Abstract
Anthrax toxin neutralization assays are used to measure functional antibody levels elicited by anthrax vaccines in both preclinical and clinical studies. In this study, we investigated the magnitude and molecular nature of Fc gamma (Fcγ) receptor-dependent toxin neutralization observed in commonly used forms of the anthrax toxin neutralization assay. Significantly more Fcγ receptor-dependent neutralization was observed in the J774A.1 cell-based assay than in the RAW 264.7 cell-based assay, a finding that could be due to the larger numbers of Fcγ receptors that we found on J774A.1 cells by using flow cytometry. Thus, the extent to which Fcγ receptor-dependent neutralization contributes to the total neutralization measured by the assay depends on the specific cell type utilized in the assay. Using Fcγ receptor blocking monoclonal antibodies, we found that at least three murine Fcγ receptor classes, IIB, III, and IV, can contribute to Fcγ receptor-dependent neutralization. When antibodies elicited by immunization of rabbits with protective-antigen-based anthrax vaccines were analyzed, we found that the magnitude of Fcγ receptor-dependent neutralization observed in the J774A.1 cell-based assay was dependent on the concentration of protective antigen utilized in the assay. Our results suggest that the characteristics of the antibodies analyzed in the assay (e.g., species of origin, isotype, and subclass), as well as the assay design (e.g., cell type and protective antigen concentration), could significantly influence the extent to which Fcγ receptor-dependent neutralization contributes to the total neutralization measured by anthrax toxin neutralization assays. These findings should be considered when interpreting anthrax toxin neutralization assay output.
Anthrax is a serious and potentially fatal disease caused by Bacillus anthracis. After uptake and germination of anthrax spores within the host, the bacteria elaborate large quantities of anthrax toxin, which is believed to be essential for disease progression (12, 16, 19). The tripartite toxin is composed of a receptor-binding component, protective antigen (PA), and two distinct enzymatic components, lethal factor (LF) and edema factor (16). LF, a Zn2+-dependent metalloprotease, combines with PA to form lethal toxin (LT), which is cytotoxic to certain cell types, such as macrophages from susceptible species (2, 6, 7). Edema factor, an adenylate cyclase, combines with PA to form edema toxin, which exerts its effects on cells by dramatically increasing cyclic AMP levels (15).
B. anthracis is considered a serious bioterror threat. For this reason, a considerable amount of effort has been devoted to the development of new vaccines and therapeutics for anthrax. While one licensed anthrax vaccine is currently available in the United States, efforts are under way to develop new-generation anthrax vaccines composed of purified recombinant PA (rPA) (9). Antibodies generated by such vaccines would be expected to neutralize the action of both LT and edema toxin and thus protect against disease.
The efficacy of new anthrax vaccines cannot be measured directly in humans because such clinical studies are either not feasible or not ethical to conduct. Therefore, in the United States, animal protection data, obtained using two relevant animal species, may serve as primary data to support human efficacy of new-generation anthrax vaccines, as described in the U.S. Government regulation (21 CFR 601, subpart H) commonly known as the “Animal Rule” (3). In order to apply the Animal Rule, animal protection data must be extrapolated to humans in a scientifically sound manner. In the case of anthrax, aerosol infection of nonhuman primates and rabbits is thought to adequately reflect human disease (4, 5). Thus, these two animal models will likely serve as the basis for pivotal animal protection studies. Immunogenicity data will play a critical role in application of the Animal Rule to anthrax vaccines, since serological data from the animals and humans will serve to bridge the animal protective response to human efficacy (5).
One serological assay that is likely to be used to assess vaccine immunogenicity in pivotal animal and human studies is the anthrax toxin neutralization assay (TNA), which measures functional antibody levels to anthrax toxin (10, 17). In this assay, the toxin is added to susceptible cells in the presence and absence of neutralizing antibodies. The effect of the toxin on the cells in the presence of antibodies is compared to that observed in their absence. Several forms of the TNA exist, with common forms designed to assess neutralization of the cytotoxic action of LT on either J774A.1 cells or RAW 264.7 cells, both of which are mouse macrophage-like cell lines. The RAW 264.7 cell-based assay has been used primarily in anthrax toxin research (25), whereas the J774A.1 cell-based assay has also been used in clinical vaccine studies and to measure antibody levels in clinical samples (9, 26).
