Abstract
Oncogenic transformation of hematopoietic cells by the Bcr-Abl oncoprotein directly involves the activation Jak2 tyrosine kinase and the Stat5 transcription factor. Both proteins are normally linked to the interleukin (IL)-3/granulocyte-macrophage colony-stimulating factor receptors for growth and survival. Since fibroblastic cells are not targets of BCR-ABL-induced oncogenesis, we determined whether forced expression of the IL-3 receptor would allow oncogenic transformation of NIH 3T3 broblasts known to be resistant to transformation by BCR-ABL. NIH 3T3 cells transduced with the human IL-3 receptor α and β chains were highly susceptible to oncogenic transformation by expression of BCR-ABL. Forced expression of both receptor chains but not either one alone allowed efficient foci formation of NIH 3T3 cells expressing BCR-ABL (triple positive cells), and these cells formed colonies in soft agar, whereas BCR-ABL + NIH 3T3 cells lacking IL-3 receptor expression did not. Signaling studies indicate that the BCR-ABL/IL-3 receptor + NIH 3T3 cells utilize the Gab2/PI-3 kinase pathway activated by Jak2, and the Stat5 pathway activated separately by Bcr-Abl, whereas BCR-ABL+ NIH 3T3 cells lacking the IL-3 receptor do not utilize the Jak2 pathway, but still maintain activation of Stat5. The Bcr-Abl kinase inhibitor imatinib mesylate (1 µm) and two Jak2 kinase inhibitors strongly inhibited agar colony formation and the activation of Gab2 caused by Jak2. All of these findings indicate that Bcr-Abl oncoprotein requires the IL-3 receptor/Jak2/Stat5 pathways for oncogenic transformation of NIH 3T3 fibroblasts.
Keywords: BCR-ABL, interleukin-3 receptor, leukemia, oncogenic transformation
Introduction
In general fibroblasts such as NIH 3T3 cells are resistant to oncogenic transformation by the BCR-ABL oncogene (Daley et al., 1987). However, Rat1 fibroblasts, which have been transduced with the Myc oncogene, are greatly stimulated to form foci after transduction of the BCR-ABL oncogene (Lugo and Witte, 1989), indicating that Bcr-Abl is unable to activate critical pathways in fibroblasts. Bcr-Abl is known to abrogate the requirement for interleukin (IL)-3 in various hematopoietic cell lines that require IL-3 for proliferation and survival (Sirard et al., 1994). Bcr-Abl also induces tyrosine phosphorylation of the β chain of the IL-3 receptor (IL-3 Rβ) (Wilson-Rawls et al., 1996; Watanabe et al., 1997). In this regard, Yokota et al. (1993) showed that forced expression of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor α and β chain cDNAs in NIH 3T3 cells generated a hematopoietic cell-like phenotype. The Bcr-Abl oncoprotein activates the Stat5 pathway independent of Jak2 (Ilaria and Van Etten, 1996; Klejman et al., 2002). In addition, Bcr-Abl activates Jak2 in a manner that does not lead to activation of Stat5 (Xie et al., 2001, 2002), and this Bcr-Abl/Jak2 pathway leads to activation of c-Myc (Samanta et al., 2006). Therefore, we hypothesized that fibroblasts would become susceptible to oncogenic transformation by BCR-ABL following forced expression of cDNAs of the α and β chains of the IL-3 receptor.
