has actions that would be considered protective against development of atherosclerosis such as down-regulation of vascular adhesion-factors 3, inhibition of foam cell formation4 and smooth muscle migration, and has anti-inflammatory effects on macrophages 1. These lines of evidence support the hypothesis that adiponectin has a protective role in preventing the development of cardiovascular disease.
However, studies examining the relationship between adiponectin and cardiovascular disease in humans have had inconsistent results, possibly due to differences in sex, race/ethnicity or age of the respective study populations. The Health Professions Follow-up study revealed that higher levels of adiponectin were associated with decreased risk of myocardial infarction in primarily White men 5. A few other studies also supported the inverse association of adiponectin and cardiovascular disease 6, 7, but these studies included only male participants. Conversely, higher adiponectin concentrations have been associated with higher mortality in heart failure patients8, 9. Wannamethee et al reported that higher adiponectin levels were associated with increased all-cause and cardiovascular mortality in older men without cardiovascular disease or with heart failure, but observed no association in those with cardiovascular disease without heart failure10. While higher adiponectin levels predicted reduced risk of nonfatal myocardial infarction in men only, higher adiponectin concentrations were associated with increased risk of both cardiovascular and all-cause mortality in a study from older White adults in the Rancho Bernardo Study 11. Lawlor et al reported that adiponectin did not predict cardiovascular events in women enrolled in the British Women’s Heart and Health Study 12. Kanaya et al found that higher adiponectin levels were associated with increased cardiovascular risk in older Black subjects but not Whites 13, while Lindsay showed no association of adiponectin with cardiovascular disease among Native Americans 14. Findings from a meta-analysis suggests that the magnitude of the association between adiponectin levels and cardiovascular disease may not be as strong as has been previously considered15.
We sought to address some of the inconsistencies in the literature by analyzing prospective data collected from a cohort of healthy, older White and Black men and women enrolled in the Health, Aging, and Body Composition (Health ABC) Study. Our goal was to determine whether total adiponectin concentrations are associated with total mortality and cardiovascular mortality if we account for differences in body composition that exist between men and women and Whites and Blacks. We also examined whether these associations differ by sex, race, smoking status, diabetes, weight loss, and use of medications known to affect adiponectin.
METHODS
Study Participants and Design
The Health Aging, and Body Composition study (Health ABC) investigates whether changes in body composition act in a common pathway by which multiple conditions increase risk of functional decline and mortality. Participants enrolled in the study were 3,075 men and women between the ages of 69 and 79 years at baseline who were recruited between April 1997 and June 1998 from a random sample of Medicare beneficiaries from 2 clinical sites (Pittsburgh, PA and Memphis, TN). To be eligible for the study, participants had to report no difficulty in walking one-quarter mile, climbing 10 steps, or performing basic activities of daily living. Individuals requiring assistive devices for ambulation, subjects with difficulty performing activities of daily living, or life-threatening cancers requiring active-treatment within the past 3 years, and those planning to leave the area within 3 years were excluded from the study. Participants in interventional studies were ineligible.
The study was approved by the institutional review boards of the University of California, San Francisco, the University of Pittsburgh, and the University of Tennessee. All study participants were provided written informed consent to participate in the study.
Predictor Variables
Participants underwent venipuncture at the baseline visit after an overnight fast. Serum samples were frozen at −70° C and stored at McKesson BioServices, Rockville, MD. Adiponectin was assayed in 2002–2003 from serum specimens that were frozen approximately 6–7 yr earlier. Total circulating levels of adiponectin were measured in duplicate by RIA (Linco Research, St. Charles, MO) with an intra-assay coefficient of variation of 1.8–3.6%.
Body Composition Measurements
Weight was measured using a standard balance beam scale, and height was measured using a stadiometer. Total adiposity was estimated by calculating BMI (weight in kilograms divided by height in meters squared). Total fat mass was measured using whole-body dual-energy x-ray absorptiometry (QDR 4500A; Hologic Inc, Bedford, Mass). Arm, leg, trunk, and total body fat were measured using dual-energy x-ray absorptiometry, and total percentage of body fat was calculated.
Regional adiposity was measured by computed tomography using the Somatom Plus 4 (Siemens, Erlangen, Germany), Picker PQ 2000S (Marconi Medical Systems, Cleveland, Ohio), or 9800 Advantage (General Electric, Milwaukee, Wis) scanners and standardized protocols. Visceral fat and subcutaneous abdominal fat were measured at the L4-L5 level with participants in the supine position. Fat areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using interactive data language development software (RSI, Boulder, CO). Visceral fat was manually distinguished from subcutaneous fat using the internal abdominal wall fascial plane. Intermuscular fat area and thigh subcutaneous fat area were measured using computed tomography at the mid-thigh level between the greater trochanter and the intercondyloid fossa. Intermuscular adipose tissue, or myosteatosis, was distinguished from subcutaneous adipose tissue by the deep fascial plane surrounding the thigh muscles.