Antibody-mediated neutralization in the TNA can occur by “classic” toxin neutralization or, with appropriate cell types, by Fc gamma (Fcγ) receptor-dependent neutralization (31). We define “classic” toxin neutralization as the blocking of a critical step in toxin action, such as receptor binding, pore formation, or enzyme activity. This type of neutralization can be observed with any cell type that is susceptible to the toxin. In contrast, Fcγ receptor-dependent neutralization would be observed only in assays that use cell types that express Fcγ receptors. One such cell type is the macrophage, the cell type that is used for LT neutralization assays because the macrophage, unlike most other cell types, is susceptible to the cytotoxic action of LT. The molecular basis for Fcγ receptor-dependent neutralization is not well understood; however, it may be the result of Fcγ receptor-mediated endocytosis of immune complexes leading to their degradation, sequestration of PA monomers at the cell surface, and/or enhancement of antibody-mediated neutralization by increasing the effective concentration of neutralizing antibodies at the cell surface. Of note, others have observed that nonneutralizing or marginally neutralizing monoclonal antibodies (MAbs) can actually enhance the cytotoxicity of LT on J774A.1 cells through an Fc receptor-dependent mechanism (20). In that case, it was postulated that the Fc receptor-MAb-PA complex decreased the off-rate of PA from the cell surface. Because the antibody did not effectively neutralize PA, stabilization of PA on the cell surface—its target site of action—had the effect of increasing LT toxicity. By similar logic, in the case of neutralizing antibodies, the Fc receptor would be expected to stabilize the antibody at the cell surface, increasing its effective concentration at the very site at which PA accumulates before it combines with LF and exerts its effects on the cell.
In order to properly interpret TNA output from pivotal animal and clinical studies and to begin to assess the relevance of the assay output to in vivo protective mechanisms, we need a thorough understanding of the mechanisms of neutralization that contribute to the total neutralization observed in the assay. In this study, we further investigated Fcγ receptor-dependent neutralization in order to better define the molecular basis for this type of toxin neutralization. We also examined the effect of assay parameters, such as cell type and PA concentration, on Fcγ receptor-dependent neutralization in order to better understand the effects that these parameters have on assay output.
MATERIALS AND METHODS
Cell lines and other reagents.
The murine macrophage-like cell line RAW 264.7 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Anti-rPA rabbit reference polyclonal serum (NR-3839), B. anthracis rPA (NR-140), recombinant LF (NR-142), and murine macrophage-like cell line J774A.1 cells (NR-28) were obtained from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH (Bethesda, MD). Rat anti-mouse CD16/CD32 MAb clone 2.4G2 (Fcγ receptor class II [FcγRII] and FcγRIII blocking MAb) was obtained from BD Pharmingen (Franklin Lakes, NJ). MAb 2.4G2 functionally blocks FcγRIIB and FcγRIII but will not functionally block FcγRIV, even though it can bind to this receptor class (11, 13, 18, 22, 30). MAb 9E9, which specifically blocks FcγRIV, was kindly provided by Jeffrey Ravetch, Rockefeller University (New York, NY). Phycoerythrin (PE)-conjugated anti-FcγRI (anti-CD64, clone X54-5/7.1) and PE-conjugated anti-FcγRII/III/IV (anti-CD16/CD32, clone 2.4G2) were also obtained from BD Pharmingen. Different strains of BALB/c mice, wild type, Fcer1g (FcγRI/III/IV knockout [KO]), and Fcgr2b (FcγRIIB KO), were obtained from Taconic Laboratories (Germantown, NY). The Fcer1g mouse is deficient in the gamma chain subunit common to FcγRI, FcγRIII, and FcγRIV, which is necessary for the assembly and cell surface expression of the intact receptors (23).
Cell culture.
Murine macrophage-like cell lines J774A.1 and RAW 264.7 were grown in Dulbecco's modified Eagle medium (containing high glucose and sodium pyruvate) supplemented with 5% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 25 U/ml of penicillin, 25 μg/ml of streptomycin sulfate, and 10 mM HEPES. Bone marrow-derived macrophages were collected from femurs of mice (wild type, FcγRI/III/IV KO, and FcγRIIB KO on the BALB/c background). Bone marrow cells were grown in the complete Dulbecco's modified Eagle medium mentioned above, supplemented with 10% conditioned L929 culture supernatant in 96-well plates. Bone marrow cells were grown for 5 days to confluence and used for TNAs. Since LT is cytotoxic for macrophages from only certain strains of mice (1, 7), only specific genetic backgrounds, such a BALB/c background, were useful in these studies. Cell culture reagents were obtained from Gibco (Carlsbad, CA).
Anthrax TNAs.
TNAs were performed essentially as described previously (26), using J774A.1 cells and RAW 264.7 cells. Briefly, cells were plated in 96-well flat-bottomed plates (40,000 cells/well) and incubated for 17 to 19 h at 37°C in a 5% CO2 incubator. Neutralization of LT cytotoxicity was measured by assessing cell viability with twofold serial dilutions of the rabbit polyclonal serum (NR-3839). Twofold serum sample dilutions prepared in a 96-well plate were preincubated with a constant amount of LT (PA at 50 ng/ml plus LF at 40 ng/ml), unless otherwise indicated. This concentration of LT kills approximately 95% of the cells in the absence of any neutralizing serum sample. After a 30-min incubation of rabbit polyclonal serum with LT, the mixtures were transferred to the 96-well cell plate and cells were incubated for 4 h at 37°C. Following incubation, MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] was added to plates. After 2 h of incubation, cells were lysed by the addition of solubilization buffer (90% isopropanol, 0.5% sodium dodecyl sulfate [wt/vol], and 38 mM HCl). The plates were read for optical density by use of a microplate reader at A570. A four-parameter logistic regression model was used to fit the data points generated when the optical density was plotted versus the logarithm of the reciprocal of the serum dilution. The inflection point, which indicates 50% neutralization, was reported as the mean effective dilution (ED50). For TNAs performed using purified immunoglobulin G (IgG) and F(ab′)2, twofold serial dilutions of IgG or F(ab′)2 were incubated with LT, followed by transfer to the cell plate. Data were analyzed as described above, and the inflection point was reported as the mean effective concentration (EC50). For TNAs performed in the presence of blocking MAb 2.4G2 or 9E9, cells were preincubated with MAbs at a concentration of 10 μg/ml for 15 min prior to the addition of LT and the serum sample mix. For experiments to determine the effect of PA concentration on the extent of the Fcγ receptor-mediated neutralization, PA was used at a concentration of 50 ng/ml or 400 ng/ml, whereas LF was used at 40 ng/ml.