In this study, we found that expression of the α and β chains of the human IL-3 receptor allowed BCR-ABL to efficiently transform NIH 3T3 cells as measured by foci formation and colony formation ability in soft agar. Colony formation of these cells was efficiently inhibited by low doses (1 µm) of the Bcr-Abl kinase inhibitor imatinib mesylate (IM), and two Jak2 kinase inhibitors (AG490 and hexabromocyclohexane).
Results
Forced expression of human IL-3R α and β chains and BCR-ABL in NIH 3T3 cells
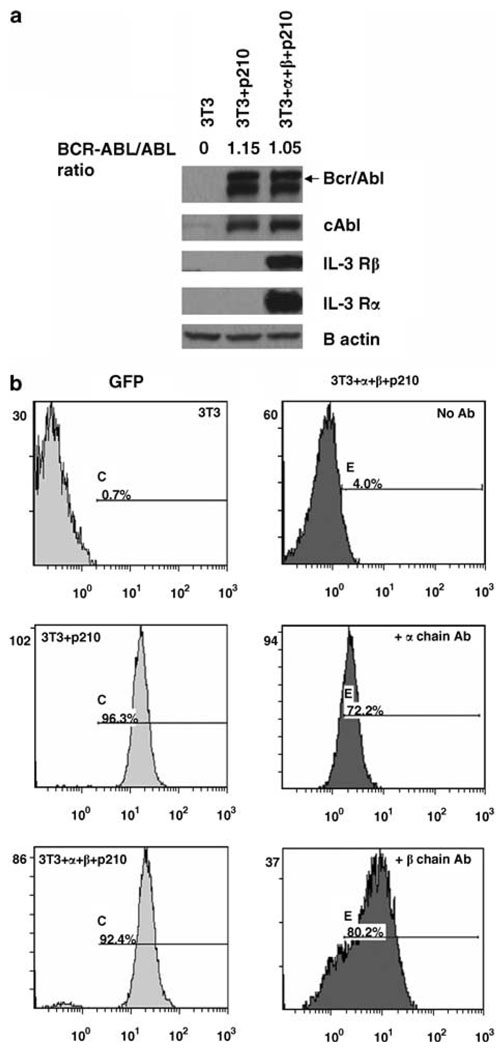
BCR-ABL + NIH 3T3 (3T3 + p210) cells were generated by stably transducing NIH 3T3 cells with cDNA of BCR-ABL and green fluorescent protein (GFP) using the MigR1 retrovirus. Similarly, 3T3 cells expressing BCR-ABL/GFP were transfected with a cDNA encoding the human IL-3 receptor chains using stable selection of cells expressing both the human IL-3 Rα (hygromycin selection) and IL-3 receptor β subunit (IL-3 Rβ) (zeocin selection). These triple positive cells were termed 3T3 + α + β + p210 cells. After drug selection and GFP sorting, stable transfectants of 3T3p210 cells and 3T3 + α + β + p210 cells were obtained. We examined the expression of BCR-ABL, IL-3 Rα and IL-3 Rβ in two cell clones by western blotting assays (Figure 1a). Anti-Abl 8E9 western blots showed that 3T3 + p210 cells and 3T3 +α + β + p210 cells expressed the BCR-ABL oncoprotein. Also, flow cytometry results showed that 96.3% of 3T3 + p210 cells and more than 92.4% of 3T3 + α + β + p210 cells exhibited GFP-positive signal, which is indicative of Bcr-Abl protein expression (Figure 1b, left panels). Expression of human IL-3 Rα and IL-3Rβ chains were observed in 3T3 + α + β + p210 by western blotting (Figure 1a). In order to determine the cellular localization of IL-3 Rα and IL-3 Rβ in 3T3 transfectants, intact 3T3 + α + β + p210 cells were stained with or without fluorescent antibody of IL-3 Rα and IL-3 Rβ. Nearly 72.2 and 80.2% of triple positive cells expressed the IL-3 Rα and IL-3 Rβ chains on their cell surface, respectively (Figure 1b, right panels). These results indicate that the Bcr-Abl protein is expressed in these IL-3 receptor + NIH 3T3 cells, and IL-3 receptor chains were expressed on the cell surface.
Figure 1.
Expression of human interleukin (IL)-3 Rα and IL-3 Rβ in 3T3+ α + β + P210 cells. (a) Western blotting results show the expression of Bcr-Abl, IL-3 Rα and IL-3 Rβ chains in NIH 3T3 cell transfectants. Whole cell lysates were blotted with anti-Abl antibody 8E9, or human anti-IL-3 receptors antibodies. (b) Flow cytometry assay of NIH 3T3, 3T3 +P210 and 3T3+ α + β + P210 cells after staining with conjugated mouse anti-human IL-3 Rα or IL-3 Rβ antibodies. A total of 10 000 events were analysed in each case.
