Abstract
Chlamydia trachomatis infection can lead to pelvic inflammatory disease, ectopic pregnancy (EP), infertility, and chronic pelvic pain in women. Activins and inducible nitric oxide synthase (iNOS) are produced by the human fallopian tube, and we speculate that tubal activins and iNOS may be involved in the immune response to C. trachomatis in humans and their pathological alteration may result in tubal pathology and the development of EP. Blood and fallopian tubes were collected from 14 women with EP. Sera were analyzed by enzyme-linked immunosorbent assay to detect antibodies against chlamydial heat shock protein 60 (chsp60) and the major outer membrane protein of C. trachomatis. Confirmation of C. trachomatis serology was made using the microimmunofluorescence test. The patients were classified into three groups according to their serological results, and immunohistochemistry and quantitative reverse transcription-PCR were performed to investigate the expression of candidate molecules by tubal epithelial cells among the three groups. This is the first study to show an increase in the expression of activin βA subunit, type II receptors, follistatin, and iNOS within the human fallopian tube of EP patients who were serologically positive for C. trachomatis. A similar expression profile was observed in the fallopian tubes with detectable antibodies only against chsp60. These results were shown at the mRNA and protein levels. We suggest that tubal activin A, its type II receptors, follistatin, and NO could be involved in the microbial-mediated immune response within the fallopian tube, and their pathological expression may lead to tubal damage and the development of EP.
Chlamydia trachomatis infection is the most common sexually transmitted bacterial infection throughout the world (75), with the highest incidence in teens and young adults (61). The majority of C. trachomatis infections are asymptomatic, and therefore a significant proportion of those infected remain undiagnosed and can develop complications. Untreated cases of chlamydial infection can result in chronic pelvic pain in women, pelvic inflammatory disease (PID), ectopic pregnancy (EP), and infertility, and C. trachomatis can be transmitted during labor and cause conjunctivitis and pneumonitis in the newborn.
EP is the major cause of maternal morbidity and mortality in early pregnancy, and it has been serologically associated with C. trachomatis in several studies (5, 8, 19). Serum chlamydia immunoglobulin G (IgG) antibody testing (CAT) against chlamydial major outer membrane protein (MOMP) is used to screen for tubal pathology in the routine fertility workup (16). Serum IgG antibodies remain detectable for many years after initial infection (16, 54), even following antibiotic treatment (21, 54). Therefore, CAT is considered a useful tool to reflect previous C. trachomatis infection which may have occurred years earlier. However, CAT does not reveal the course of the infection and the extent of tubal damage and therefore is not useful in discriminating between clearance and persistence of a C. trachomatis infection (16, 34).
Heat shock proteins are highly conserved proteins present in almost all prokaryotic and eukaryotic organisms. They are members of a family of stress response proteins which protect the cells from a variety of insults (36). The chlamydia heat shock protein 60 (chsp60) is a chlamydia genus-specific protein, serving as a strong antigenic target for the immune system (32). Anti-chsp60 antibodies are significantly more common in women with tubal pathology than in those without tubal pathology (11). Sziller et al. have reported that anti-chsp60 antibodies were significantly higher in tubal pregnancy (68). Furthermore, antibodies to a single epitope that corresponds to amino acids 260 to 271 of chsp60 were absolutely specific for ectopic pregnancies (68). Antibodies against this epitope of chlamydial hsp60 have also been shown to react with the corresponding regions in human hsp60 (76). Endogenous hsp60 production is induced by proinflammatory mediators, and therefore any inflammatory stimulus would induce self-hsp60 expression and reactivate an hsp60-sensitized lymphocyte response, which may progress to autoimmune pathological events with adverse clinical consequences (68).
Wang and Grayston (73a) were the first to develop the microimmunofluorescence (MIF) test which is based on purified chlamydial elementary bodies spotted on a glass slide. The MIF test was developed for typing the C. trachomatis strains in serotypes and for seroepidemiological studies. Until now, the MIF test has been considered the “gold standard” for the serological diagnosis of chlamydial infections (18).
Nucleic acid amplification tests are considered the most sensitive method for screening and diagnosis of persistent chlamydial infections. Detection rates of chlamydial DNA have varied considerably in different studies of fallopian tubes from patients with EP (range, 0.03 to 70%; mean, 26%) (4, 15, 33, 47, 70). Chlamydial mRNA was also detected by reverse transcription-PCR (RT-PCR) in one study that included 10 patients, and the authors suggested that their results indicated the presence of metabolically active C. trachomatis organisms (15). However, several other studies that included a total of 189 patients were unable to detect chlamydia using both cell culture and PCR in tubal tissue from ectopic pregnancies, although serological markers indicated prior chlamydial infections in many of these cases (5, 8, 19, 37, 50).
The tubal damage associated with C. trachomatis infection is mainly due to an inadequate immune response and inflammation of infected mucosal tissues. Chronic inflammation of infected tissues is thought to be mediated by immune modulators that are constantly attempting but unable to eliminate the chlamydial organisms from the host (52).
Activins are members of the transforming growth factor β family, and they are secreted by the tubal epithelial cells (60). Activins are homodimers of inhibin β subunits (βA and βB), and the different dimerization of subunits gives rise to three glycoproteins: activin A (βA-βA), activin B (βB-βB), and activin AB (βA-βB). Activins are tightly regulated by their high-affinity binding protein, follistatin, and they act through serine/threonine kinase transmembrane receptors (60). Activins have been identified as novel players in inflammation and repair and are most likely involved in the pathogenesis of inflammatory and fibrotic human diseases (74). Activin A expression has been reported to increase in several inflammatory diseases, such as septicemia (41), inflammatory bowel disease (23), rheumatoid arthritis (17), and asthma (31). Similar to transforming growth factor β (35), activin A can have both pro- and anti-inflammatory actions, depending on both cellular and temporal contexts (53).
Inducible nitric oxide synthase (iNOS) is regulated by cytokines, and it generates nitric oxide and other toxic nitrogen radicals that are lethal to a variety of intracellular pathogens (9, 27). In vivo studies have shown that iNOS is not essential for the clearance of microbiologically detected infection in mice, but it may provide protection against chronic C. trachomatis infection and the associated complications (56). It has been reported that the courses of genital chlamydial infection in iNOS−/− and iNOS+/+ mice were identical, and both models resolved C. trachomatis infection in a similar fashion (25, 56). However, iNOS−/− mice sustained greater pathological outcome subsequent to infection than iNOS+/+ controls (56). A strong positive correlation between elevated iNOS activity and protection from chronic disease sequelae (e.g., hydrosalpinx formation and infertility) (56, 58) and prevention of chlamydial systemic spread has also been reported (25).