Twenty-one participants were missing information for total fat mass, 113 for total percentage fat, 119 for abdominal visceral fat, 209 for abdominal subcutaneous fat, 64 for thigh intermuscular fat, and 64 for thigh subcutaneous fat.
Covariates
Racial group, age, and sex were assessed by self-report during the initial interview. On self-administered questionnaires, participants reported their smoking history as never, former, or current smoker and their alcohol consumption as current drinker, former drinker, or never drinker. Comorbid conditions reported at baseline included asthma/chronic obstructive pulmonary disease, cancer, chronic kidney disease, congestive heart failure, coronary artery disease, diabetes, hypertension, and stroke/transient ischemic attack.
Seated systolic and diastolic blood pressures were measured by a manual sphygmomanometer. Hypertension was defined by self-report of a diagnosis and use of an antihypertensive medication or if systolic blood pressure was at least 140 mm Hg or if diastolic blood pressure was at least 90 mm Hg. Diabetes was defined by self-report of diabetes diagnosis or use of diabetes drug or if fasting plasma glucose was at least 126 mg/dl or 2-hour post-challenge glucose was at least 200 mg/dl. Fasting lipoprotein levels (Vitros 950 analyzer; Johnson & Johnson, New Brunswick, NJ), and fasting and 2-hour post-challenge plasma glucose by automated glucose oxidase reaction (YSI 2300 Glucose Analyzer; YSI, Yellow Springs, OH) were measured. Fasting serum insulin was measured by RIA (Pharmacia, Uppsala, Sweden) only among participants without known diabetes at the baseline examination. Cystatin C, a measure of kidney function that estimates glomerular filtration rate better than creatinine or estimated glomerular filtration rate16, was measured by particle-enhanced immunonephelometric assay (N Latex Cystatin C) using a BNII nephelometer (Dade Behring Inc, Deerfield, IL). Plasma high-sensitivity C-reactive protein (CRP) concentration was measured in duplicate by ELISA (Calbiochem, San Diego, CA) and was standardized according to the World Health Organization First International Reference Standard with a sensitivity of 0.08 μg/ml.
Seventy-seven participants were missing information for LDL cholesterol, 27 for HDL cholesterol, 35 for triglycerides, 103 for fasting glucose and 38 for CRP. Individuals with known diabetes did not have oral glucose tolerance testing or fasting insulin measures (366 for fasting insulin, 566 for 2-hour glucose).
Outcome Variables
The two main outcomes of interest were cardiovascular (CVD) mortality and total mortality from the adjudicated outcomes data set completed through August 31, 2005. All deaths were adjudicated by a central committee for immediate and underlying causes of death as determined by established criteria including review of death certificate, all recent hospital records, and interview with the next of kin. Cardiovascular mortality was defined as atherosclerotic cardiovascular disease (definite fatal myocardial infarction, definite fatal cardiovascular heart disease, or possible fatal cardiovascular heart disease), stroke, atherosclerotic disease other than coronary or cerebrovascular, and other cardiovascular disease (e.g., valvular heart disease).
Statistical Analyses
Initially, univariate associations of baseline covariates with total mortality and CVD mortality were assessed using unadjusted Cox models. We log-transformed HDL-cholesterol, fasting insulin, and trigylcerides to meet linearity assumptions, and rescaled overall and regional adiposity measures by their standard deviations to directly compare associations with these predictors. We also assessed the correlation among all fat variables both overall and by sex to identify and eliminate collinearity. Appropriate functional form of adiponectin with total mortality and CVD mortality was determined using penalized B-splines. Splines indicated that untransformed adiponectin was the most appropriate, and adiponectin was modeled per standard deviation for more meaningful interpretation.
We used multivariable Cox proportional hazards models to examine adjusted associations between adiponectin and total and CVD mortality. In nested sequences of models using adiponectin per standard deviation and only those with all covariate values (n=2,548), we adjusted for age, sex, race, site, smoking, hypertension, diabetes, prevalent CHD, and LDL-cholesterol. Then, we additionally adjusted our models for measures of overall and regional adiposity including BMI, abdominal visceral fat, thigh intermuscular fat, and thigh muscle area. Finally, models were adjusted for HDL-cholesterol, insulin, and triglycerides to examine any potential mediating effects of these metabolic factors.