BCR-ABL-induced oncogenic transformation of NIH 3T3 cells is enhanced by co-expression of IL-3 receptors
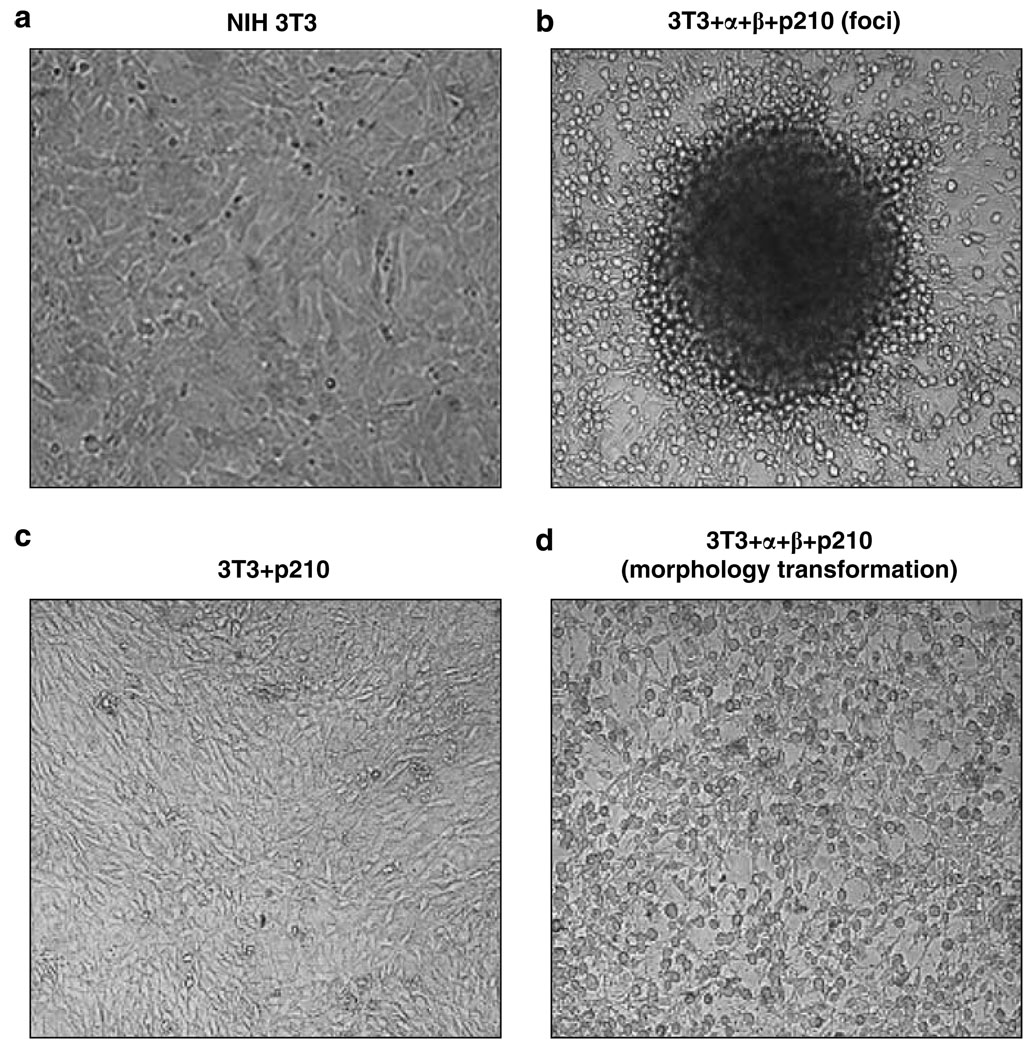
The constitutively activated ABL tyrosine kinase activity of BCR-ABL oncoprotein is the driving force responsible for cytokine independent growth and cells transformation of hematopoietic cells (Deininger et al., 2000). To determine whether or not the BCR-ABL can induce oncogenic transformation of NIH 3T3 cells, we examined cells for changes in cell morphology. Expression of BCR-ABL alone had little effect on the morphology of NIH 3T3 cells. However, co-expression of BCR-ABL and the IL-3 receptor chains induced significant changes in the morphology of NIH 3T3 cells, and caused the cells to form foci (Figure 2). We compared foci formation ability of these cultures. NIH 3T3 cells expressing both BCR-ABL and the IL-3 receptor chains formed foci, whereas BCR-ABL + NIH 3T3 cells did not (Table 1).
Figure 2.
BCR-ABL-induced foci formation and morphological changes in NIH 3T3 cells require expression of α and β chains of the human IL-3 receptor. (a) NIH 3T3 without BCR-ABL gene. (b) Foci formation in α and β chain expressing NIH 3T3 cells infected with the BCR-ABL retrovirus. (c) NIH 3T3 cells infected with the BCR-ABL virus (no change in growth pattern of NIH 3T3 cells). (d) Morphological transformation of α and β chain expressing NIH 3T3 cells infected with the BCR-ABL retrovirus. Note that only NIH 3T3 cells expressing both α and β chains of the human IL-3 receptor and BCR-ABL show morphological transformed foci (panel b) and rounded cells (panel d).
Table 1.
Foci formation in NIH-3T3 cells by BCR-ABL requires expression of α and β chains of the human IL-3 receptor
| Cell types (NIH3T3) | No. foci (10 days) | No. foci (27 days) |
|---|---|---|
| 3T3 + α + β + p210 | 2.25 | 17.5 |
| 3T3 + p210 | 0 (P = 0.07) | 0.75 (P = 0.00001) |
| 3T3 | 0 (P = 0.07) | 0 (P = 0.0001) |
P-value of the t-test of the 3T3 cell line or the 3T3 + p210 cell line is compared to 3T3 + α + β + p210 cell line.
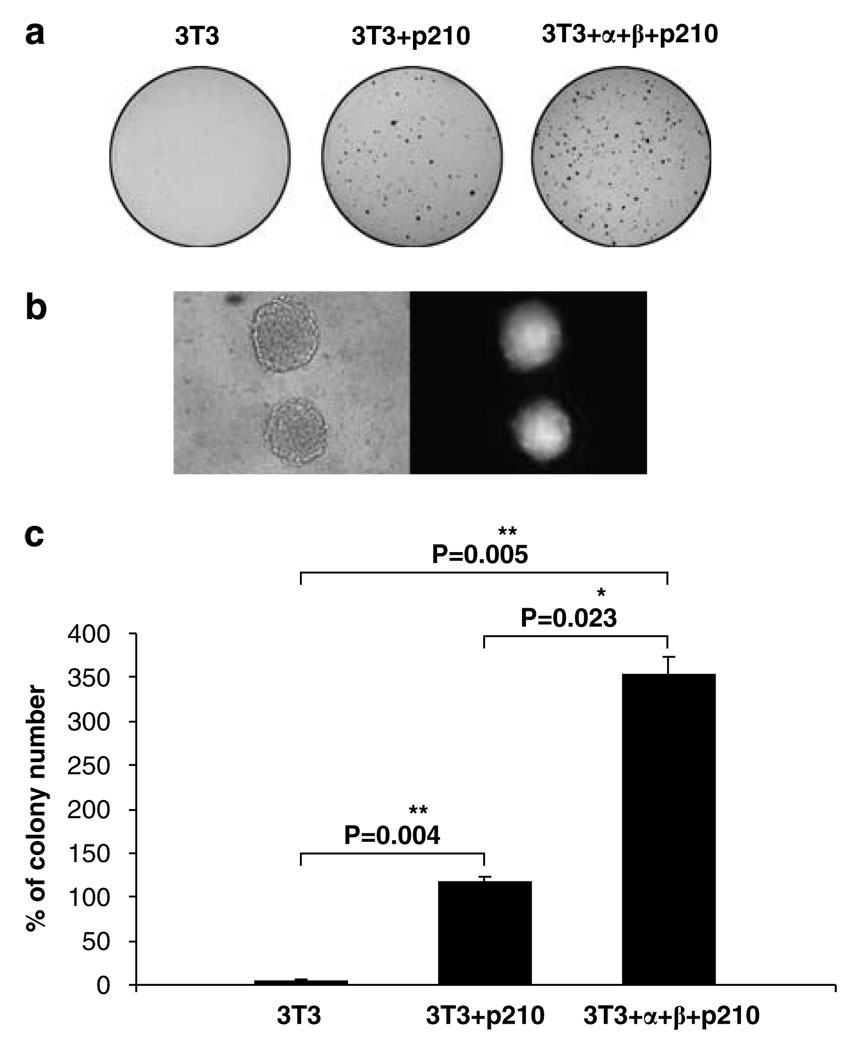
Next we compared anchorage-independent growth of these cells by the soft agar colony formation. Only, a very small number of colonies were observed with NIH 3T3 cells (Figure 3a). The colony number formed by 3T3 + α + β + p210 cells was quite vigorous. Colony formation 3T3 + p210 cells (about 116 colonies) was three times less than that of 3T3 + α + β + p210 cells (about 352) (Figure 3c). The GFP-positive signal detected in the colonies of 3T3 + α + β + p210 cells confirmed the presence of BCR-ABL in soft agar colonies (Figure 3b). Together, these experiments indicate that co-expression of IL-3 receptor chains and BCR-ABL in NIH 3T3 fibroblasts induced an oncogenic phenotype.