We have previously reported an increase in the expression of activin A and its related molecules (61) and iNOS (2) by human tubal epithelial cells collected from EP patients. Therefore, we speculate that tubal activins and iNOS may be involved in the immune response to chlamydia-induced tubal chronic inflammation and that pathological alteration in the expression of these proteins by fallopian tube epithelial cells may result in tubal pathology and the subsequent development of ectopic pregnancy.
MATERIALS AND METHODS
Patients and tissue collection.
The study was approved by South Sheffield Ethics Committee, and informed written consent was obtained prior to the collection of tissue samples. All specimens were collected at the Jessop Wing, Royal Hallamshire Hospital in Sheffield, United Kingdom.
All participants had a period of amenorrhoea (6 to 8 weeks) and suffered from abdominal pain and/or vaginal bleeding. A positive pregnancy test increased the suspicion of EP, and the patients were referred to the Early Pregnancy Assessment Unit, Royal Hallamshire Hospital, Sheffield, United Kingdom. The diagnosis of EP was based on serial measurement of serum concentrations of beta human chorionic gonadotropin and transvaginal ultrasound. All participants had no sign of previous infection in their clinical notes. The participants were selected to join the study according to the following criteria: (i) women diagnosed with EP following spontaneous conception and for whom salpingectomy is planned on clinical management grounds, (ii) clinical determination that the patient is hemodynamically stable, and (iii) clinical decision to undertake unilateral salpingectomy.
(i) Fallopian tube samples.
Fresh fallopian tube samples were obtained at the time of salpingectomy from 14 women diagnosed with EP (median age, 29 years; range, 24 to 36 years; mean gestational age calculated from the last menstrual period, 6.5 weeks ± 1.5 week). All participants in this group conceived spontaneously and were not taking exogenous progesterone.
(ii) Venous blood samples.
Blood samples were collected from all participants to investigate the serological markers of C. trachomatis. All blood samples were delivered as nonheparinized whole blood and had been centrifuged briefly to sediment out the red blood cells and to separate the serum, which was then stored in −20°C freezers until processed.
Sampling and processing.
The fallopian tubes were excised at least 1 cm away from the implantation site to avoid collecting any embryonic or trophoblastic tissue and to ensure the integrity of tubal morphology and function. Each sample was then divided into three equal pieces using DNase- and RNase-free equipment (baked at 200°C for 4 h), with one piece being immediately fixed in 10% buffered formalin for immunohistochemistry and the other parts snap-frozen and stored at −80°C for the nucleic acid amplification tests.
Genomic DNA extraction.
Genomic DNA was extracted from the fallopian tube samples using the QIAamp DNA minikit (Qiagen, Crawley, United Kingdom) according to the manufacturer's instructions. The quality and quantity of extracted DNA were assessed with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) and typically had an A260/A280 ratio of 1.7 to 1.9. Extracted DNA was stored at −80°C until used.
Single PCR and N-PCR procedures. (i) Human genomic DNA.
Detection of human genomic DNA was performed by PCR using primers PCO3 (62) and PCO6 (33) (Table 1) specific for the human β-globin gene. The PCR was performed on 10 μl of the extracted DNA sample in a final reaction mixture of 25 μl. The final reaction mixture contained 2.5 mM MgCl2, 1× KCl PCR buffer, 200 μM deoxynucleoside triphosphates (dTNPs), 50 pmol of each primer, and 1 U Taq DNA polymerase. The PCR consisted of 40 cycles, comprising denaturation at 94°C for 1 min, primer annealing at 55°C for 40 s, and primer extension at 72°C for 40 s. Before the PCR assay, the tubes were incubated at 94°C for 4 min, and after PCR, they were incubated at 72°C for 8 min. The expected PCR product of 326 bp was visualized on a 0.8% agarose gel after ethidium bromide staining. This PCR was used to show the presence of human DNA, the success of DNA extraction, and the absence of PCR inhibitors.
TABLE 1.
Sequences of PCR primers used for the detection of β-globin, C. trachomatis, activin βA and βB subunits, follistatin, ActRIIA, ActRIIB, iNOS, and β-actin
| Primer | Sequence
|
|
|---|---|---|
| Forward | Reverse | |
| β-Globin | PCO3: 5′-ACACAACTGTGTTCACTAGC-3′ | PCO6: 5′-CATCAGGAGTGGACAGATCC-3′ |
| C. trachomatis 1 | T1: 5′-GGACAAATCGTATCTCGG-3′ | T2: 5′-GAAACCAACTCTACGCTG-3′ |
| C. trachomatis 2 | T3: 5′-ATTAACCCTCACTAAAGGGA-3′ | T4: 5′-GCCATGTCTATAGCTAAAGC-3′ |
| βA subunit | 5′-CCACTCAACAGTCATCAACCACTA-3′ | 5′-TGGGCACACAGCACGATT-3′ |
| βB subunit | 5′-AGCCTCCAGGATACCAGCAA-3′ | 5′-TCTCCGACTGACAGGCATTTG-3′ |
| Follistatin | 5′-GCCACCTGAGAAAGGCTACCT-3′ | 5′-ATCTTCACAGGACTTTGCTTTGATAC-3′ |
| ActRIIA | 5′-AGGAGGAAATTGGCCAGCAT-3′ | 5′-CAATGGTTTCACAGAGCATTGC-3′ |
| ActRIIB | 5′-TTCAGTTCCACACCACAAATCC-3′ | 5′-ACACTACCATTAAGATCATGTCCAGTTC-3′ |
| INOS | 5′-CCTCAAGTCTTATTTCCTAACGTT-3′ | 5′-CCGATCAATCCAGGGTGCTA-3′ |
| β-Actin | 5′-ATCCCCCAAAGTTCACAATG-3′ | 5′-GTGGCTTTTAGGATGGCAAG-3′ |
(ii) N-PCR for the detection of C. trachomatis DNA.