We checked for evidence of statistical interaction between adiponectin and variables known to be associated with adiponectin concentrations including sex, race, diabetes, smoking status and BMI category for the total mortality outcome. We also examined whether weight loss and use of certain medications may modify the association between adiponectin and mortality. We stratified our analysis for weight loss >5% of baseline body weight vs. those who had stable or increase in weight during the follow-up period. We also stratified our analysis by medication status categorizing participants who used any medication known to increase adiponectin (insulin, thiazolidenedione, angiotensin receptor blockers, angiotensin converting enzyme inhibitors, beta blockers, and/or statins) at baseline as a medication user vs. non-user. In a final analysis, we evaluated the association between baseline adiponectin and mortality per year and by tertile of follow-up time.
The proportional hazards assumption for all Cox models was verified by examining plots of Schoenfeld residuals versus transformed time and assessing interactions of the all predictors with log transformed time, both individually and all together for a global test of the proportional hazards assumption. We used SAS Version 9.1 (SAS Institute, Cary, NC) and SPlus Version 6.1 (Insightful Corp., Seattle, WA) to perform the analyses and p-values less than 0.05 were considered statistically significant.
RESULTS
Of 3,075 participants enrolled in the study, there were a total of 679 (22.1%) deaths of which 247 (38.0%) were from cardiovascular disease after mean 6.6 ±1.6 years of follow-up. A total of 3,044 of the participants had total serum adiponectin levels measured at baseline (934 white men, 846 white women, 542 black men, and 722 black women).
The average age of the cohort was 73.6±2.9 years and the mean BMI was 27.1±4.8 kg/m2 (men 27.0±3.9, women 27.7±5.4 kg/m2). Black women had a significantly higher BMI than White women (29.6±5.8 vs. 26.0±4.5 kg/m2, p<0.001). Abdominal visceral fat was higher in men than women (155.0±71.7 vs. 131.3±60.3 cm2, p<0.001) and higher in Whites than Blacks (152.1±69.3 vs 129.7±61.5 cm2, p<0.001). Thigh total muscle area was greater in men than women (132.0±22.5 vs. 92.8±18.2 cm2, p<0.001) and greater in Blacks than Whites (117.8±28.2 vs. 107.5±27.1 cm2, p<0.001). Mean total adiponectin levels were significantly higher in women than in men (9.5±5.6 vs. 13.3±7.4 μg/ml, p<0.001), and higher in Whites than in Blacks (12.8±7.0 vs. 9.5±6.3 μg/ml, p<0.001).
Table 1 presents associations between adiponectin tertiles and baseline covariates. Higher concentrations of adiponectin were significantly associated with older age, female sex, alcohol use, chronic kidney disease, and higher HDL-cholesterol. Lower concentrations of adiponectin were significantly associated with Black race, diabetes, hypertension, existing coronary heart disease, cardiovascular disease, and higher levels of BMI, waist circumference, abdominal visceral fat, thigh intermuscular fat and muscle area, fasting and 2-hour glucose, insulin, triglycerides, and CRP.
Table 1.
Association between adiponectin tertiles and baseline covariates*, Health ABC 1997–1998
| Tertile 1 ≤7.0 μg/ml n=1016 | Tertile 2 7.1 – 12.9 μg/ml n=938 | Tertile 3 ≥13.0 μg/ml n=1092 | p-for-trend | |
|---|---|---|---|---|
| Age, mean/SD | 73.4±2.7 | 73.5±2.9 | 74.0±3.0 | <0.001 |
| Female sex | 372 (36.1) | 458 (48.8) | 738 (67.6) | <0.001 |
| Black race | 586 (57.7) | 382 (40.7) | 298 (27.3) | <0.001 |
| Current Smoker | 112 (11.0) | 94 (10.0) | 108 (9.9) | 0.66 |
| Any alcohol use | 456 (44.9) | 498 (53.1) | 553 (50.6) | <0.001 |
| Comorbid Diseases: | ||||
| Diabetes | 382 (37.6) | 205 (21.9) | 126 (11.5) | <0.001 |
| Hypertension | 681 (67.0) | 599 (63.9) | 649 (59.4) | 0.001 |
| Chronic kidney disease | 184 (18.1) | 199 (21.2) | 270 (24.7) | 0.001 |