Figure 3.
NIH 3T3 cells expressing α and β chains of the IL-3 receptor and P210 BCR-ABL form colonies in soft agar. (a) Colony growth of NIH 3T3, 3T3 + P210 and 3T3 + α + β + P210 cells. Pictures were captured 3 weeks later after cells were plated into soft agar by 700 cells per 60 mm dish. (b) Microscopic pictures of colonies from 3T3 + α + β + P210 cells after colonies grew for 2 weeks, right panel, GFP fluorescence. Magnification × 20. (c) Quantification of soft agar colonies formed by NIH 3T3, 3T3 + P210 and 3T3+ α + β + P210 cells.
Oncogenic transformation of NIH 3T3 cells expressing the IL-3 receptor and BCR-ABL requires the BCR-ABL tyrosine kinase
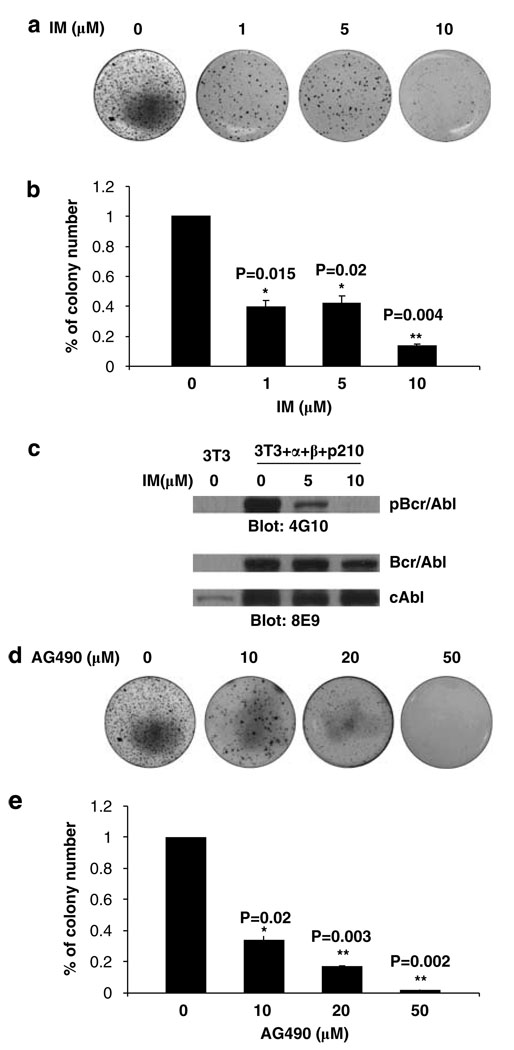
We next determined whether transformation of IL-3 receptor + NIH 3T3 cells depended on Bcr-Abl tyrosine kinase activity. To address this question, soft agar colony assays were conducted with 3T3 + α + β + p210 cells in the presence of different doses of imatinib mesylate (IM), a potent inhibitor of the Abl kinase activity of Bcr-Abl. Soft agar colony results showed that IM strongly inhibited colony growth of 3T3 + α + β + p210 cells in a dose-dependent manner (Figure 4a). The colony number decreased significantly under the treatment with 1 µm IM (≈342) or 5 µm IM (≈360) compared with the nontreatment group (≈870) (Figure 4b). Only a very small number of colonies were seen in 3T3 + α + β + p210 cells exposed to 10 µm IM. We also examined the Bcr-Abl phosphotyrosine activity in 3T3 + α + β + p210 cells after treatment with different doses of IM (5 and 10 µm) for 24h by western blotting. The 4G10 western blots showed that Bcr-Abl tyrosine phosphorylation was strongly inhibited at a dose of 5 µm IM treatment (Figure 4c). Of interest, the level of c-Abl protein was increased in BCR-ABL + cells expressing IL-3 receptors and BCR-ABL + cells (Figure 4c). Since IM inhibited both soft agar colony growth and Bcr-Abl kinase activity, we conclude that oncogenic transformation of BCR-ABL/IL-3 receptor + NIH 3T3 cells was driven by the tyrosine kinase of the Bcr-Abl oncoprotein.
