Abstract
Background
Chronic fatigue syndrome (CFS) is a complex and controversial condition responsible for marked functional impairment. Infectious mononucleosis (IM) may be a predisposing factor for CFS. Among adults after IM, 9-12% may have symptomatic fatigue 6 months later. Rates of CFS in the general adolescent population are low (0.2%).
Objective
To prospectively characterize the course and outcome of CFS in adolescents during a 2 year period following IM.
Design/Methods
301 adolescents (12-18 years) with IM were identified and screened for non-recovery 6 months following IM using a telephone screening interview. Non-recovered adolescents underwent a medical evaluation, and had follow-up screening at 12 and 24 months following IM. Following blind review, final diagnoses of CFS were made at 6, 12 and 24 months using established pediatric criteria.
Results
6, 12 and 24 months following IM, 13%, 7% and 4%, respectively, of adolescents met criteria for CFS. Most individuals recovered with time; only 2 adolescents with CFS at 24 months seemed to have recovered or had an explanation for CFS at 12 months but then were reclassified as CFS at 24 months. All 13 adolescents with CFS 24 months following IM were female and on average reported greater fatigue severity at 12 months. Reported use of steroid therapy during the acute phase of IM did not increase the risk of developing CFS.
Conclusions
IM thus may be a risk factor for CFS in adolescents. Female gender and greater fatigue severity, but not reported steroid use during the acute illness, were associated with the development of CFS in adolescents. Further research is needed to determine other predictors of persistent fatigue following IM.
Keywords: adolescent health, chronic fatigue, epidemiology, Epstein-Barr virus, mononucleosis
Introduction
Chronic fatigue syndrome (CFS) is a complex and controversial condition involving severe fatigue and disabling musculoskeletal and cognitive symptoms (1). Chronic fatigue accounts for marked functional impairment and educational disruption among adolescents (2-5). Studies of adults have indicated that acute infectious mononucleosis (IM) may be a predisposing factor for the development of CFS. Buchwald and colleagues (6) followed previously healthy subjects older than 16 years for 6 months following IM: 12% had not fully recovered, reporting symptoms of fatigue and impaired functioning, as is seen in patients with CFS. Nearly identical results were reported in a prospective cohort study of adults from Australia, where CFS 6 and 12 months following IM or two other infections common in Australia (Q fever and Ross River virus) were 11% and 9%, respectively (7). White and colleagues (8,9) found that 9% of subjects aged 16-65 years with IM were fatigued and complained of excessive sleeping at 6 months; in some cases these patients were depressed as well.
The observation that CFS may follow IM appears particularly frequently in adolescent samples (e.g., [4]). Rates of acute, mononucleosis-like illness preceding chronic fatigue have been documented in about three-quarters of adolescents with CFS, with nearly half evidencing active mononucleosis infection at symptom onset (10-12).
There have been no prospective studies of post-infectious fatigue in an adolescent population, and none that have followed subjects longer than 6 months. We therefore initiated a 2 year prospective study of CFS following IM in adolescents.
Methods
We enrolled adolescents in the greater Chicago area with monospot-positive acute IM, the presumption being that the vast majority of these cases were caused by Epstein-Barr virus infection (13). The adolescents were identified via school nurses (middle school, high school and college/university), pediatric practices, including the Pediatric Practice Research Group (14) and the Virology Laboratory of Children’s Memorial Hospital. All prescribed treatments were recorded. Six months following their IM diagnosis, a telephone screening interview identified those not fully recovered and 50 recovered controls willing to come for a clinical evaluation. Adolescents not fully recovered and the controls underwent a clinical evaluation 6, 12 and 24 months post-IM. All aspects of the study were approved by the Institutional Review Boards of Children’s Memorial Research Center and the College of Applied Sciences of the University of Illinois at Chicago.
Definitions
We used the Jason et al. (15) revision of the Fukuda (1) criteria to diagnose CFS. When a well-recognized underlying condition, such as primary depression, could explain the subject’s symptoms, we classified him/her as having “CFS-explained”.