| Coronary heart disease | 252 (24.8) | 210 (22.4) | 192 (17.6) | <0.001 |
| Cardiovascular disease | 307 (30.2) | 262 (27.9) | 259 (23.7) | <0.001 |
| Laboratory Values: (mean/SD) | ||||
| Fasting glucose, mg/dL | 116.4±41.5 | 102.2±31.6 | 95.0±24.7 | <0.001 |
| 2-hour glucose, mg/dL | 148.9±65.0 | 137.0±57.0 | 122.2±42.4 | <0.001 |
| Fasting insulin, μU/ml | 10.8±8.8 | 8.7±5.9 | 6.4±4.0 | <0.001 |
| Total cholesterol, mg/dL | 198.7±40.9 | 203.7±37.9 | 205.8±36.6 | 0.58 |
| HDL-cholestorol, mg/dL | 47.0±13.8 | 53.2±16.3 | 61.4±17.3 | <0.001 |
| LDL-cholesterol, mg/dL | 121.1±36.6 | 123.2±33.7 | 120.6±33.6 | 0.09 |
| Triglycerides, mg/dL | 159.2±107.1 | 138.2±68.8 | 119.5±59.1 | <0.001 |
| C-reactive protein, mg/L | 3.3±4.7 | 3.0±4.3 | 2.7±5.1 | <0.001 |
| Cystatin C, mg/L | 1.0±0.33 | 1.0±0.28 | 1.0±0.40 | 0.10 |
| Body Composition: (mean/SD) | ||||
| Body mass index, kg/m2 | 28.9±4.5 | 27.8±4.8 | 25.1±4.5 | <0.001 |
| Waist circumference, cm | 103.4±12.0 | 100.7±13.9 | 95.1±12.2 | <0.001 |
| Total body fat, % | 34.1±7.5 | 35.4±7.8 | 35.5±7.9 | 0.43 |
| Abd visceral fat area, cm2 | 163.1±67.6 | 150.2±65.5 | 117.7±59.4 | <0.001 |
| Thigh intermuscular fat area, cm2 | 11.3±6.8 | 10.8±7.1 | 9.3±6.4 | <0.001 |
| Thigh total muscle area, cm2 | 125.9±26.7 | 113.0±25.8 | 97.6±24.0 | <0.001 |
data represent n(%) or mean±SD
Table 2 shows the bivariate association between the baseline covariates and total mortality and cardiovascular mortality. As expected, women had lower risk of total and cardiovascular mortality (HR 0.60, 95% CI 0.52–0.70 and HR 0.63, 95% CI 0.49–0.81, respectively) compared to men. Black participants had significantly higher risk of total and cardiovascular mortality (HR 1.48, 95% CI 1.27–1.72 and HR 1.57 95% CI 1.22–2.02, respectively) compared to Whites. Current tobacco use was associated with higher mortality risk, and alcohol use showed a trend towards decreased risk of total mortality. Chronic diseases such as asthma/chronic obstructive pulmonary disease, chronic kidney disease, congestive heart failure, coronary artery disease, diabetes, hypertension, and stroke/transient ischemic attack were all bivariately associated with higher total and cardiovascular mortality. Cancer was not associated with mortality during this length of follow-up. With regards to medication use, ACE inhibitor, diuretic, and insulin use were associated with higher mortality risks. Oral estrogen users had decreased risk of total and cardiovascular mortality in this unadjusted analysis. Increasing levels of total, HDL-, and LDL-cholesterol were associated with lower total mortality risk. Increasing levels of fasting plasma glucose, 2-hour glucose, CRP, and cystatin-C was associated with higher mortality risk. Unadjusted levels of total adiponectin had a trend towards increased total and cardiovascular mortality risk (HR 1.07, 95% CI 0.98–1.16 for total mortality; HR 1.14, 95% CI 0.99–1.31 for CVD mortality).
Table 2.
Unadjusted associations of baseline covariates with 5-year mortality, Health ABC study
| Total Mortality HR (95% CI) | CVD Mortality* HR (95% CI) | |
|---|---|---|
| Age, per year | 1.09 (1.06–1.12) | 1.06 (1.01–1.10) |
| Female sex (vs. male) | 0.60 (0.52–0.70) | 0.63 (0.49–0.81) |
| Black race (vs. Whites) | 1.48 (1.27–1.72) | 1.57 (1.22–2.02) |
| Study site Pittsburgh (vs. Memphis) | 0.83 (0.72–0.97) | 0.80 (0.62–1.02) |
| Current Smoker (vs. never/past) | 2.17 (1.78–2.64) | 1.95 (1.39–2.72) |
| Alcohol use (any vs. none) | 0.87 (0.74–1.01) | 0.95 (0.74–1.22) |
| Comorbid Diseases: | ||
| Asthma/COPD | 1.29 (1.01–1.64) | 1.49 (1.03–2.18) |
| Cancer | 1.14 (0.97–1.33) | 1.07 (0.77–1.48) |
| Congestive heart failure | 3.65 (2.76–4.83) | 5.92 (4.06–8.63) |
| Coronary artery disease | 1.76 (1.50–2.07) | 2.41 (1.86–3.11) |
| Diabetes | 1.62 (1.37–1.89) | 1.72 (1.31–2.23) |