IgG purification and F(ab′)2 preparation.
IgG was purified from the rabbit reference polyclonal serum (NR-3839) by using a Nab Spin kit (Pierce, Rockford, IL) according to the manufacturer's instructions. Briefly, rabbit polyclonal serum was incubated with an immobilized protein G column for 10 to 15 min. After IgG binding, the column was washed five times and bound IgG was eluted using a low-pH elution buffer into a tube containing neutralization buffer. Purified IgG was stored in 1× phosphate-buffered saline (PBS). F(ab′)2 fragments were prepared from the purified rabbit polyclonal serum IgG described above by use of an ImmunoPure F(ab′)2 preparation kit (Pierce). Briefly, approximately 10 mg of IgG in 1 ml of digestion buffer was incubated with immobilized pepsin for about 3 h in a shaking water bath at 37°C. F(ab′)2 fragments were then purified using an immobilized protein A column. Additional purification was performed using a Bio-Gel P-100 column (Bio-Rad, Hercules, CA). Purified F(ab′)2 fragments were concentrated and stored in 1× PBS. The concentrations of the purified IgG and F(ab′)2 fragments were determined using absorbance at 280 nm and extinction coefficients of 1.43 and 1.48, respectively.
Flow cytometry.
J774A.1 and RAW 264.7 cells were maintained in culture as described above and harvested at 60 to 80% confluence to perform flow cytometric analysis. Cells were washed and suspended in cold flow cytometry buffer (FB), defined as Ca2+- and Mg2+-free PBS, supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and incubated for 30 min on ice to inhibit nonspecific binding of antibodies. Cells were incubated with either PE-conjugated anti-FcγRI or PE-conjugated anti-FcγRII/III/IV for 30 min on ice at appropriate concentrations, determined separately by titration to ensure saturation of all receptors. After one wash with FB, the cell suspension was stained to discriminate live from dead cells by using a commercially available kit according to the manufacturer's instructions (Live/Dead staining kit; Invitrogen, Carlsbad, CA). A second wash with FB was performed before the samples were stained for other cell surface markers. The following antibodies were used: anti-CD19 (clone 1D3), anti-CD45 (clone 30-F11), and anti-CD11b (clone M1/70). Appropriate fluorochrome-labeled, isotype-matched antibodies were used as controls for surface staining. After 30 min of incubation, cells were washed and fixed in 0.5% paraformaldehyde (EMS, Hatfield, PA). Twenty thousand total events were counted using an analytical LSR II flow cytometer (Becton Dickinson, San Jose, CA). The starting point for all analyses was the exclusion of dead (Pacific Blue positive) cells and of aggregates by comparison of forward scatter A versus forward scatter H. Cells were then analyzed for CD45 and CD11b, characteristic markers of leukocytes and macrophages, respectively, and CD19, a B-cell marker used as negative control. Live CD45+ CD11b+ CD19− cells, representing 98 to 99% of the cells, were then evaluated for expression of FcγRI and FcγRII/III/IV, and the mean fluorescence of these entire populations was calculated. Data analyses were performed using FlowJo (Tree Star, Inc.) software, v7.1.3.
Statistical analyses.
All statistical analyses were performed using GraphPad Prism 5 software. ED50s, EC50s, and ED50 ratios were log transformed prior to analysis. For the determination of ED50 (or EC50) ratios, the ED50 (EC50) values were established using a constrained four-parameter logistic model in which the slope parameters and the asymptotes were equal in the two curves.
RESULTS
Fcγ receptor-dependent neutralization in J774A.1 cells and RAW 264.7 cells.