Figure 4.
Soft agar colony growth of NIH 3T3 cells expressing α and β chains of the IL-3 receptor and P210 BCR-ABL was inhibited by imatinib (IM) or AG490. (a) NIH 3T3 cells expressing IL-3 Rα, IL-3 Rβ and P210 BCR-ABL were seeded into soft agar containing different doses of imatinib (1, 5 and 10 µm). Colonies were stained with crystal blue 3 weeks later. (b) Percentage of agar colony numbers in the presence of imatinib compared to the colony numbers in the absence of imatinib. (c) Bcr-Abl tyrosine phosphorylation following imatinib treatment for 24h; (d) Crystal blue staining agar colonies formed by NIH 3T3 cells expressing the α and β chain of the IL-3 receptor and P210 BCR-ABL in the presence and absence of 10, 20, 50 µm AG490. (e) Percentage of agar colony numbers in AG490-treated cells compared to -untreated cells. Colony counts were assessed on each individual samples twice, P-value is compared with untreated group.
Jak2 activation is required for BCR-ABL-mediated transformation of IL-3 receptor expressing NIH 3T3 cells
It is known that Jak2 forms a complex with Bcr-Abl and the oncogenic effects of BCR-ABL require Jak2 (Miyamoto et al., 2001; Xie et al.,. 2001, 2002; Samanta et al., 2006). In normal hematopoietic cells Jak2 is associated with and activated by the IL-3/IL-5/GM-CSF common β chain (Quelle et al., 1994). We hypothesized that Jak2 activity would be required for the transformation of 3T3 + α + β + p210 cells induced by BCR-ABL expression. To test this possibility, we examined the soft agar colony formation of 3T3 + α + β + p210 cells in the presence the Jak2 inhibitor AG490 and the new Jak2 inhibitor hexabromocyclohexane (Sandberg et al., 2005). The results showed that AG490 dramatically inhibited colony number of 3T3 + α + β + p210 cells (Figure 4d) by threefold (10 µm, colony number about 292) and sixfold (20 µm, colony number about 149) when compared with no treatment of cells (colony number 870). A total of 50 µm of AG490 totally inhibited colony formation. Similar results were observed by treating triple positive cells with another Jak2 inhibitor hexabromocyclohexane (Supplementary Figure 1). These results indicate that Jak2 activation is required for Bcr-Abl to induce oncogenic transformation of 3T3 + α + β + p210 cells.
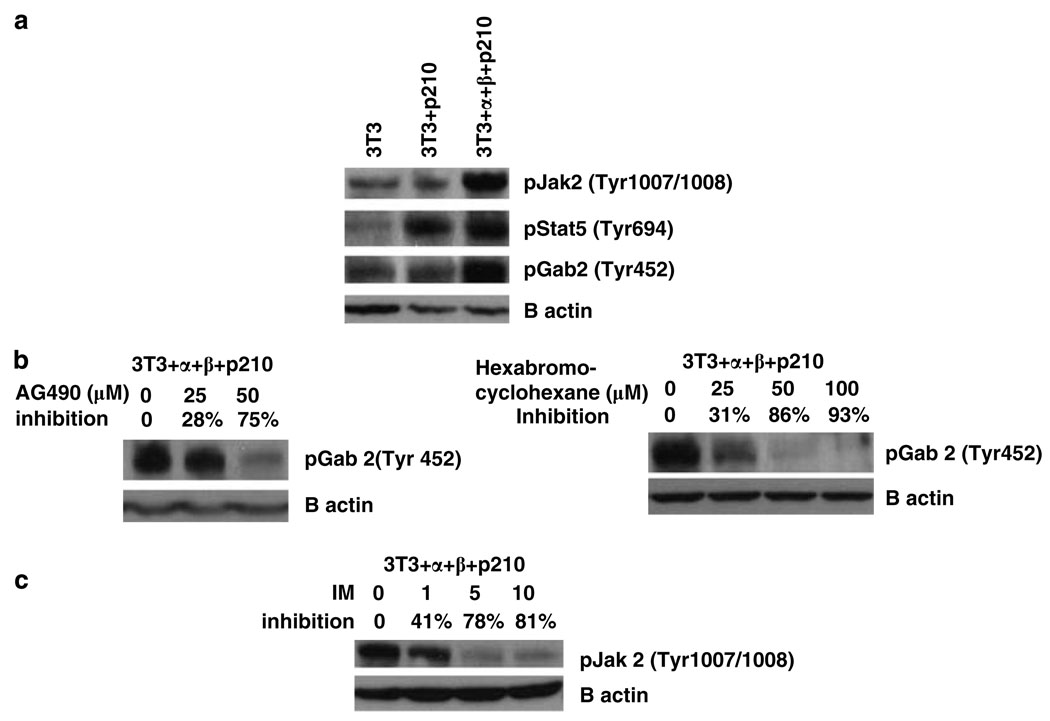
We determined whether Jak2 and Stat5 are activated in 3T3 + α + β + p210 cells and whether Bcr-Abl + 3T3 cells that lack the IL-3 receptor expression also utilize Jak2 and Stat5 (Figure 5). The results indicated that the 3T3 + α + β + p210 cells strongly activate Jak2, whereas Bcr-Abl + cells lacking the receptors do not activate Jak2 over above NIH 3T3 cells. Surprisingly, the IL-3 receptor expression is not required for activation of Stat5, as both BCR-ABL + NIH 3T3 cells and the triple positive cells had higher levels of activated Stat5 than NIH 3T3 cells (Figure 5a). Importantly, imatinib treatment of triple positive cells strongly inhibited phosphorylation of Jak2 on Y1007/1008 (Figure 5c).