| Hypertension | 1.31 (1.09–1.58) | 1.69 (1.27–2.24) |
| Stroke/TIA | 1.70 (1.34–2.14) | 1.77 (1.21–2.58) |
| Current Medication Use: | ||
| ACE inhibitor | 1.41 (1.16–1.71) | 1.76 (1.31–2.37) |
| Aspirin | 1.11 (0.95–1.30) | 1.29 (1.00–1.66) |
| Beta-blocker | 1.04 (0.84–1.30) | 1.36 (0.98–1.88) |
| Diuretic | 1.22 (1.03–1.44) | 1.76 (1.36–2.28) |
| Estrogen (oral) | 0.44 (0.32–0.61) | 0.43 (0.25–0.74) |
| Insulin | 1.80 (1.32–2.46) | 1.72 (1.02–2.90) |
| Statin | 1.01 (0.81–1.26) | 1.28 (0.91–1.80) |
| Thiazolidenedione | 0.71 (0.18–2.83) | 0.95 (0.13–6.75) |
| Laboratory Values: | ||
| Total cholesterol, mg/dL** | 0.85 (0.78–0.92) | 0.87 (0.77–0.99) |
| Triglycerides, mg/dL† | 0.90 (0.77–1.06) | 0.85 (0.65–1.12) |
| HDL-cholestorol, mg/dL† | 0.68 (0.53–0.88) | 0.58 (0.38–0.89) |
| LDL-cholesterol, mg/dL** | 0.88 (0.82–0.96) | 0.95 (0.83–1.08) |
| Fasting plasma glucose, mg/dL† | 1.89 (1.43–2.48) | 2.47 (1.61–3.77) |
| 2-hour glucose, mg/dL† | 1.35 (1.07–1.71) | 1.32 (0.89–1.96) |
| Fasting insulin, μU/ml† | 0.94 (0.81–1.08) | 0.91 (0.72–1.16) |
| Albumin, g/L** | 0.87 (0.80–0.94) | 0.87 (0.77–0.99) |
| Cystatin C, mg/L** | 1.21 (1.17–1.25) | 1.20 (1.14–1.27) |
| C-reactive protein, mg/L† | 1.35 (1.24–1.47) | 1.23 (1.07–1.42) |
| Adiponectin, μg/ml** | 1.07 (0.98–1.16) | 1.14 (0.99–1.31) |
| Body Composition**: | ||
| Body mass index, kg/m2 | 0.91 (0.84–0.99) | 0.95 (0.84–1.08) |
| Waist circumference, cm | 0.97 (0.90–1.05) | 0.99 (0.87–1.12) |
| Abd visceral fat area, cm2 | 1.05 (0.97–1.14) | 1.08 (0.95–1.22) |
| Thigh intermuscular fat area, cm2 | 1.09 (1.02–1.17) | 1.09 (0.97–1.22) |
| Thigh total muscle area, cm2 | 1.05 (0.98–1.14) | 1.05 (0.92–1.18) |
Cardiovascular disease mortality includes all CHD, all stroke, and all CVD deaths combined
analyzed per standard deviation increase;
analyzed as natural log
We also examined bivariate associations between body composition measures and total and cardiovascular mortality stratified by sex. A higher BMI (HR 0.91, 95% CI 0.84–0.99 per SD) was associated with a lower total mortality risk, while no association was seen with BMI and cardiovascular mortality. With regards to the regional adiposity measures, thigh intermuscular fat area was associated with trends toward increased mortality risk (HR 1.09, 95% CI 1.02–1.17 per SD). Both higher levels of abdominal visceral fat and thigh muscle area, a measure of lean body mass, had a trend towards association with both outcomes.
Prior to model building, we evaluated the Pearson correlations between all body composition measurements separately for men and women. These correlations did not differ significantly by sex. Using a combined analysis of the whole cohort, we identified all correlated body composition variables to ensure that two highly correlated variables (r>0.60) were not included in our adjusted models.
In table 3, the association between adiponectin and total and cardiovascular mortality was evaluated in sequentially adjusted models. While the unadjusted association between adiponectin and each outcome had a trend towards increased risk, adjustment for sex revealed the increased risk of both total mortality (HR 1.18, 95% CI 1.08–1.29) and cardiovascular mortality (HR 1.25, 95% CI 1.09–1.55) with each increasing SD of adiponectin. Similarly, just adjusting for race alone also revealed increased risk of adiponectin with mortality as well (HR 1.11, 95% CI 1.02–1.21 for total mortality; HR 1.21, 95% CI 1.05–1.39 for CVD mortality). Additional adjustment for covariates, confounders, and potential mediators including body composition and variables associated with the metabolic syndrome (i.e. HDL, triglycerides, and insulin) did not diminish this association. Adiponectin remained strongly associated with increased risk of total mortality (HR 1.23, 95% CI 1.11–1.37, per SD) and CVD mortality (HR 1.36, 95% CI 1.14–1.61, per SD) in fully adjusted models.