The nested PCR (N-PCR) consisted of two rounds of amplifications using two sets of primers, and it was carried out as previously described (22). The expected PCR product of 320 bp was examined on a 0.8% agarose gel after ethidium bromide staining and was confirmed by sequencing and comparison of the amplicon to GenBank accession no. 144462. C. trachomatis serovar LGV1 DNA was used as a positive control, and distilled deionized water in place of DNA template was used as a PCR-negative control to demonstrate whether there was any contamination with C. trachomatis DNA.
To verify the absence of inhibitors, 9 μl of each eluted tubal DNA sample was spiked with 1 μl of diluted positive control at a concentration of 1:105, resulting in a final concentration of 1:106 for spiked samples, which were then processed for N-PCR.
Serological markers for C. trachomatis. (i) IgG against C. trachomatis MOMP.
An enzyme immunoassay kit (AniLabsystems, Finland) was used for the detection of IgG antibodies against C. trachomatis MOMP. The test is considered species specific as it is based on synthetic peptides derived from the C. trachomatis-specific variable domain of MOMP. This allows the screening and diagnosis of C. trachomatis infections without interference of C. pneumoniae antibodies (45).
All samples were processed in duplicate according to the manufacturer's instructions. Briefly, sera were diluted 1:9 in sample diluent provided with the kit and were subsequently tested in microplates coated with chlamydia-specific recombinant MOMP fragments. The plates were incubated for 30 min at 37°C in a humid chamber. The plates were washed five times with wash solution (400 μl/well) and tapped dry. To each well, 200 μl of conjugate (horseradish peroxidase-conjugated goat anti-human IgG) was added, and the plates were incubated for 30 min at 37°C. Again the plates were washed five times with wash solution (400 μl/well) and tapped dry. To each well, 200 μl of tetramethylbenzidine substrate was added, and the plates were incubated for 15 min at room temperature in a dark place. Finally, 100 μl of 0.45 mol/liter sulfuric acid was added to stop the color reaction. The optical density of the plates was measured in a spectrophotometer at 450 nm. Threshold indexes were calculated according to the manufacturer's instructions, and the cutoff value for a positive test was 1.4.
(ii) MIF test.
IgG antibodies to C. trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci were detected using the Chlamydia IgG SeroFIA kit (Savyon Diagnostics, Ltd., Ashdod, Israel). The kit is a macroimmunofluorescence assay based on the principles of the MIF test. For this purpose, all samples were diluted by adding 10 μl of the serum in 630 μl of serum diluent provided in the kit. This was followed by the incubation of 10 μl from each diluted sample on the microscope slides, dotted with three chlamydial antigens, for 30 min at 37°C in a moist chamber. The slides were washed four times with wash buffer and twice with distilled water and incubated with conjugated fluorescein-labeled rabbit anti-human IgG for 30 min at 37°C. Again the slides were washed four times with wash buffer and twice with distilled water. Mounting fluid was added to the slides, and a coverslip was placed on the slides. Positive reactions, inclusions presenting a brilliant apple green fluorescence, were identified with the aid of a fluorescence microscope (Olympus Vanox with a B2 filter) at a ×400 magnification. All slides were evaluated independently by two readers. In case of disagreement, the judgment of a third reader was decisive.
(iii) IgG against chsp60.
An enzyme-linked immunosorbent assay kit (Medac, Germany) was used for the detection of IgG antibodies against C. trachomatis hsp60. The test is considered species specific as it uses a recombinant hsp60 from C. trachomatis. All samples were processed in duplicate according to the manufacturer's instructions. Briefly, 10 μl of each sample was diluted in 490 μl sample diluent provided with the kit, and subsequently 50 μl was tested in microplates coated with recombinant hsp60 from C. trachomatis. The plates were incubated for 60 min at 37°C in a humid chamber. The plates were washed three times with wash solution (200 μl/well) and tapped dry. To each well, 50 μl of conjugate (horseradish peroxidase-conjugated goat anti-human IgG) was added, and the plates were incubated for 60 min at 37°C. Again the plates were washed three times with wash solution (200 μl/well) and tapped dry. To each well, 50 μl of tetramethylbenzidine substrate was added, and the plates were incubated for 30 min at room temperature in a dark place. Finally, 100 μl of 0.45 mol/liter sulfuric acid was added to stop the color reaction. The optical density at 450 nm of the plates was measured in a spectrophotometer. Threshold indexes were calculated according to the manufacturer's instructions, and the cutoff value for a positive test was 1.1.
Immunohistochemistry.
The immunohistochemistry protocol was carried out as previously described (60). The sections were observed on a Labor Lux microscope (Leitz, Wetzlar, Germany) at magnifications of ×100, ×250 and ×400. A positive reaction was characterized by the presence of brown staining. Each section was examined by two observers who were blinded to the source of tissue, and the intensity of staining was measured according to the following semiquantitative scale: 0, negative; 1, equivocally positive; 2 to 4, weakly positive; 5 to 7, positive; and 8 to10, strongly positive. Representative sections were photographed using an Olympus digital camera at ×250 and ×400 magnifications.
Real-time PCR. (i) Laser capture microscopy.
One of the snap-frozen pieces was collected randomly, and the epithelial cells were microdissected using a laser capture microdissector (Arcturus, Hertfordshire, United Kingdom) to study the levels of mRNA expression as previously described (60).
(ii) cDNA synthesis.
RNA extraction and cDNA synthesis were carried out as using the RNeasy FFPE kit (Qiagen, Ltd., Crawley, United Kingdom) and the RETROscript kit (Applied Biosystems, Warrington, United Kingdom) as previously described (60).
(iii) Quantitative PCR.
PCR was performed with cDNAs, Power SYBR green master mix (Applied Biosystems, Warrington, United Kingdom), and primers (Metabion, Germany) (Table 1) as previously described (60). Universal human RNA (Stratagene, Amsterdam, The Netherlands) was used as a positive control, and two negative controls were included, one with a minus-reverse transcription (minus-RT) control. All experiments were performed in triplicate.
Results were analyzed using Mx3000P (Stratagene, Hemel Hempstead, United Kingdom). Relative levels of expression of mRNA quantities of the different molecules were compared between the MIF-positive, chsp60-only-positive, and absolute C. trachomatis-negative fallopian tube epithelial cells collected from EP. The threshold cycle (2−ΔΔCt) values were normalized against threshold value of human β-actin. The results are expressed as means ± standard deviations. Statistical analysis was performed using one-way analysis of variance, and P < 0.05 was considered significant.
RESULTS
N-PCR for the detection of C. trachomatis DNA.