Fcγ receptor-dependent toxin neutralization has been documented to occur in the J774A.1 cell-based TNA (31). Fcγ receptor-dependent neutralization might also be expected to be observed in the RAW 264.7 cell-based TNA, since these cells express Fcγ receptors (28). To determine the contribution of Fcγ receptor-dependent neutralization in each of these two forms of the assay, we compared the neutralizing capacities of equimolar amounts of a purified IgG preparation and its corresponding F(ab′)2 fragments in the two different cell-based TNAs. The difference in neutralization observed for purified IgG and its corresponding F(ab′)2 fragments in a particular assay represents the contribution of Fcγ receptor-dependent neutralization for that assay, since F(ab′)2 fragments, which retain the bifunctional binding of the IgG antibody, lack an Fc portion (14) and therefore cannot bind to the Fcγ receptor on the cell surface. The IgG preparation used in this study was isolated from a polyclonal antiserum (NR-3839) pooled from rabbits immunized with an rPA vaccine. Our reasons for using rabbit antiserum for this study were twofold. First, rabbits will be utilized for pivotal protection and immunogenicity studies that will serve, in part, as the basis for approval of new anthrax vaccines. Second, the quantities of rabbit immune serum available to us were larger than those of immune sera from other species, such as human, nonhuman primate, or mouse, which is of particular importance when purified IgG and F(ab′)2 fragments are needed. Of note, Fcγ receptor-dependent neutralization was observed previously with immune sera from mice, rabbits, and humans, indicating that the murine Fcγ receptors can bind to the constant region of IgG molecules of multiple species, despite the fact that the primary structures of the Fc region differ between species (31).
As seen in Fig. 1A, the purified IgG preparation neutralized LT more efficiently than the corresponding F(ab′)2 fragments in the J774A.1 cell-based LT TNA. The geometric mean (GM) ratio of EC50s for F(ab′)2:IgG based on four independent experiments was 3.0, with a 95% confidence interval (95% CI) of 2.2 to 4.2. The fact that the lower limit of the 95% CI for the ratio was clearly greater than 1.0 indicates that Fcγ receptor-dependent neutralization contributes significantly to the total neutralization observed in the J774A.1 cell-based assay. When the neutralization by the purified IgG preparation was compared to that of the corresponding F(ab′)2 fragments in the RAW 264.7 cell-based TNA (Fig. 1B), the GM ratio of the EC50s for F(ab′)2:IgG was 1.3, with a 95% CI of 1.3 to 1.4, as determined in three independent assays. The GM ratio of the EC50s for F(ab′)2:IgG obtained in the J774A.1 cell-based assay was significantly different than that observed for the RAW 264.7 cell-based assay (P < 0.01, unpaired t test), indicating that Fcγ receptor-dependent neutralization plays a considerably larger role in the J774A.1 cell-based assay.
FIG. 1.
Contribution of Fcγ receptor-dependent neutralization in J774A.1 cell-based and RAW 264.7 cell-based TNAs. IgG was purified from the polyclonal antiserum NR-3839; F(ab′)2 fragments were prepared from this IgG preparation. The indicated concentrations of either IgG or F(ab′)2 fragments were examined for their ability to neutralize LT in either the J774A.1 cell-based TNA (A) or the RAW 264.7 cell-based TNA (B). Each point corresponds to the mean of the values obtained for three independent sample preparations run on the same plate, with the standard error of the mean indicated by the error bar. For each independent assay, the samples were run on duplicate plates. The figures are representative of four and three independent assays for the J774A.1 and RAW 264.7 cell-based assays, respectively, with each independent assay run on different days.
Quantification of Fcγ receptors on J774A.1 cells and RAW 264.7 cells.
The finding that the two macrophage-based TNAs manifest Fcγ receptor-dependent neutralization to differing extents prompted us to further investigate the root cause for this difference. A simple explanation for the difference in Fc receptor-dependent neutralization observed with the two assays might be a difference in the numbers of Fcγ receptors present on the surfaces of the two different cell types. Fc receptors are classified based on the isotypes of antibodies that they recognize. Fcγ receptors bind the most prevalent antibody isotype, IgG (27). In the mouse, four different classes of Fcγ receptors have been identified: FcγRI, FcγRIIB, FcγRIII, and FcγRIV (23). Murine monocytes and macrophages have been reported to express all four classes of Fcγ receptors (23); thus, both J774A.1 cells and RAW 264.7 cells, which were derived from BALB/c mice, might be expected to express all four classes of Fcγ receptors.
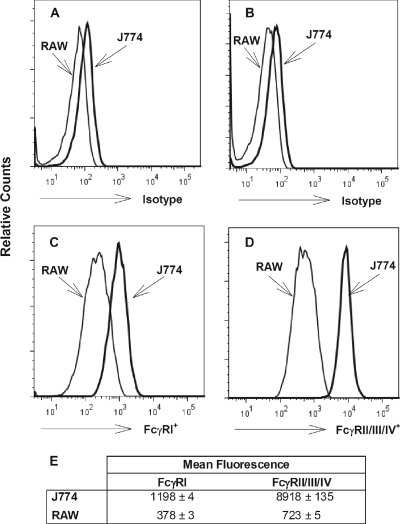
We used flow cytometry to determine whether the quantities of Fcγ receptors on J774A.1 cells and RAW 264.7 cells differ. Using an antibody specific for FcγRI or an antibody that binds FcγRIIB, FcγRIII, and FcγRIV, we examined the relative levels of abundance of these groups of receptors on the two different cell types. Both cell types expressed FcγRI and FcγRIIB/III/IV (Fig. 2). The mean fluorescence of FcγRI binding was about two times greater for J774A.1 cells than for RAW 264.7 cells, while about a 10-fold difference between the two types of cells was observed for FcγRIIB/III/IV binding. Thus, a simple explanation for the difference in the extents of Fcγ receptor-mediated neutralization observed between the J774A.1 cell-based assay and the RAW 264.7 cell-based assay may be the greater number of Fcγ receptors, especially FcγRIIB/III/IV, on J774A.1 cells.