Table 3.
Association between Adiponectin and Total and Cardiovascular Mortality with Sequential Adjustment for Explanatory Variables in Staged Models
| Models | Total Mortality HR (95% CI) | CVD Mortality* HR (95% CI) |
|---|---|---|
| Unadjusted Adiponectin μg/ml, per SD** | 1.07 (0.98–1.16) | 1.14 (0.99–1.31) |
| Model 1a | 1.21 (1.11–1.33) | 1.35 (1.16–1.55) |
| Model 2b | 1.20 (1.09–1.33) | 1.35 (1.15–1.58) |
| Model 3c | 1.23 (1.11–1.37) | 1.36 (1.14–1.61) |
Cardiovascular mortality includes all CHD, all stroke, and all CVD deaths combined
analyzed per standard deviation increase of adiponectin, 6.87 μg/ml
adjusted for age, sex, race, site, smoking, hypertension, diabetes, prevalent CHD, and LDL-cholesterol, cystatin C, and current oral estrogen use
Model 1 + adjusted for BMI, abdominal visceral fat, thigh intermuscular fat, and thigh muscle area (all per SD)
Model 2 + adjusted for HDL-cholesterol, insulin, and triglycerides (all natural log transformed)
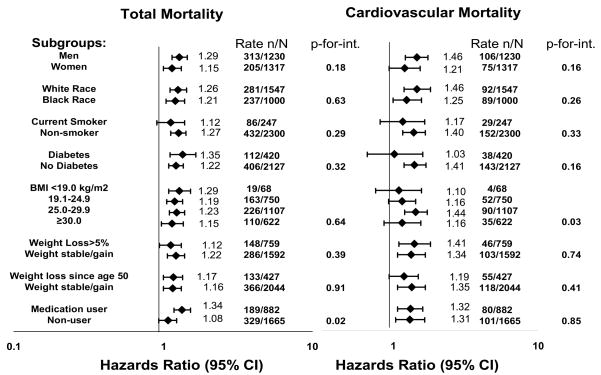
We attempted to further understand and explain the association between adiponectin and mortality by evaluating subgroups where adiponectin levels may vary. We found no evidence of interaction between adiponectin with sex, race, smoking status, or diabetes in fully adjusted models. (Figure) Since weight status is associated with adiponectin levels, we evaluated potential interactions between baseline BMI and weight loss as well. Those with overweight BMI had somewhat increased risk of CVD mortality (p-for-interaction=0.03). We examined two separate subgroups for weight loss. The first examined whether participants had lost weight (>5% from baseline) during the follow-up period of the study vs. weight stable or weight gain. The second variable examined whether participants had lost weight between age 50 and the baseline clinical examination (mean age 74 years). We found no evidence of interaction between prior weight loss or prospective weight loss since study enrollment with adiponectin levels for either mortality outcome. We also determined whether using a medication that increases adiponectin levels, such as insulin, a thiazolidinedione, an angiotensin converting enzyme inhibitor, an angiotensin II receptor blocker, a beta blocker, or a statin would modify the association between adiponectin and mortality. A total of 882 (35%) participants were using one or more of these medications with a baseline adiponectin of 11.2±6.7 μg/ml vs. 12.1±6.9 μg/ml among non-medication users (p<0.001). Participants on any of these medications had higher risk of total mortality with increasing adiponectin levels than those not using any of these medications (p-for-interaction=0.02), but there was no difference by medication use for the CVD mortality outcome (p-for-interaction=0.85). Lastly, we examined whether this increased risk of adiponectin with mortality varied by time. We separated our follow-up time into tertiles, and found that association between adiponectin and total mortality was significant primarily in the earliest tertile of follow-up time (HR 1.29, 95% CI 1.16 – 1.43 in a fully adjusted model), with a similar magnitude of risk in the second (HR 1.10, 0.76 – 1.57) and most distant tertile (HR 1.33, 0.75 – 2.33) of follow-up that did not reach statistical significance.
Figure.
The association between adiponectin (per SD) and total and cardiovascular mortality by subgroup after adjustment for age, sex, race, study site, smoking, hypertension, diabetes, prevalent coronary heart disease, LDL-cholesterol, cystatin C, estrogen use, BMI, abdominal visceral fat, thigh intermuscular fat, thigh muscle area, HDL-cholesterol, insulin, and triglycerides (without adjustment for the subgroup variable of interest). We present p-for-interaction for each subgroup. *Medication use refers to any participant taking a medication that increases adiponectin concentrations: insulin, a thiazolidinedione, an angiotensin converting enzyme inhibitor, an angiotensin II receptor blocker, a beta blocker, or a statin medication. Those not on any of these medications are termed as medication non-user.