DNA samples from all fallopian tubes were positive for the human β-globin gene, and the expected PCR product of 326 bp was visualized on 0.8% agarose gel.
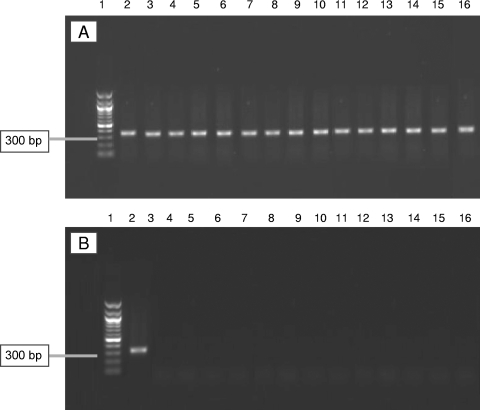
All tissue samples from the 14 patients with EPs were negative for C. trachomatis DNA using N-PCR (Fig. 1A). Spiked tubal DNA samples containing a known dilution of DNA from C. trachomatis were used as internal inhibition controls for each sample to detect inhibition of the amplification reaction. All spiked samples were positive, suggesting that there was no inhibition of the amplification reaction and that chlamydial DNA could be detected (Fig. 1B).
FIG. 1.
PCR results from C. trachomatis plasmid in tubal samples and their corresponding spiked DNA samples. (A) A 0.8% agarose gel showing the 1-kb ladder (lane 1) and the 320-bp N-PCR product of the C. trachomatis plasmid in all spiked tubal DNA samples (lanes 2 to 16) from EP patients; (B) a 0.8% agarose gel showing the 1-kb ladder (lane 1), the 320-bp N-PCR product of the C. trachomatis plasmid-positive control (lane 2), and negative samples from the EP group (lanes 3 to 16).
Serological markers of C. trachomatis.
Five patients were positive for C. trachomatis MOMP IgG and were also positive for IgG antibodies against C. trachomatis by MIF. Eight patients had IgG antibodies for chsp60, and five were negative to all markers. IgG antibodies against C. psittaci were not detected in any sample (Table 2).
TABLE 2.
Summary of individual patient results for chlamydial DNA and serological markers
| Assay | Result for patient:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| MOMP IgG | + | − | + | − | − | − | − | + | − | − | − | + | − | + |
| MIF | +a | − | +a | − | − | − | − | +b | − | − | − | +a | − | +a |
| HSP-60 | − | + | + | + | − | − | − | + | + | − | − | + | + | + |
| N-PCR | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
Antibodies to C. trachomatis were detected.
Antibodies to C. trachomatis and C. pneumoniae were detected.
When individual patients were considered, four patients were positive for all serological markers and one patient was positive by MOMP and MIF only. However, one patient (patient 8) showed cross-reactivity between C. trachomatis and C. pneumoniae IgG by MIF, although the maximum recommended titration (1:512) was used (Table 2). Four patients were only found positive for chsp60, and five were found negative for all serological markers of C. trachomatis (Table 2).
Immunohistochemistry.
The patients were classified into three groups according to their C. trachomatis serological results. The first group (CT+) included five patients who had detectable IgG antibodies against chlamydial MOMP and/or chsp60 and who were found positive by MIF. The second group (CHSP60+) included four patients who only had detectable IgG antibodies against chsp60. The third group (CT−) included five patients with EP and who were found negative by the three serological markers. Immunohistochemistry was performed to investigate the expression of activins, their type II receptors, follistatin, and iNOS among the three groups, with the CT− group acting as a control.
A positive antigen-antibody reaction was characterized by a brown staining, and all target molecules were found to be expressed in the cytoplasm of the tubal epithelial cells (Fig. 2 to 4). The intensity and the distribution of the immunostain of all molecules were higher in the CT+ and CHSP60+ patients than in the CT− group, except for the levels of activin βB subunit, which were similar among the three groups (Table 3).
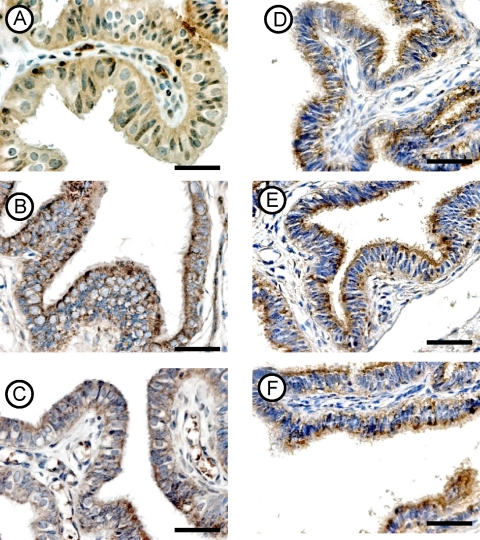
FIG. 2.
Immunohistochemical localization of the activin βA (A, B, and C) and βB (D, E, and F) subunits in fallopian tubes from the CT− (top row), CT+ (middle row), and CHSP60+ (bottom row) groups. Magnifications for all panels, ×400. Scale bar, 10 μm.
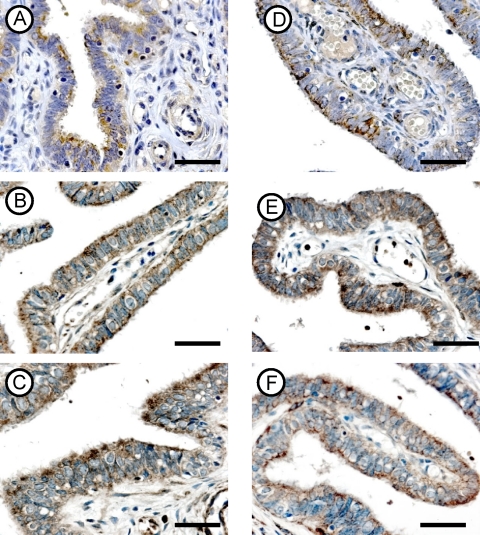
FIG. 4.
Immunohistochemical localization of follistatin (A, B, and C) and iNOS (D, E, and F) in fallopian tubes from the CT− (top row), CT+ (middle row), and CHSP60+ (bottom row) groups. Magnifications for all panels, ×400. Scale bar, 10 μm.
TABLE 3.