FIG. 2.
Quantification of Fcγ receptors on J774A.1 and RAW 264.7 cells, determined using flow cytometry. Flow cytometric analysis was performed as described in Materials and Methods. Representative histograms of live CD45+ CD11b+ CD19− cells analyzed for FcγRI (C) and FcγRII/III/IV (D) are shown, with comparison to the appropriate isotype-matched, fluorochrome-labeled control antibodies for each (FcγRI [A] and FcγRII/III/IV [B]). The arithmetic mean fluorescent intensities ± standard deviations from triplicate samples are also shown (E).
Identification of specific Fcγ receptor classes that play a role in toxin neutralization.
We next attempted to identify specific Fcγ receptor classes that can play a role in Fcγ receptor-dependent neutralization. We first examined the ability of MAb 2.4G2, an FcγRIIB/III blocking antibody, to inhibit the Fcγ receptor-dependent neutralization observed with the polyclonal rabbit serum sample, NR-3839. As shown in Fig. 3A, the presence of MAb 2.4G2 significantly decreased the neutralization observed in the J774A.1 cell-based assay, as manifested by a decrease in the ED50 of the serum sample examined. Three independent replicates of this experiment were conducted, and the GM ratio of the ED50s obtained with or without MAb 2.4G2 was 2.2, with a 95% CI of 1.8 to 2.6. This result indicates that FcγRIIB and/or FcγRIII can contribute to the Fcγ receptor-dependent neutralization observed in the TNA. In order to determine whether FcγRIV might also contribute to Fcγ receptor neutralization, we next examined the effect of MAb 9E9, a MAb that functionally blocks FcγRIV (22). As seen in Fig. 3B, MAb 9E9 significantly decreased the anthrax toxin neutralization observed in the assay. The GM ratio of the ED50s obtained with or without MAb 9E9 determined in three independent experiments was 1.5, with a 95% CI of 1.3 to 1.6, indicating that FcγRIV contributed modestly to the Fcγ receptor-dependent neutralization.
FIG. 3.
Toxin neutralizing activity of rabbit polyclonal serum NR-3839, measured in the presence and absence of anti-FcγRII/III or anti-FcγRIV. TNAs were performed essentially as described in Materials and Methods. The indicated dilutions of NR-3839 were used to neutralize LT in J774A.1 cell-based TNAs in the absence (−) or presence (+) of anti-FcγRII/III (MAb 2.4G2) (A) and anti-FcγRIV (MAb 9E9) (B). Each point corresponds to the mean of the values obtained for two independent sample preparations each run on a separate plate, with the standard error of the mean indicated by the error bar. Each figure is representative of three independent assays, each run on different days.
The individual contributions of FcγRIIB and FcγRIII to the neutralization of LT cytotoxic action on murine macrophages could be distinguished further by using bone marrow-derived macrophages derived from mice with mutations in genes encoding specific Fcγ receptor subunits. We first confirmed that the neutralization observed with wild-type bone marrow-derived macrophages was sensitive to MAb 2.4G2. As shown in Fig. 4, MAb 2.4G2 shifts the neutralization curve to the left and decreases the ED50 of NR-3839 by about a factor of 2, similar to that seen with J774A.1 cells. The individual roles of FcγRIIB and FcγRIII were then distinguished using bone marrow-derived macrophages from two different strains of KO mice, Fcer1g and Fcgr2b. The Fcer1g strain is referred to as the FcγRI/III/IV knockout (KO) strain. The Fcgr2b mouse is deficient in the FcγRIIβ protein of FcγRIIB (29) and is referred to as the FcγRIIB KO strain. Cells from both strains of mice exhibited a shift in ED50 upon addition of the FcγRIIB/III blocking antibody. The GM ratios of the ED50s obtained with NR-3839 with or without MAb 2.4G2 for the FcγRI/III/IV KO strain and the FcγRIIB KO strain were 1.7 (95% CI of 1.3 to 2.0) and 2.2 (95% CI of 1.8 to 2.7), respectively. Since the FcγRIIB KO strain does not express FcγRIIB but does express FcγRIII, the component of neutralization blocked by MAb 2.4G2 is due to FcγRIII when cells from this KO strain are used in the assay. Since the FcγRI/III/IV KO strain expresses only FcγRIIB, the Fcγ receptor-dependent component of neutralization seen with cells from this KO strain is due to FcγRIIB. Because, in each case, the lower limit of the 95% CI of the GM ratio of the ED50s is greater than 1.0, these results indicate that both FcγRIIB and FcγRIII can contribute to toxin neutralization. The results reported above were obtained using a polyclonal rabbit serum pool (NR-3839) to neutralize the action of the toxin. Similar results were obtained using either human or nonhuman primate polyclonal serum pools (data not shown).