Figure 5.
Jak2, Gab2 and Stat5 are tyrosine phosphorylated in NIH 3T3 cells expressing α and β chains of the IL-3 receptor and P210 BCR-ABL. (a) The cell lysates from NIH 3T3, 3T3 + P210 and 3T3+ α + β + P210 cell lines were immunoblotted with phospho-Jak2 (Tyr 1007/1008) antibody, phospho-Gab2 (Tyr 452) antibody and phospho-Stat5 (Tyr 694) antibody. (b) 3T3+ α + β + P210 cells were treated with different concentrations of AG490 (left panel) or 1,2,3,4,5,6-hexabromocyclohexane (right panel) for 24 h. Cell lysates were immunoblotted with phospho-Gab2 (Tyr 452) antibody. (c) 3T3+ α + β + P210 cells were treated with different concentration of imatinib for 24h. Cell lysates were immunoblotted with phospho-Jak2 (Tyr 1007/1008) antibody.
Because our previous studies have shown that activated Jak2 is necessary for activation of the Gab2/PI-3 kinase pathway driven by Bcr-Abl by inducing the phosphorylation of Gab2 on YxxM residue(s) (Samanta et al., 2006), we examined the involvement of pTyr (YxxM) Gab2 levels in these NIH 3T3 cells. Phosphotyrosine Gab2 (YxxM) was low in NIH 3T3 cells and BCR-ABL + 3T3 cells, but highly elevated in the BCR-ABL triple positive cells (Figure 5a). In addition, inhibition of Jak2 by either AG490 or hexabromocyclohexane strongly inhibited Gab2 (Tyr 452) phosphorylation in the BCR-ABL triple positive cells (Figure 5b). We note that, although Akt activation as measured by pSer473 occurred in all three cells at high levels, none were dependent on Jak2 alone (not shown), indicating that NIH 3T3 cells have one or more pathways that do not depend on the Bcr-Abl/receptor/Jak2 pathway for Akt activation.
Discussion
Bcr-Abl is a leukemia-inducing protein, which causes oncogenic transformation of myeloid progenitors in Philadelphia chromosome (Ph)-positive chronic myeloid leukemia and lymphoid progenitors in Ph + acute lymphoid leukemia. Transgenic mouse models indicate that although BCR-ABL is expressed in every cell of the transgenic mouse, mice develop only hematology malignancies (Groffen et al., 1993). A number of years ago it was shown that Bcr-Abl expression does not efficiently transform fibroblasts (Daley et al., 1987). Importantly, transformation of Rat-1 fibroblasts is an exception but these cells are a special isolate of Rat-1 cells (Lugo and Witte, 1989) and only poorly show colony formation unless cells are also transformed by the Myc oncogene (Lugo and Witte, 1989). These results indicate that Bcr-Abl has little oncogenic effects on nonhematopoietic cells.
We addressed this issue and hypothesized that if nonhematopoietic cells could be forced to take on a hematopoietic phenotype, BCR-ABL might be able to cause malignant transformation. Yokota et al. (1993) demonstrated that NIH 3T3 cells transfected with the human GM-CSF receptor chains had a phenotype that resembled hematopoietic cells. We chose to focus on the human IL-3 receptor expression in NIH 3T3 cells, as a number of studies showed that Bcr-Abl expression can prevent IL-3-dependent cells from undergoing cell death when maintained in the absence of IL-3 (Sirard et al., 1994; Wilson-Rawls et al., 1996).
Our findings show that NIH 3T3 cells expressing both the receptor chains of the IL-3 receptor are oncogenically transformed by Bcr-Abl. Thus, these cells have altered morphology and form foci in cell culture. In addition, these cells readily form colonies in soft agar. Importantly, colony formation is strongly inhibited by IM, which is consistent with the ability of Bcr-Abl to strongly activate tyrosine phosphorylation of various target proteins in these cells. In contrast, NIH 3T3 cells expressing BCR-ABL alone had normal morphology, did not form significant numbers of foci and were very inefficient at forming colonies in soft agar.
Since the expression of the IL-3 receptor chains was critical for the oncogenic transformation of NIH 3T3 cells by BCR-ABL, we asked whether Jak2 is involved in the oncogenic behavior of these cells. We have previously shown that mouse and human BCR-ABL + hematopoietic cells require Jak2 for the expression of the oncogenic phenotype (Xie et al., 2001, 2002; Samanta et al., 2006). Importantly, Jak2 plays a critical role in activating the PI-3 kinase, Akt, and NF-kB and inactivating GSK3-β. The end result of this Jak2 signaling is that Bcr-Abl is able to elevate c-Myc through the PI-3 kinase Akt–GSK3 pathway (Xie et al., 2002; Samanta et al., 2006). Our current studies show that Jak2 inhibitors AG490 and hexabromocyclohexane strongly inhibited colony formation in soft agar of NIH 3T3 cells expressing BCR-ABL and the IL-3 receptor at doses below the typical IC50 for Jak2 inhibitor (50 µm) (Samanta et al., 2006). Of interest, the triple positive cells contain highly activated Jak2 and high levels of pTyr (YxxM) Gab2, indicating that the Bcr-Abl/Jak2/Gab2 (YxxM) pathway is intact in triple positive cells but not in BCR-ABL + NIH 3T3 cells that lack the receptors. However, Jak2 inhibition, although reducing activated Gab2, did not affect the level of activated Akt, indicating the Akt activation occurs through more than one signaling pathway in triple positive cells.