DISCUSSION
In a cohort of elderly Black and White Americans, total circulating adiponectin levels were found to be significantly associated with increased risk of both total and cardiovascular mortality after adjusting only for sex or race. This association was not diminished after adjusting for other covariates including body composition and other variables associated with the metabolic syndrome. The association was robust and did not vary by sex, race, smoking, diabetes, BMI, weight loss, medication use, or length of follow-up.
Adiponectin levels are affected by sex, race, and age. Women have higher levels of adiponectin and higher percentage of high molecular weight (HMW) adiponectin complexes than men, which may be a result of the sex differences in body composition and adipocyte size and number17. In addition, both total and HMW adiponectin are reduced by testosterone18. Blacks have significantly lower levels of circulating adiponectin than Whites19, possibly related to the differences in body composition between the races. Advancing age is associated with higher levels of adiponectin20. Once adjusted for either sex or race the results of our study are consistent with other published studies showing higher adiponectin levels are associated with increased cardiovascular mortality and all-cause mortality10, 21.
Body composition and levels of adiposity vary greatly between the young and old, men and women, Blacks and Whites. The body composition of an aging adult changes to less lean mass and more fat mass. Visceral fat, subcutaneous abdominal fat, intramuscular fat, and intrahepatic fat all increase with age. These changes are a result of hormonal changes associated with aging, decreased resting metabolic rate, and especially decreases in physical activity 22. There are also differences in body composition across sex and race. Women have greater fat content than men and preferentially accumulate fat in the gluteofemoral region. Men preferentially accumulate fat in the abdominal area, especially visceral fat 23. Blacks generally have smaller visceral fat area than whites 23, 24. We hypothesized that body composition variations may help to explain some the inconsistencies in the association between adiponectin to cardiovascular disease and mortality in previous studies. In the present study, we did not find that adjustment for these carefully measured body composition variables attenuated the association between adiponectin and mortality, nor did the association vary by BMI category.
We also investigated the relationships between adiponectin and mortality in several subgroups in which adiponectin levels might be affected including demographic characteristics, smoking, diabetes, BMI status, weight loss, and medication use. The only subgroup that showed a significant interaction with adiponectin was among those using medications known to increase adiponectin levels25. However, this interaction was not observed for CVD mortality, the most likely cause of death among those using these medications for the treatment of diabetes and cardiovascular disease. Both medication users and non-users had similarly increased risk of mortality with higher adiponectin levels.
Our results contradict the results of several studies that have reported that higher adiponectin levels are cardioprotective5–7, 26. One explanation for this contradiction is that adiponectin may affect unmeasured biological factors that have opposite effects on cardiovascular disease and mortality. Overweight people who survive to old age may have characteristics that protect them from adverse effects of being overweight27. Weight loss is associated with increased adiponectin levels28, however, we found that participants who lost >5% of body weight during follow-up had similarly increased risk of mortality with higher adiponectin levels at baseline. Higher adiponectin levels in the elderly may be a marker for a catabolic process leading to weight loss seen prior to death8; however this explanation appears unlikely to be the case for the well-functioning, physically active adults studied here. Another explanation for the inconsistent results seen to date may be related to the fact that most studies measure total circulating adiponectin levels and do not separately measure the presence of high or low molecular weight complexes. A higher ratio of higher molecular weight adiponectin to total circulating adiponectin is best correlated with enhanced insulin sensitivity1. The proportion of HMW adiponectin to total adiponectin varies by age 29, sex 30, and race31, 32. However, a recent study found no association between HMW adiponectin or the ratio of HMW/total adiponectin and incident CHD events33. We are not aware of any studies that have examined the association between HMW adiponectin and mortality.
There are several limitations to our analysis. The Health ABC cohort is comprised of older adults who were recruited from their communities and did not have advanced physical or cognitive impairment. The findings of this study may not be generalizable to younger populations or the frail elderly. Other factors associated with body weight, such as dietary intake, physical activity, or physical fitness, may affect the association of adiponectin with mortality34. Moreover, we had only one baseline measurement of adiponectin. This “snap-shot” in time may fail to capture the complexity of compensatory mechanisms that are involved in regulating adiponectin concentrations prior to death.
In conclusion, in this prospective analysis of elderly Black and White men and women revealed a positive association between adiponectin and total and cardiovascular mortality. Comorbid diseases, detailed measures of body composition, metabolic covariates, weight loss, and medication use did not explain this increased association between adiponectin and mortality. Differences in demographic characteristics between different populations studied may in part explain previous inconsistencies in the associations of adiponectin with cardiovascular and total mortality.