FIG. 4.
Fcγ receptor dependence of anthrax toxin neutralization, determined using bone marrow-derived macrophages from wild-type (WT) and KO mice. Primary cultures of bone marrow-derived macrophages were prepared from WT, FcγRIIB KO, and FcγRI/III/IV KO strains of BALB/c mice. The macrophages were exposed to LT and various dilutions of polyclonal rabbit serum NR-3839, either in the absence (−) or in the presence (+) of the FcγRII/III blocking MAb 2.4G2. Each point corresponds to the mean of the values obtained for two independent sample preparations each run on a separate plate, with the standard error of the mean indicated by the error bar. Each figure is representative of three independent assays, each run on different days.
Dependence of Fc receptor-mediated neutralization on PA concentration.
Fcγ receptor-dependent neutralization is thought to occur through the interaction of Fcγ receptors, toxin-specific antibodies, and the toxin. For the case in which antibodies were induced by vaccination with an rPA, toxin-specific antibodies would target PA. Our results, described above, suggest that the number of Fcγ receptors available on the cell surface can influence the magnitude of Fcγ receptor-dependent neutralization that is observed. We next investigated whether the PA concentration used in the TNA might also affect the magnitude of the Fcγ receptor-dependent neutralization observed. We examined the toxin neutralizing capacity of rabbit polyclonal antiserum NR-3839 when the PA concentration used in the assay was either fixed at 50 ng/ml (used in the experiments described above) or increased to 400 ng/ml. As shown in Fig. 5A and B, the extent of Fcγ receptor-dependent neutralization observed in the J774A.1-based LT TNA exhibits a dependence on PA concentration. The GM ratio of ED50s for neutralization with or without FcγRIIB/III blocking MAb 2.4G2 increased from 2.6 (GM of three determinations) at a [PA] of 50 ng/ml to 5.3 (GM of three determinations) at a [PA] of 400 ng/ml, a statistically significant difference (P < 0.01; unpaired t test). The ratio of ED50s for neutralization with or without MAb 2.4G2 was not dependent on LF concentration (data not shown), as might be expected since the antibodies are specific for PA. In contrast to the [PA] dependence seen in the J774A.1-based LT TNA, the RAW 264.7-based LT TNA did not exhibit this striking dependence, as shown in Fig. 5C and D. For this assay, the GM ratios of ED50s for neutralization with or without MAb 2.4G2 were similar at [PA] of 50 ng/ml and 400 ng/ml, i.e., 1.3 (GM of three determinations) versus 1.4 (GM of three determinations), respectively.
FIG. 5.
Dependence of Fcγ receptor-mediated neutralization on the concentration of PA used in the TNA. The indicated dilutions of NR-3839 in the absence (−) or presence (+) of anti-FcγRII/III (MAb 2.4G2) were examined for their ability to neutralize LT in either the J774A.1 cell-based TNA (A and B) or the RAW 264.7 cell-based TNA (C and D), using a PA concentration of either 50 ng/ml (A and C) or 400 ng/ml (B and D). Each point corresponds to the mean of the values obtained for three independent sample preparations run on the same plate, with the standard error of the mean indicated by the error bar. For each independent assay, the samples were run on duplicate plates. Each figure is representative of three independent assays, each run on different days.
DISCUSSION
The anthrax TNA has been used, and is expected to continue to be used, to evaluate the immunogenicity of new PA-based anthrax vaccines in clinical studies. Therefore, a solid understanding of the nature and contributions of different types of toxin neutralization measured by the assay is essential in order to properly interpret assay output.
Our results indicate that Fcγ receptor-dependent neutralization manifests itself to differing extents depending on the cell line used in the assay. Two of the most common cell lines used in TNAs, J774A.1 cells and RAW 264.7 cells, are of myeloid origin and express Fcγ receptors on their surface. In the case of the J774A.1 cell-based TNA, Fcγ receptor-dependent toxin neutralization contributes in a significant and substantial way to the total neutralization observed in the assay. In contrast, in the RAW 264.7 cell-based TNA, Fcγ receptor-dependent neutralization contributes much less to the total neutralization measured in the assay. These results indicate that the two assays may provide fundamentally different information about toxin neutralization. The basis for the difference in the two cell-based assays may be simply the larger numbers of Fcγ receptors on J774A.1 cells than on RAW 264.7 cells. Such a result would imply that differences in cell growth conditions that lead to altered expression of Fcγ receptors on the cell surface also have the potential to affect assay output for either of these two cell-based assays.