We emphasize that activation of Stat5 as measured by formation pTyr694 Stat5 occurred in both triple positive cells and Bcr-Abl + 3T3 cells but not to any great extent in 3T3 cells (Figure 5a). Thus, it appears that activation of Stat5 is independent of the IL-3 receptor. Previous findings by two groups have shown that Stat5 activation is directed by Bcr-Abl oncoprotein (Ilaria and Van Etten, 1996; Klejman et al., 2002). Our previous studies indicate that Stat5 activation is independent of Jak2 activation in BCR-ABL + 32D cells (Xie et al., 2001), which is in agreement with the earlier findings of Ilaria and Van Etten (1996).
This cell system will provide a model to probe the detailed reactions required for Bcr-Abl oncogenesis. For example, we can determine which sequences within the IL-3 receptor chains are required for transformation, and which receptor tyrosine residues are needed for oncogenic transformation and the accessory signaling pathways involving Jak2/Gab2/PI-3 kinase pathway. Further studies are underway to answer these questions.
Materials and methods
Reagents
AG490 was purchased from LC Laboratories (Woburn MA, USA; catalog no. 134036-52-5). Imatinib was obtained from the MD Anderson Pharmacy. 1,2,3,4,5,6-hexabromocyclohexane was purchased from EMD BioScience (Calbiochem, San Diego, CA, USA; catalog no. 420132). Hygromycin (Hygromycin B from Streptomyces hygroscopicus) was purchased from Sigma-Aldrich (Louis MO, USA; catalog no. H3274). Zeocin was from Invitrogen (Carlsbad CA, USA; catalog no. R250-01). Mouse anti-human IL-3 Rα monoclonal antibody and rabbit anti-human IL-3/IL-5/GM-CSF receptor common β chain polyclonal antibody used for western blotting were purchased from BD Bioscience (San Jose, CA, USA). Phycoerythrin (PE)-conjugated mouse anti-human IL-3 Rα antibody and mouse anti-human IL-3/IL-5/GM-CSF receptor common β chain antibody applied in flow cytometry assays were purchase from eBioscience (San Diego, CA, USA). Anti-Abl SH2 domain monoclonal antibody (8E9) was produced by our own laboratory. Anti-phosphotyrosine antibody (4G10) were purchased from Upstate (Billerica, MA, USA). Phospho-Jak2 (Try1007/1008) antibody, phospho-Gab2 antibody (Tyr 452) and phosphor-Stat5 (Tyr 694) antibody were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA).
Cell lines
The mouse NIH 3T3 fibroblast cell line was cultured in 1 × Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) and 100 µg ml−1 penicillin/streptomycin. All cell lines were maintained in a 5.5% CO2 incubator at 37°C.
Plasmid and cell transfection
PME-DUK-1 vector, which contained the IL-3 Rα cDNA ligated with hygromycin drug selection gene and KH97 vector, which had IL-3 Rβ cDNA ligated with zeocin selection gene, were kindly provided by Dr James McCubrey (East Carolina Medical School, Greenville, NC, USA). PME-DUK-1 and KH97 were transfected into NIH 3T3 cell by FuGene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN, USA). Cells were allowed to recover for 48 h in DMEM medium with 10% FBS without either hygromycin or zeocin. After 2 days, hygromycin and zeocin were added to the normal medium to a final concentration of 200 µg ml−1 and 400 µg ml−1, respectively. Most of the cells died rapidly within 48 h and double drug-resistant cell lines were obtained within 14–18 days; cells were transferred into selection medium supplemented with hygromycin and zeocin, 4 weeks later, stable positive clones grew out and were maintained in drug selection medium for all experiments.
Foci formation
A total of 5 × 104 cells were plated in each well in six-well plates. Foci were counted after 10 or 27 days of culture by crystal violet staining and foci were confirmed to be GFP positive by fluorescent microscopy. Percent GFP fluorescence of cells seeded was determined by analysis of 10 000 cells in a flow cytometry. Percentage of BCR-ABL-positive cells can be inferred from the percentage of GFP-positive NIH 3T3 cells infected with Migr1 retrovirus containing both the GFP gene and the BCR-ABL gene in the same viral transcript. Numbers of foci are average number of foci from three wells per 104 GFP-positive cells.
Soft agar colony formation
Soft agar colony formation assay was performed following the previous protocol. A total of 1000 cells were mixed with 0.35% upper gel supplemented with indicated concentrations of either imatinib (1, 5 and 10 µm) or AG490 (10, 20 µm) or hexabromocyclohexane (50, 75 and 100 µm) and were set in each well of six-well plates. The culture medium containing corresponding concentration of either imatinib or AG490 or hexabromocyclohexane was changed every 3 days. After 3 weeks, pictures of colonies were taken by a digital camera after staining with 0.005% blue violet. Each treatment was triplicate. Colonies numbers were counted at least twice and the data were imported into Excel for graphing and statistics analysis.