Acknowledgments
Funding/Support and Role of the Sponsor. Alka M. Kanaya was funded in part by K23HL080026 and R21DK068608. The Health ABC study was funded via contracts with the National Institute on Aging contract #s: N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and included substantial involvement of NIA staff in data collection, analysis, interpretation, review, and approval of the manuscript. Dr. Kanaya had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005 Feb;257(2):167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 2.Motoshima H, Wu X, Sinha MK, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002 Dec;87(12):5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 3.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103(8):1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 5.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Jama. 2004 Apr 14;291(14):1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 6.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003 Jan 1;23(1):85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 7.Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007 Feb;92(2):571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 8.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005 Sep 20;112(12):1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 9.Tamura T, Furukawa Y, Taniguchi R, et al. Serum adiponectin level as an independent predictor of mortality in patients with congestive heart failure. Circ J. 2007 May;71(5):623–630. doi: 10.1253/circj.71.623. [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007 Jul 23;167(14):1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 11.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007 Jan 15;165(2):164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005 Oct;90(10):5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 13.Kanaya AM, Wassel Fyr C, Vittinghoff E, et al. Serum adiponectin and coronary heart disease risk in older Black and White Americans. J Clin Endocrinol Metab. 2006 Dec;91(12):5044–5050. doi: 10.1210/jc.2006-0107. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay RS, Resnick HE, Zhu J, et al. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005 Mar;25(3):e15–16. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 15.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006 Aug 15;114(7):623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 16.Herget-Rosenthal S, Trabold S, Pietruck F, Holtmann M, Philipp T, Kribben A. Cystatin C: efficacy as screening test for reduced glomerular filtration rate. Am J Nephrol. 2000 Mar-Apr;20(2):97–102. doi: 10.1159/000013564. [DOI] [PubMed] [Google Scholar]
- 17.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004 Feb;53 (Suppl 1):S143–151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 18.Xu A, Chan KW, Hoo RL, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005 May 6;280(18):18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 19.Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004 Jan;53(1):1–3. doi: 10.1016/j.metabol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003 Apr;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 21.Laughlin GA, Barrett-Connor E, May S. Sex-specific association of the androgen to oestrogen ratio with adipocytokine levels in older adults: the Rancho Bernardo Study. Clin Endocrinol (Oxf) 2006 Oct;65(4):506–513. doi: 10.1111/j.1365-2265.2006.02624.x. [DOI] [PubMed] [Google Scholar]
- 22.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005 Nov;82(5):923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 23.Despres JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000 Aug;20(8):1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 24.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996 Sep;45(9):1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 25.Swarbrick MM, Havel PJ. Physiological, pharmacological and nutritional regulation of circulating adiponectin concentrations in humans. Metabolic Syndrome and Related Disorders. 2008 doi: 10.1089/met.2007.0029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shioji K, Moriwaki S, Takeuchi Y, Uegaito T, Mutsuo S, Matsuda M. Relationship of serum adiponectin level to adverse cardiovascular events in patients who undergo percutaneous coronary intervention. Circ J. 2007 May;71(5):675–680. doi: 10.1253/circj.71.675. [DOI] [PubMed] [Google Scholar]
- 27.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001 May 14;161(9):1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 28.Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti- inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86(8):3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 29.Kato K, Osawa H, Ochi M, et al. Serum total and high molecular weight adiponectin levels are correlated with the severity of diabetic retinopathy and nephropathy. Clin Endocrinol (Oxf) 2008 Mar;68(3):442–449. doi: 10.1111/j.1365-2265.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- 30.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004 Mar 26;279(13):12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 31.Retnakaran R, Hanley AJ, Zinman B. Does hypoadiponectinemia explain the increased risk of diabetes and cardiovascular disease in South Asians? Diabetes Care. 2006 Aug;29(8):1950–1954. doi: 10.2337/dc06-0867. [DOI] [PubMed] [Google Scholar]
- 32.Sinha MK, Songer T, Xiao Q, et al. Analytical validation and biological evaluation of a high molecular-weight adiponectin ELISA. Clin Chem. 2007 Dec;53(12):2144–2151. doi: 10.1373/clinchem.2007.090670. [DOI] [PubMed] [Google Scholar]
- 33.Sattar N, Watt P, Cherry L, Ebrahim S, Smith GD, Lawlor DA. High molecular weight adiponectin is not associated with incident coronary heart disease in older women: a nested prospective case control study. J Clin Endocrinol Metab. 2008 Feb 26; doi: 10.1210/jc.2007-2603. [DOI] [PubMed] [Google Scholar]
- 34.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. Jama. 2005 Apr 20;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]