We found that three murine Fcγ receptor classes, FcγRIIB, FcγRIII, and FcγRIV, can play roles in Fcγ receptor-dependent neutralization. Our data do not exclude the possibility that FcγRI might also contribute to Fcγ receptor-dependent neutralization. Murine Fcγ receptor types are known to bind to the four different murine IgG subclasses in a differential manner. FcγRI displays high affinity for the Fc region of murine IgG and has a restricted subclass specificity (2a ≫ 1, 2b, and 3), whereas FcγRIIB and FcγRIII have low to intermediate affinity for the antibody constant region but display a broader subclass specificity (1 = 2a = 2b ≫ 3) (8). FcγRIV is a relatively recently discovered Fcγ receptor type which displays a low to intermediate affinity for IgG and restricted subclass specificity (2a = 2b) (21). While the specificity of murine Fcγ receptor types for mouse IgG subclasses is well established, less is known about the specificity and affinity of mouse Fcγ receptor types for IgG molecules of other species. Just as specificities and affinities vary for the different mouse subclasses, specificities and affinities might vary for the different IgG molecules of the different species. This raises the cautionary note that the extent to which Fcγ receptor-dependent neutralization contributes to the total amount of neutralization observed in the TNA may depend on the characteristics of the specific serum sample examined, e.g., species of origin, antibody isotype composition, antibody subclass composition, or target epitopes. Factors that could affect serum antibody characteristics likely include the dosing schedule, the adjuvant, and the nature of the antigenic composition of the vaccine. Therefore, care must be taken when interpreting results of an assay in which Fcγ receptor-dependent neutralization plays a role. These results could have particular significance for interpreting the output of the J774A.1 cell-based assay when the assay is used to analyze the neutralizing action of a MAb, since the specific characteristics of the antibody may significantly affect assay output.
In this study, we also found that the extent to which Fcγ receptor-dependent toxin neutralization contributes to the total neutralization measured by the J774A.1 cell-based TNA is strikingly dependent on the concentration of PA used in the assay. FcγRII/III-dependent neutralization contributed to a greater extent when PA was employed at a concentration of 400 ng/ml than when it was employed at 50 ng/ml. For most of the experiments presented in this report, we chose to use PA at a concentration of 50 ng/ml since this concentration has been used in assays to evaluate clinical samples (9, 24, 26). Interestingly, we did not observe this PA concentration dependence for the RAW 264.7 cell-based assay. We do not know the reason why RAW 264.7 cells respond differently than J774A.1 cells in this respect. Additional studies are needed to shed light on this phenomenon.
In conclusion, our results suggest that elements of assay design, such as cell type used and PA concentration, can significantly influence the extent to which Fc receptor-mediated neutralization plays a role in the TNA. Because differences in assay parameters can significantly affect assay output, the use of a standardized form of the assay would facilitate the comparison of assay results between different laboratories. In addition, our findings indicate that a form of the TNA that displays a significant component of Fcγ receptor-dependent neutralization, such as the J774A.1-cell based assay, may provide different information about anthrax toxin neutralization than one which displays little or no component of Fcγ receptor-dependent neutralization, such as the RAW 264.7 cell-based assay. This possibility raises the question of which assay might be the best to use for the evaluation of new PA-based vaccines in the clinic and in pivotal animal studies. The answer to this question will depend on the results of studies which elucidate the role that Fc receptor-dependent neutralization plays in protection against B. anthracis infection in vivo.
Acknowledgments
This work was supported in part by CBER/FDA-NIAID/NIH interagency agreement YI-AI-6153-01.
The following reagents were obtained from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: anthrax LF, recombinant from B. anthracis, NR-142; anthrax PA, recombinant from B. anthracis, NR-140; J774A.1 monocyte/macrophage (mouse) Working Cell Bank, NR-28; and rabbit anti-PA reference serum pool, NR-3839. MAb 9E9 was the generous gift of Jeffrey Ravetch, Rockefeller University. We thank Tod Merkel, Vanessa Kelly, and Siobhán Cowley for expert help and advice.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Boyden, E. D., and W. F. Dietrich. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38:240-244. [DOI] [PubMed] [Google Scholar]
- 2.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 3.FDA. 2002. New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Fed. Regist. 67:37988-37998. [PubMed] [Google Scholar]
- 4.FDA. 2002. Anthrax vaccines: efficacy testing and surrogate markers of immunity workshop. http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/TranscriptsMinutes/UCM054606.pdf.
- 5.FDA. 2007. Anthrax vaccines: bridging correlates of protection in animals to immunogenicity in humans. http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/TranscriptsMinutes/UCM054424.pdf.