Immunohistochemical staining of activin βA and βB subunits, receptor types IIA and IIB, follistatin, and iNOS in the CT-, CT+, and CHSP60+ groups
| Protein | Mean ± SD staining for groupa:
|
||
|---|---|---|---|
| CT− | CT+ | CHSP60+ | |
| Activin βA | 6.35 ± 1.18 | 9.25 ± 0.91* | 9.25 ± 0.68* |
| Activin βB | 5.65 ± 0.98 | 6.05 ± 0.88 | 6.68 ± 1.07 |
| Receptor type IIA | 4.5 ± 0.94 | 7.55 ± 1.14* | 8.43 ± 1.31* |
| Receptor type IIB | 6.35 ± 1.13 | 9.1 ± 0.78* | 9.3 ± 0.8* |
| Follistatin | 4.9 ± 1 | 8.45 ± 1.1* | 9.12 ± 0.95* |
| iNOS | 6.6 ± 1.31 | 8.95 ± 0.75* | 9.43 ± 0.62* |
The values shown represent the mean of the immunohistochemical staining ± standard deviation on a scale from 0 to 10. *, P < 0.05 compared to CT−.
(i) Expression of the activin βA subunit.
The antibody clearly labeled the cytoplasm of the tubal epithelial layer in the three groups and showed similar distributions. However, the intensity of the immunoexpression was stronger in the CT+ (Fig. 2B) and CHSP60+ (Fig. 2C) groups than in the tubal samples collected from the CT− group (Fig. 2A). No difference was noted between the CT+ and the CHSP60+ groups.
(ii) Expression of the activin βB subunit.
The βB subunit was localized in the epithelial cells of all tubal samples and did not show any difference in either the distribution or the intensity of expression between the three studied groups (Fig. 2D to F).
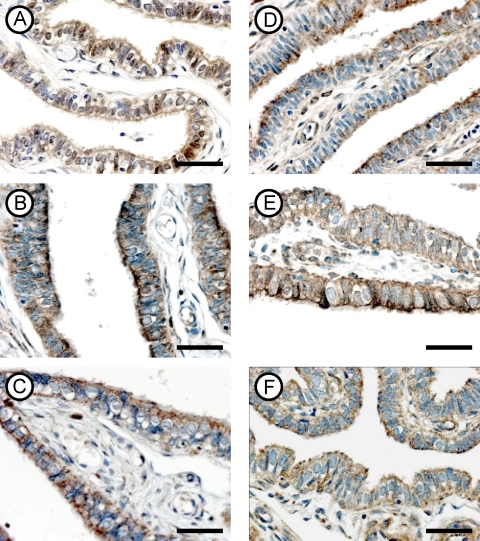
(iii) Expression of activin receptor type IIA.
The antibody against activin type IIA receptor clearly labeled the cytoplasm of all the epithelial cells in the three groups, and there was no difference in the distributions of the immunostain (Fig. 3A to C). However, the intensity of staining was stronger in the CT+ (Fig. 3B) and CHSP60+ (Fig. 3C) groups than in the tubes collected from the CT− group (Fig. 3A). The intensities of expression were similar between the CT+ and CHSP60+ groups.
FIG. 3.
Immunohistochemical localization of activin type IIA (A, B, and C) and IIB (D, E, and F) receptors in fallopian tubes from the CT− (top row), CT+ (middle row), and CHSP60+ (bottom row) groups. Magnifications for all panels, ×400. Scale bar, 10 μm.
(iv) Expression of activin receptor type IIB.
The immunolocalization of activin type IIB receptor revealed that the protein was expressed in the cytoplasm of the epithelial layer. The distribution of the immunostain in the CT− group was patchy and did not cover all cells from the epithelial layer (Fig. 3D). Additionally, the intensity of immunostaining was stronger in the CT+ (Fig. 3E) and CHSP60+ (Fig. 3F) groups than in the CT− group (Fig. 3D). No difference was found between the CT+ and CHSP60+ groups.
(v) Expression of follistatin.
All tubal epithelial cells from the three groups were stained, and the antibody clearly labeled the cytoplasm of the tubal epithelium (Fig. 4A to C). The intensity of the immunostaining to the follistatin antibody was stronger in the CT+ (Fig. 4B) and CHSP60+ (Fig. 4C) groups than in the CT− group (Fig. 4A). No difference was found between the CT+ and CHSP60+ groups.
(vi) Expression of iNOS.
Positive staining was observed in all tissue samples for the iNOS antibody. The antibody clearly labeled the cytoplasm of the tubal epithelial cells in all groups. The immunostain of iNOS in the CT− groups was mainly localized in the apical region of the cytoplasm (Fig. 4D). In the CT+ and CHSP60+ groups, the immunostain was localized in the cytoplasm of all epithelial cells (Fig. 4E and F). The intensity of expression was higher in the CT+ (Fig. 4E) and CHSP60+ (Fig. 4F) groups than in the CT− group (Fig. 4D). No difference was found between the CT+ and CHSP60+ groups.
Quantitative RT-PCR.
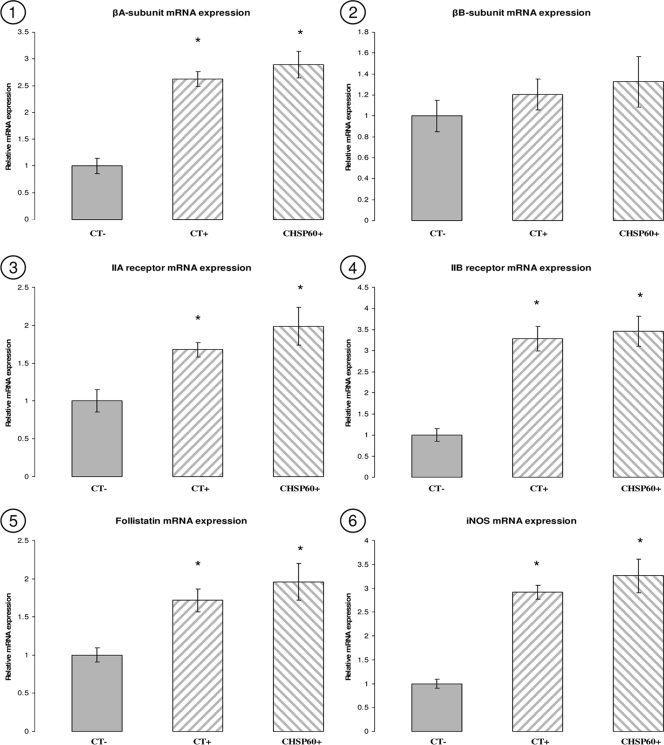
The relative levels of mRNA expression of activin βA and βB subunits, type IIA and IIB receptors, follistatin, and iNOS were determined by SYBR green quantitative RT-PCR analysis in all the groups. Results from β-actin failed to show any statistically significant variation between the different samples (P > 0.05), which indicates that the loading of RNA from the different samples was similar. Using the 2−ΔΔCt method, mRNA expression results were normalized against β-actin and expressed as change (fold) compared to the CT− group. Statistical analysis was performed using one-way analysis of variance. Our results demonstrated that the expression of activin βA subunit, activin type IIA and type IIB receptors, follistatin, and iNOS genes was significantly higher in the CT+ and CHSP60+ patients (P < 0.05) than in the CT− group (Fig. 5). However, there was no significant statistical variation in the expression of these genes between the CT+ and CHSP60+ groups (P > 0.05). Activin βA subunit, type IIA receptor, and follistatin increased by twofold in the CT+ and CHSP60+ groups in comparison to the CT− group. Type IIB receptor and iNOS gene increased by threefold in the same groups compared to the CT− group. The activin βB subunit failed to show any significant changes between the three groups (P > 0.05). The gene expression of all molecules was found to correlate with the results obtained from immunohistochemistry.