Evaluations
The 6 month clinical evaluation consisted of a complete history, physical examination and laboratory screening by one of two physicians (BZK or CJM) well versed in CFS (16). Preprinted checklists with inclusion/exclusion criteria, historical (e.g., education, activities, drug use, menstrual history) and physical examination findings important for diagnosing CFS and related disorders (e.g., fibromyalgia by the presence of tender points, orthostatic blood pressure assessment) were used. The clinical evaluation also included laboratory tests to rule out medical causes of CFS (e.g., urine toxicology and thyroid function tests) that were sent on each subject. Using the data from the clinical evaluation, the physicians made a diagnosis of CFS, CFS-explained, or recovered on each subject. These diagnoses were then reviewed by an expert panel before being permanently assigned to the subject. Self-report measures were used to complete the Chalder Fatigue Scale (17).
At 12 and 24 months following the diagnosis of IM, subjects evaluated at 6 months were evaluated in their homes. The home evaluation included blood, urine, and saliva testing and the same history, interviews, and self-report measures used at six months to complete the Chalder Fatigue Scale.
Analysis
Chi square tests were used to evaluate the significance of categorical data. T-tests or Kruskal Wallis tests, as appropriate, were used to evaluate continuous data. The significance of fatigue over time in relation to a diagnosis of CFS at 6, 12 and 24 months was evaluated using Student’s t-test or the Friedman test for the 13 adolescents who retained a diagnosis of CFS at 24 months. The significance of steroid use was evaluated using chi-square analysis.
Results
There were 301 adolescents enrolled in the study. Six months following their IM diagnosis, 286 (95%) completed a telephone screening interview. Based on the screening interview, 70 of these adolescents (24%) were assessed as not fully recovered. A clinical evaluation was completed on 53 (76%) of these 70 not fully recovered adolescents; 12 refused, 3 had exclusionary diagnoses (primary depression, transverse myelitis, anorexia) and 2 did not meet study criteria (the fatigue predated the IM or the subject was not able to complete the 6 month evaluation in a timely fashion). There was no significant difference in sex, family socioeconomic status or subject age between the group that completed the 6 month evaluation (N=53), the group (N=12) that refused or the group (N=5) that was excluded (data not shown).
Following the 6 month clinical evaluation, 39 of the 53 not fully recovered subjects who underwent clinical evaluation were classified as having CFS (13% of the original sample of 301 adolescents). Compared with the other 262 enrolled subjects in the cohort, 35 of the 39 subjects with CFS at 6 months were female (90%, vs 68%, p=0.01 by Fisher’s exact test). There was no difference in race or socioeconomic status between the entire cohort and the subjects who went on to develop CFS (data not shown).
Among the 14 other subjects completing the 6 month clinical evaluation, 1 had recovered between the time of the phone interview and the time he/she was seen in Clinic and 13 were classified as CFS explained (1 abused drugs, on more careful questioning 1 subject’s fatigue predated the mononucleosis, 1 had an eating disorder, 1 had an unrelated medical illness, 6 had underlying psychiatric diagnoses, 2 had psychiatric and sleep disorders, and 1 had an intercurrent acute parvovirus infection following IM, so symptoms could not be solely explained by infectious mononucleosis). There was no difference in family socioeconomic status or subject age between the group diagnosed with CFS (N=39) and the group (N=13) with CFS-explained (data not shown). All subjects evaluated at 6 months were at least Tanner stage 4.