- 6.Friedlander, A. M. 1986. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 261:7123-7126. [PubMed] [Google Scholar]
- 7.Friedlander, A. M., R. Bhatnagar, S. H. Leppla, L. Johnson, and Y. Singh. 1993. Characterization of macrophage sensitivity and resistance to anthrax lethal toxin. Infect. Immun. 61:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gessner, J. E., H. Heiken, A. Tamm, and R. E. Schmidt. 1998. The IgG Fc receptor family. Ann. Hematol. 76:231-248. [DOI] [PubMed] [Google Scholar]
- 9.Gorse, G. J., W. Keitel, H. Keyserling, D. N. Taylor, M. Lock, K. Alves, J. Kenner, L. Deans, and M. Gurwith. 2006. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA 102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine 24:5950-5959. [DOI] [PubMed] [Google Scholar]
- 10.Hering, D., W. Thompson, J. Hewetson, S. Little, S. Norris, and J. Pace-Templeton. 2004. Validation of the anthrax lethal toxin neutralization assay. Biologicals 32:17-27. [DOI] [PubMed] [Google Scholar]
- 11.Hirano, M., R. S. Dvis, W. D. Fine, S. Nakamura, K. Shimizu, H. Yagi, K. Kato, R. P. Stephan, and M. D. Cooper. 2007. IgEb immune complexes activate macrophages through FcgammaRIV binding. Nat. Immunol. 8:762-771. [DOI] [PubMed] [Google Scholar]
- 12.Inglesby, T. V., D. A. Henderson, J. G. Bartlett, E. Eitzen, A. M. Friedlander, J. Hauer, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russel, and K. Tonat. 1999. Anthrax as a biological weapon. JAMA 281:1735-1745. [DOI] [PubMed] [Google Scholar]
- 13.Jakus, Z., R. Nemeth, J. S. Verbeek, and A. Mocsai. 2008. Critical but overlapping role of FcgammaRIII and FcgammaRIV in activation of murine neutrophils by immobilized immune complexes. J. Immunol. 180:618-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamoyi, E., and A. Nisonoff. 1983. Preparation of F(ab′)2 fragments from mouse IgG of various subclasses. J. Immunol. Methods 56:235-243. [DOI] [PubMed] [Google Scholar]
- 15.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations in eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leppla, S. H. 1999. The bifactorial Bacillus anthracis lethal and oedema toxins, p. 243-263. In J. H. Freer (ed.), Comprehensive sourcebook of bacterial protein toxins. Academic Press, London, United Kingdom.
- 17.Li, H., S. D. Soroka, T. H. Taylor, K. L. Stamey, K. W. Stinson, A. E. Freeman, D. R. Abramson, R. Desai, L. X. Cronin, J. W. Oxford, J. Caba, C. Pleatman, S. Pathak, D. S. Schmidt, V. A. Semenova, S. K. Martin, P. P. Wilkins, and C. P. Quinn. 2008. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J. Immunol. Methods 333:89-106. [DOI] [PubMed] [Google Scholar]
- 18.Mancardi, D. A., B. Iannascoli, S. Hoos, P. England, M. Daeron, and P. Bruhns. 2008. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J. Clin. Investig. 118:3738-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed, N., J. Li, C. S. Ferreira, S. F. Little, A. M. Friedlander, G. L. Spitalny, and L. S. Casey. 2004. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 72:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimmerjahn, F., P. Bruhns, K. Horiguchi, and J. V. Ravetch. 2005. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23:41-51. [DOI] [PubMed] [Google Scholar]
- 22.Nimmerjahn, F., and J. V. Ravetch. 2008. Analyzing antibody-Fc-receptor interactions. Methods Mol. Biol. 415:151-162. [DOI] [PubMed] [Google Scholar]
- 23.Nimmerjahn, F., and J. V. Ravetch. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34-47. [DOI] [PubMed] [Google Scholar]
- 24.Omland, K. S., A. Brys, D. Lansky, K. Clement, F. Lynn, and the Participating Laboratories. 2008. Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species. Clin. Vaccine Immunol. 15:946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, S., and S. H. Leppla. 2000. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr. Purif. 18:293-302. [DOI] [PubMed] [Google Scholar]
- 26.Quinn, C. P., P. M. Dull, V. Semenova, H. Li, S. Crotty, T. H. Taylor, E. Steward-Clark, K. L. Stamey, D. S. Schmidt, K. W. Stinson, A. E. Freeman, C. M. Elie, S. K. Martin, C. Greene, R. D. Aubert, J. Glidewell, B. A. Perkins, R. Ahmed, and D. S. Stephens. 2004. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J. Infect. Dis. 190:1228-1236. [DOI] [PubMed] [Google Scholar]
- 27.Ravetch, J. V., and J.-P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9:457-492. [DOI] [PubMed] [Google Scholar]
- 28.Ravetch, J. V., A. D. Luster, R. Weinshank, J. Kochan, A. Pavlovec, D. A. Portnoy, J. Hulmes, Y. E. Pan, and J. C. Unkeless. 1986. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science 234:718-725. [DOI] [PubMed] [Google Scholar]
- 29.Takai, T., M. Ono, M. Hikida, H. Ohmori, and J. V. Ravetch. 1996. Augmented humoral and anaphylactic responses in FcgammaRII-deficient mice. Nature 379:346-349. [DOI] [PubMed] [Google Scholar]
- 30.Unkeless, J. C. 1979. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 150:580-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitale, L., D. Blanset, I. Lowy, T. O'Neill, J. Goldstein, S. Little, G. P. Andrews, G. Dorough, R. K. Taylor, and T. Keler. 2006. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect. Immun. 74:5840-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]