FIG. 5.
Quantitative RT-PCR analysis of activin βA (panel 1) and βB (panel 2) subunits, follistatin (panel 3), type IIA (panel 4) and IIB (panel 5) receptors, and iNOS (panel 6) in the fallopian tubes collected from the CT−, CT+, and CHSP60+ groups. *, P < 0.05 compared to CT−.
DISCUSSION
In this study, we examined the prevalence of C. trachomatis infection in samples collected from EP patients using N-PCR and serological markers. We also studied whether the observed increase in the expression of activins and iNOS by tubal epithelial cells collected from EP patients was associated with C. trachomatis infection.
This is the first study to show an increase in the expression of the activin βA subunit, type II receptors, follistatin, and iNOS within the human fallopian tube bearing an EP in patients who were serologically positive for C. trachomatis. Furthermore, a similar expression profile was observed in the fallopian tubes of patients with detectable antibodies only against chsp60. These results were shown at the mRNA and protein levels, as confirmed by quantitative RT-PCR and immunohistochemistry.
A limitation of our study is that it presents data from a small sample size (14 patients), and increasing the number of participants would increase the statistical power of the study. However, previous published reports investigating the role of C. trachomatis infection in the pathogenesis of EP generated their results from a similar sample size, emphasizing the difficulties of patient recruitment (8, 61). Nevertheless, we were able to detect a significant difference between the different groups (P < 0.05) at the mRNA and protein levels, suggesting that these candidate molecules have a role in microbial-mediated immune response within the human fallopian tube. A further limitation of the study is that immunohistochemistry is a semiquantitative and subjective technique, and it lacks standardization. Thus, discrepancies in the result may arise from inter- and/or intraobservers’ variations in the interpretation of the immunostains (73). However, immunohistochemistry is widely used in routine diagnostic work and for scientific research in pathology and cytology (51, 63, 73).
Nucleic acid amplification tests, which include N-PCR, are more typically used for screening and diagnosing chlamydial infections. The plasmid target DNA is present only in C. trachomatis and at 7 to 10 copies per genome; therefore, targeting it with PCR increases the sensitivity and specificity of the test (4, 5, 18). We targeted plasmid DNA using N-PCR, but chlamydial DNA was not detected in any of our 14 patients with ectopic pregnancy. All samples were stored at −70°C and heated at ≥95°C to remove inhibitors (72), which could lead to false-negative results (14). PCR of human β-globin and spiked tubal DNA samples with a known dilution of DNA from C. trachomatis showed all specimens to be positive, suggesting the absence of inhibitors and detection of the target organism.
Similar findings were reported by five previous studies that were unable to detect C. trachomatis DNA in 189 tubal samples collected from EP patients with positive serological markers of C. trachomatis (5, 8, 19, 37, 50). In contrast, C. trachomatis DNA was found in 0.03 to 70% of the cases in another five studies (4, 15, 33, 47, 70). One explanation for the nondetection of chlamydial DNA in our case group could be the small sample size. However, the two studies with the highest positivity rates (67% and 70%) (4, 15) used fresh material, and the numbers of patients were low (n = 24 and n = 10), similar to our study. It has been suggested that this difference in results could be technical in nature, such as loss of chlamydial DNA during sampling or extraction, or it may represent genuine differences between patients (5). Another explanation could be that patients whose fallopian tubes were negative for C. trachomatis had a cervical or endometrial chlamydial infection, sites that were not investigated in the present study (4).
EP was initially associated with C. trachomatis through serological markers (8, 19). Our results showed that four patients had detectable IgG antibodies against all the three serological markers. Four patients had only detectable IgG antibodies against chsp60. The MIF test has been considered the “gold standard” for the serological diagnosis of chlamydial infections, and the discrimination between the different species of chlamydia (18). The specificity of the MIF test can be estimated by the ability to discriminate between C. trachomatis and C. pneumoniae reactivity (5, 18). Our results showed that five patients had IgG antibodies against C. trachomatis by MIF. IgG antibodies against C. pneumoniae were detected in one patient from the ectopic group (patient 8) by MIF, and they showed cross-reactivity with C. trachomatis, even though the maximum recommended titration (1:512) was used, suggesting possible multiple infections.
C. trachomatis hsp60 is a strong antigenic target, and it may mediate immune responses that lead to tubal pathology (68). It has been suggested that antibodies against chsp60 reflect chronic chlamydial infection (5, 68). Our results showed that antibodies against chsp60 were detected in eight patients with EP. Anti-chsp60 antibodies are significantly more common in women with tubal pathology than in those without tubal pathology (11). Sziller et al. have also reported that anti-chsp60 antibodies were significantly increased in tubal pregnancy and that antibodies against amino acids 260 to 271 of the chsp60 were absolutely specific for the women with EPs (68). It has also been suggested that chlamydial hsp60 may trigger an autoimmune response to host heat shock proteins because they could cross-react with epitopes on the human homologue (68).