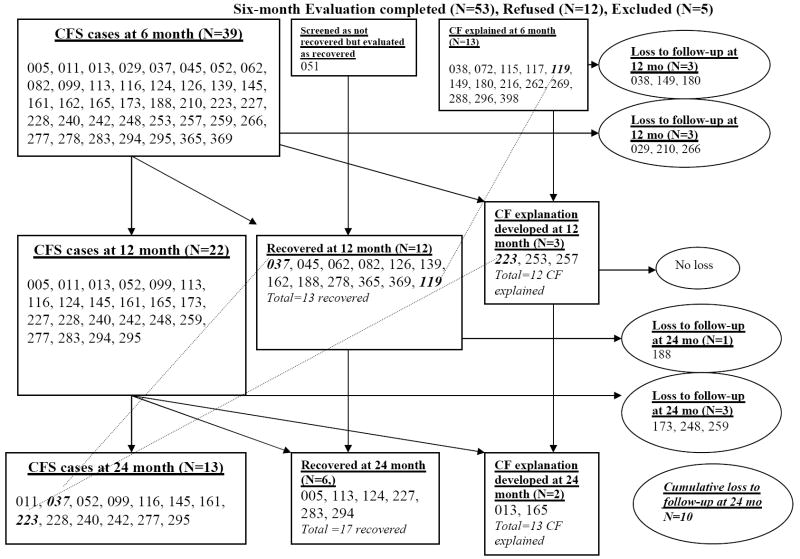
Thirty-six subjects diagnosed with CFS at the 6 month evaluation were re-evaluated at 12 months (3 of the CFS patients diagnosed at 6 months were lost to follow-up): 11 recovered, 3 were now classified as CFS-explained (2 abused drugs and 1 had a pregnancy and miscarriage), leaving 22 with a diagnosis of CFS (7% of the original sample; all females). At the 24 month evaluation, 3 more subjects with CFS were lost to follow-up since the 12 month evaluation. Of the 19 remaining subjects diagnosed with CFS at 12 months: 6 more recovered, 2 developed a reason for their CFS (psychiatric and/or eating disorders), 1 subject thought to have recovered at 12 months developed symptoms again and was reclassified as CFS, and 1 subject thought to have CFS-explained at 12 months (the subject with the pregnancy and miscarriage) now met criteria for CFS and had no predisposing reason, leaving 13 subjects (all females) who were classified as CFS 24 months following monospot-positive IM (4% of the original sample of 301 adolescents). Figure 1 documents the CFS status of subjects seen at 6, 12 and 24 months.
Figure 1. Follow-up summary for screened non-recovered participants (N=70).
Three digit numbers in the Figure represent unique patient identifiers used throughout the study.
At all time points examined, significantly more persistently fatigued subjects were female (Table 1). When the course of fatigue over time was examined for the 13 adolescents who retained the diagnosis of CFS at 24 months, using the Chalder Fatigue Scale, there was a general trend for the fatigue to peak at 12 months (data not shown). There were no significant associations between treatment of the acute episode of mononucleosis with steroids and the development of CFS at 6, 12 or 24 months: 11 of 39 (28%) of adolescents who developed CFS at 6 months and 11 of 50 controls (22%) were treated with steroids, as were 7 of 22 (32%) with CFS at 12 months, and 4 of 13 (31%) among those with CFS at 24 months (all p>.05).
Table 1.
Frequencies and Percentages of CFS by Gender
| CFS at 6 months * | CFS at 12 months | CFS at 24 months | |
|---|---|---|---|
| Female | 35 (11.6%) | 22 (7.3%) | 13 (4.3%) |
| Male | 4 (1.3%) | 0 | 0 |
| Total | 39 (12.9%) | 22 (7.3%) | 13 (4.3%) |
6 months Chi-square = 8.21, p = 0.004
Discussion
In our study, 6, 12 and 24 months following IM, 13%, 7% and 4%, respectively, of adolescents met criteria for CFS following IM. Most individuals recovered with time; only 2 adolescents with CFS 24 months following IM seemed to have recovered or had an explanation for CFS at 12 months but then were reclassified as CFS at 24 months. All of those who did not recover by 12 months were female. A female preponderance in CFS has also been found in a prospective study of chronic fatigue in adolescents (18) and in a prospective cohort study of adults following IM (19). Nearly all of those who did not recover showed the greatest fatigue severity at 12 months. Steroid use during acute IM did not appear to predispose to developing CFS at 6, 12 or 24 months.
In our prospective cohort study, rates of CFS in adolescents following IM were about 20 times higher than those found in the general adolescent population (0.2% [16]), and the percent of adolescents with lingering symptoms following monospot-positive IM was similar to that seen in adults (9-12% [6-9]). IM thus may be a risk factor for CFS in both adolescents and adults, while steroid treatment of IM appears not to be. Further research is necessary to determine other predictors of persistent fatigue following IM. Our data suggest that females may be at particular risk.
Acknowledgments
We thank the Pediatric Practice Research Group (14) and all of the physicians and school nurses who referred patients into our study.
Supported by R01HD4330101A1 from the National Institute of Child Health and Human Development.
Abbreviations
- CFS
chronic fatigue syndrome
- IM
infectious mononucleosis
Footnotes
There are no conflicts of interest to report.
Presented in preliminary form at the Pediatric Academic Societies and Asian Society for Pediatric Research 2008 Joint Meeting, 3 May 2008.