Western blotting
Cells were plated on 100 mm Petri dishes with DMEM medium. When achieving 80% confluent, cells were treated with IM (1, 5 and 10 µm) or AG490 (25 and 50 µm) for 24h. Cells were lysed in 1% NP40 lysis buffer containing 50 mm Tris–HCl (pH 7.5), 150 mm NaCl, 2 mm EDTA (pH 8.0), 50 mm NaF, 10 mm glycerol-2 phosphate and protease inhibitors (1 mm Na3VO3, 2 µg ml−1 leupentin, 2 µg ml−1 aprotinin (Sigma-Aldrich), 0.5 mg ml−1 benzamidine, 1 mm phenylmethylsulphonylfluoride), incubated on ice for 30 min and centrifuged at 15 000 r.p.m. for 20 min at 4 °C. The supernatant protein was quantified by Coomassie Plus blue assay reagent (Amersham Pharmacia Biotech, Piscataway, NJ, USA). A total of 60 µg of lysates was loaded to each lane of Sodium dodecyl Sulphate (SDS)–PAGE and transferred onto PVDF membrane. Membranes were blocked with 5% nonfat milk or 5% bovine serum albumin, incubated with the primary antibody at 4 °C with gentle shaking overnight. After washing, membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies at room temperature for 1 h, visualized by enhance chemiluminescence (Amersham Pharmacia Biotech).
Flow cytometry assay
Cells were digested with 0.5% trypsin-EDTA (Gibco) from 100 mm Petri dishes and centrifuged at 1000 r.p.m. for 5 min at room temperature. After washing cells twice with cold phosphate-buffered saline (PBS), cells were incubated with either PE-conjugated mouse anti-human IL-3 Rα antibody or IL-3/IL-5/GM-CSF receptor common β chain antibody at 4 °C with gentle shaking for 3 h in darkness. Cells were washed twice with cold PBS, resuspended in 300 µl PBS and examined by flow cytometry (Beckman Coulter, Fullerton CA, USA).
Supplementary Material
Acknowledgements
This research was supported in part by funds from CA49639, and funds from the Hubert L Stringer for Cancer Research.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- Daley G, McLaughlin J, Witte ON, Baltimore D. The CML-specific P210 Bcr/Abl protein, unlike v-abl does not transform NIH 3T3 fibroblast. Science. 1987;237:532–535. doi: 10.1126/science.2440107. [DOI] [PubMed] [Google Scholar]
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- Groffen J, Voncken JW, Kaartinen V, Morris C, Heisterkamp N. Ph-positive leukemia: a transgenic mouse model. Leuk Lymphoma. 1993;11 Suppl 1:19–24. doi: 10.3109/10428199309047857. [DOI] [PubMed] [Google Scholar]
- Ilaria RL, Jr, Van Etten RA. P210 and P190 (BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- Klejman A, Schreiner SJ, Nieborowska-Skorska M, Slupianek A, Wilson M, Smithgall TE, et al. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. EMBO J. 2002;21:5766–5774. doi: 10.1093/emboj/cdf562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo TG, Witte ON. The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol Cell Biol. 1989;9:1263–1270. doi: 10.1128/mcb.9.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Sugita K, Goi K, Inukai T, Lijima K, Tezuka T, et al. The JAK2 inhibitor AG490 predominantly abrogates the growth of human B-precursor leukemic cells with 11q23 translocation or Philadelphia chromosome. Leukemia. 2001;15:1758–1768. doi: 10.1038/sj.leu.2402260. [DOI] [PubMed] [Google Scholar]
- Quelle FW, Sato N, Witthuhn BA, Inhorn RC, Eder M, Miyajima A, et al. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994;14:4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta AK, Lin H, Sun T, Kantarjian H, Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 2006;66:6468–6472. doi: 10.1158/0008-5472.CAN-06-0025. [DOI] [PubMed] [Google Scholar]
- Sandberg EM, Ma X, He K, Frank SJ, Ostrov DA, Sayeski PP. Identification of 1,2,3,4,5,6-hexabromocyclohexane as a small molecule inhibitor of Jak2 tyrosine kinase autophosphorylation. J Med Chem. 2005;48:2526–2533. doi: 10.1021/jm049470k. [DOI] [PubMed] [Google Scholar]
- Sirard C, Laneuville P, Dick JE. Expression of bcr-abl abrogates factor-dependent growth of human hematopoietic M07E cells by an autocrine mechanism. Blood. 1994;83:1575–1585. [PubMed] [Google Scholar]
- Watanabe S, Itoh T, Arai K. Roles of JAK kinase in human GM-CSF receptor signals. Leukemia. 1997;11 Suppl 3:76–78. [PubMed] [Google Scholar]
- Wilson-Rawls J, Xie S, Liu J, Laneuville P, Arlinghaus RB. P210 Bcr-Abl interacts with the interleukin 3 receptor beta(c) subunit and constitutively induces its tyrosine phosphorylation. Cancer Res. 1996;56:3426–3430. [PubMed] [Google Scholar]
- Xie S, Lin H, Sun T, Arlinghaus RB. Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene. 2002;21:7137–7146. doi: 10.1038/sj.onc.1205942. [DOI] [PubMed] [Google Scholar]
- Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE, et al. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene. 2001;20:6188–6195. doi: 10.1038/sj.onc.1204834. [DOI] [PubMed] [Google Scholar]
- Yokota T, Watanabe S, Mui AL, Muto A, Miyajima A, Arai K. Reconstitution of functional human GM-CSF receptor in mouse NIH3T3 fibroblasts and BA/F3 proB cells. Leukemia. 1993;7 Suppl 2:S102–S107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.