In our study, four samples were found to be positive for chsp60 only and did not have detectable antibodies against C. trachomatis by the two other markers. Heat shock proteins are highly conserved proteins among the different species, and activation of humoral and cellular responses to microbial hsp60 (mhsp60) has been associated with a number of infectious diseases (77). Antibodies against chsp60 were significantly higher in women suffering from perihepatitis-salpingitis and did not have distinctive clinical or microbiologic findings of C. trachomatis infection (43). Several other organisms have been associated with PID, which may subsequently result in the development of salpingitis and EP (10, 20, 38, 64). Neisseria gonorrhoeae, Mycoplasma hominis, Mycoplasma genitalium, and Ureaplasma urealyticum have been isolated from the fallopian tubes or peritoneal fluid from women with PID, supporting a possible role of these organisms in the development of tubal pathology and subsequent EP or infertility (10, 64). Therefore, samples positive for chsp60 only could represent cross-reactivity with hsp60 from the above bacteria.
Activins, their receptors, follistatin, and iNOS are expressed in the human oviduct during the different phases of the menstrual cycle and in early pregnancy (59, 60). We studied the effect of C. trachomatis on the expression of activins, their type II receptors, follistatin, and iNOS in human fallopian tubes bearing an EP.
Activins have been linked with a number of disease processes associated with chronic inflammation and fibrosis (40, 74). At the present time, there is no direct evidence in the literature on whether activin A is involved in the immune response to C. trachomatis. However, cumulative data from different studies suggest that activin A may play an important role in host control of chlamydial infection (28-30, 41, 42). Our study is the first to report an increase in the expression of activin A and its related molecules in tubal specimens collected from EP patients and patients who were serologically positive for C. trachomatis.
All published studies on the role of activins in modulating inflammation were on activin A, and there is no report in the literature on whether the other activin isoforms (activin AB and activin B) have a role in this process. Additionally, gene manipulation studies have demonstrated distinct functions for each activin isoform—at least during embryo development—suggesting that activin βA and βB subunits do not functionally overlap in all settings in vivo (6, 7). Therefore, the observed nonsignificant difference in the expression of the activin βB subunit in our study suggests that the other activin isoforms do not have a role in controlling microbial-mediated immune response in the human fallopian tube.
Activin A is released in sheep and mice following the injection of lipopolysaccharide, which is a major surface component of C. trachomatis, as with other gram-negative bacteria (28, 29). Toll-like receptor 4 recognizes C. trachomatis lipopolysaccharide (55), and it is responsible for the release of activin A in sheep and mice (28, 29). Activin A also appears to be responsive to the activation of Toll-like receptor 2 (42), which is involved in the production of proinflammatory cytokine following C. trachomatis infection (48). Infection with C. trachomatis increased the production of tumor necrosis factor alpha in human cervical tissue (66), interleukin-1 in human fallopian tube bearing an EP (24), and interleukin-6 in serum from women diagnosed with EP (65). These cytokines have been reported to regulate the release of activin A in sheep and mice (28, 30). Activin A has been reported to modulate the function of B lymphocytes (49), which play an important role in controlling reinfection with C. trachomatis (44).
Infection with C. trachomatis is associated with scar formation (1). Repeated C. trachomatis infection of pigtailed macaque fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring (71). Prolonged and/or significantly increased expression of activins is involved in the development of fibrosis. Activin A was increased in liver fibrosis in rats, and it was mainly localized around the fibrotic areas (67). Increased expression of activin A was also detected in the fibrotic lung in mice, and the upregulation of activin A correlated with the appearance of fibrotic changes (39). Activin A is involved in the inflammatory and repair phases occurring in burnt skin, and it induces early scar formation (40). Our results support the previous observations (28-30, 39-42, 67) as we have shown an increase in the activin βA subunit, type II receptors, and follistatin within the human fallopian tube of EP patients and patients who were serologically associated with C. trachomatis.
iNOS is expressed in the human fallopian tube, and it synthesizes nitric oxide, which is lethal to a variety of intracellular pathogens (9, 27). In vitro models have reported an increase in iNOS activity following C. trachomatis infection (26). In vivo studies have shown that iNOS may provide protection against chronic C. trachomatis infection (56-58). It has also been reported that elevated iNOS activity positively correlates with protection from hydrosalpinx formation and prevention of the systemic spread of C. trachomatis (25). Nitric oxide also induces relaxation of fallopian tube smooth muscle (12, 13). Therefore, the observed increase in the expression of iNOS in our study suggests that iNOS may participate in the immune response to C. trachomatis infection in humans and that increased production of iNOS by tubal epithelial cells secondary to chlamydial infection may lead to a delay in tubal motility and the development of EP.
mhsps are dominant antigens, and they are extremely immunogenic in humans (46). During an infection, microbes strongly increase their mhsp60 synthesis to protect themselves from the host's immune response (3, 46). PID and subsequent salpingitis can follow infection with several other organisms as well as C. trachomatis (10, 64). mhsp60s are highly conserved between the different pathogens, and they induce the release of cytokines and provoke an immune response (69), which can lead to persistent inflammatory reactions (77), and therefore tubal damage and scar formation could also be triggered by other mhsp60s, due to the high conservation of this protein between the different pathogens. Our results showed that there was an increase in the expression of activin βA subunit, type II receptors, follistatin, and iNOS in fallopian tubes bearing an EP and in patients with only antibodies against chsp60. Furthermore, there was no difference in the levels of expression of these molecules between C. trachomatis-positive patients and those that were only positive for chsp60. Increased expression of these molecules may lead to impairment of tubal motility, an increase in tubal receptivity, and subsequently the development of EP.
We therefore suggest that tubal activin A, its type II receptors, follistatin, and nitric oxide could be involved in the microbial-mediated immune response within the fallopian tube, and their pathological expression may lead to tubal damage and the development of EP.
Footnotes
Published ahead of print on 19 August 2009.