References
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The Chronic Fatigue Syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Marshall GS, Gesser RM, Yamanish K, Starr SE. Chronic fatigue in children: Clinical features, Epstein-Barr virus and human herpes virus 6 serology and long term follow-up. Pediatr Infect Dis J. 1991;10:287–290. [PubMed] [Google Scholar]
- 3.Richards J, Turk J, White S. Children and adolescents with chronic fatigue syndrome in non-specialist settings: Beliefs, functional impairment and psychiatric disturbance. Eur Child Adolesc Psychiatry. 2005;14:310–318. doi: 10.1007/s00787-005-0477-4. [DOI] [PubMed] [Google Scholar]
- 4.Sankey A, Hill CM, Brown J, Quinn L, Fletcher A. A follow-up study of chronic fatigue syndrome in children and adolescents: Symptom persistence and school absenteeism. Clin Child Psychol Psychiatry. 2006;11:126–138. doi: 10.1177/1359104506059133. [DOI] [PubMed] [Google Scholar]
- 5.ter Wolbeek M, van Doornen LJ, Kavelaars A, Heijnen CJ. Severe fatigue in adolescents: A common phenomenon? Pediatrics. 2006;117(6):e1078–86. doi: 10.1542/peds.2005-2575. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald DS, Rea TD, Katon WJ, Russo JE, Ashley RL. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med. 2000;109:531–7. doi: 10.1016/s0002-9343(00)00560-x. [DOI] [PubMed] [Google Scholar]
- 7.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006 doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White PD, Thomas J, Amess J, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry. 1998;173:475–481. doi: 10.1192/bjp.173.6.475. [DOI] [PubMed] [Google Scholar]
- 9.White PD, Thomas JM, Kangro HO, et al. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet. 2001;358:1946–1954. doi: 10.1016/S0140-6736(01)06961-6. [DOI] [PubMed] [Google Scholar]
- 10.Feder HM, Dworkin PH, Orkin C. Outcome of 48 pediatric patients with chronic fatigue. Arch Fam Med. 1994;3:1049–55. doi: 10.1001/archfami.3.12.1049. [DOI] [PubMed] [Google Scholar]
- 11.Krilov LR, Fisher M, Friedman SB, Reitman D, Mandel FS. Course and outcome of chronic fatigue in children and adolescents. Pediatrics. 1998;102(2 Pt 1):360–366. doi: 10.1542/peds.102.2.360. [DOI] [PubMed] [Google Scholar]
- 12.Smith MS, Mitchell J, Corey L, et al. Chronic fatigue in adolescents. Pediatrics. 1991;88:195–202. [PubMed] [Google Scholar]
- 13.Blake JM, Edwards JM, Fletcher W, McSwiggan DA, Pereira MS. Measurement of heterophile antibody and antibodies to EB viral capsid antigen IgG and IgM in suspected cases of infectious mononucleosis. J Clin Pathol. 1976;29:841–7. doi: 10.1136/jcp.29.9.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBailly S, Ariza A, Bayldon B, Binns HJ for the Pediatric Practice Research Group. The origin and evolution of a regional pediatric practice-based research network: Practical and methodological lessons from the Pediatric Practice Research Group. Curr Probl Pediatr Adolesc Health Care. 2003;33:124–134. doi: 10.1067/mps.2003.13. [DOI] [PubMed] [Google Scholar]
- 15.Jason LA, Jordan K, Miike T, et al. A pediatric case definition for myalgic encephalomyelitis and chronic fatigue syndrome. J Chronic Fatigue Syndr. 2006;13:1–44. [Google Scholar]
- 16.Jordan KM, Jason LA, Mears CJ, et al. Prevalence of pediatric chronic fatigue syndrome in a community-based sample. J Chronic Fatigue Syndr. 2006;13:75–8. [Google Scholar]
- 17.Chalder T, Berelowitz C, Pawlikowska T. Development of a fatigue scale. J Psychosom Res. 1993;37:147–54. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 18.Rimes KA, Goodman R, Hotopf M, Wesseley S, Meltzer H, Chalder T. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: a prospective community study. Pediatrics. 2007 doi: 10.1542/peds.2006-2231. www.pediatrics.org/cgi/doi/10.1542/peds.2006-2231. [DOI] [PubMed]
- 19.Candy B, Chalder T, Cleare AJ, et al. Predictors of fatigue following the onset of infectious mononucleosis. Psychol Med. 2003;33:847–55. doi: 10.1017/s0033291703007554. [DOI] [PubMed] [Google Scholar]