REFERENCES
- 1.Abu el-Asrar, A. M., K. Geboes, K. F. Tabbara, S. A. al-Kharashi, L. Missotten, and V. Desmet. 1998. Immunopathogenesis of conjunctival scarring in trachoma. Eye 12:453-460. [DOI] [PubMed] [Google Scholar]
- 2.Al-Azemi, M., B. Refaat, S. Amer, B. Ola, N. Chapman, and W. Ledger. 2009. The expression of inducible nitric oxide synthase in the human fallopian tube during the menstrual cycle and in ectopic pregnancy. Fertil. Steril. doi: 10.1016/j.fertnstert.2009.04.020. [DOI] [PubMed]
- 3.Bachmaier, K., and J. M. Penninger. 2005. Chlamydia and antigenic mimicry. Curr. Top. Microbiol. Immunol. 296:153-163. [DOI] [PubMed] [Google Scholar]
- 4.Barlow, R. E., I. D. Cooke, O. Odukoya, M. K. Heatley, J. Jenkins, G. Narayansingh, S. S. Ramsewak, and A. Eley. 2001. The prevalence of Chlamydia trachomatis in fresh tissue specimens from patients with ectopic pregnancy or tubal factor infertility as determined by PCR and in-situ hybridisation. J. Med. Microbiol. 50:902-908. [DOI] [PubMed] [Google Scholar]
- 5.Bjartling, C., S. Osser, and K. Persson. 2007. Deoxyribonucleic acid of Chlamydia trachomatis in fresh tissue from the Fallopian tubes of patients with ectopic pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 134:95-100. [DOI] [PubMed] [Google Scholar]
- 6.Brown, C. W., D. E. Houston-Hawkins, T. K. Woodruff, and M. M. Matzuk. 2000. Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat. Genet. 25:453-457. [DOI] [PubMed] [Google Scholar]
- 7.Brown, C. W., L. Li, D. E. Houston-Hawkins, and M. M. Matzuk. 2003. Activins are critical modulators of growth and survival. Mol. Endocrinol. 17:2404-2417. [DOI] [PubMed] [Google Scholar]
- 8.Brunham, R. C., B. Binns, J. McDowell, and M. Paraskevas. 1986. Chlamydia trachomatis infection in women with ectopic pregnancy. Obstet. Gynecol. 67:722-726. [DOI] [PubMed] [Google Scholar]
- 9.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen, H. F., J. Fedder, M. Drasbek, P. K. Nielsen, B. Toft, H. J. Ingerslev, S. Birkelund, and G. Christiansen. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866-1874. [DOI] [PubMed] [Google Scholar]
- 11.den Hartog, J. E., J. A. Land, F. R. Stassen, A. G. Kessels, and C. A. Bruggeman. 2005. Serological markers of persistent C. trachomatis infections in women with tubal factor subfertility. Hum. Reprod. 20:986-990. [DOI] [PubMed] [Google Scholar]
- 12.Ekerhovd, E., M. Brannstrom, M. Alexandersson, and A. Norstrom. 1997. Evidence for nitric oxide mediation of contractile activity in isolated strips of the human Fallopian tube. Hum. Reprod. 12:301-305. [DOI] [PubMed] [Google Scholar]
- 13.Ekerhovd, E., and A. Norstrom. 2004. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of fallopian tube contractility. Gynecol. Endocrinol. 19:239-246. [DOI] [PubMed] [Google Scholar]
- 14.Gdoura, R., F. Daoudi, F. Bouzid, F. Ben Salah, C. Chaigneau, J. M. Sueur, F. Eb, S. Rekik, A. Hammami, and J. Orfila. 2001. Detection of Chlamydia trachomatis in semen and urethral specimens from male members of infertile couples in Tunisia. Eur. J. Contracept. Reprod. Health Care 6:14-20. [PubMed] [Google Scholar]
- 15.Gerard, H. C., P. J. Branigan, G. R. Balsara, C. Heath, S. S. Minassian, and A. P. Hudson. 1998. Viability of Chlamydia trachomatis in fallopian tubes of patients with ectopic pregnancy. Fertil. Steril. 70:945-948. [DOI] [PubMed] [Google Scholar]
- 16.Gijsen, A. P., J. A. Land, V. J. Goossens, M. E. Slobbe, and C. A. Bruggeman. 2002. Chlamydia antibody testing in screening for tubal factor subfertility: the significance of IgG antibody decline over time. Hum. Reprod. 17:699-703. [DOI] [PubMed] [Google Scholar]
- 17.Gribi, R., T. Tanaka, R. Harper-Summers, and J. Yu. 2001. Expression of activin A in inflammatory arthropathies. Mol. Cell. Endocrinol. 180:163-167. [DOI] [PubMed] [Google Scholar]
- 18.Hamdad-Daoudi, F., J. Petit, and F. Eb. 2004. Assessment of Chlamydia trachomatis infection in asymptomatic male partners of infertile couples. J. Med. Microbiol. 53:985-990. [DOI] [PubMed] [Google Scholar]
- 19.Hartford, S. L., P. D. Silva, G. S. diZerega, and M. L. Yonekura. 1987. Serologic evidence of prior chlamydial infection in patients with tubal ectopic pregnancy and contralateral tubal disease. Fertil. Steril. 47:118-121. [DOI] [PubMed] [Google Scholar]
- 20.Hebb, J. K., C. R. Cohen, S. G. Astete, E. A. Bukusi, and P. A. Totten. 2004. Detection of novel organisms associated with salpingitis, by use of 16S rDNA polymerase chain reaction. J. Infect. Dis. 190:2109-2120. [DOI] [PubMed] [Google Scholar]
- 21.Henry-Suchet, J., M. Askienazy-Elbhar, M. Thibon, C. Revol, and B. A. Akue. 1994. Post-therapeutic evolution of serum chlamydial antibody titers in women with acute salpingitis and tubal infertility. Fertil. Steril. 62:296-304. [DOI] [PubMed] [Google Scholar]
- 22.Hosseinzadeh, S., A. Eley, and A. A. Pacey. 2004. Semen quality of men with asymptomatic chlamydial infection. J. Androl. 25:104-109. [DOI] [PubMed] [Google Scholar]
- 23.Hubner, G., M. Brauchle, M. Gregor, and S. Werner. 1997. Activin A: a novel player and inflammatory marker in inflammatory bowel disease? Lab. Investig. 77:311-318. [PubMed] [Google Scholar]
- 24.Hvid, M., A. Baczynska, B. Deleuran, J. Fedder, H. J. Knudsen, G. Christiansen, and S. Birkelund. 2007. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell. Microbiol. 9:2795-2803. [DOI] [PubMed] [Google Scholar]
- 25.Igietseme, J. U., L. L. Perry, G. A. Ananaba, I. M. Uriri, O. O. Ojior, S. N. Kumar, and H. D. Caldwell. 1998. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect. Immun. 66:1282-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igietseme, J. U., I. M. Uriri, M. Chow, E. Abe, and R. G. Rank. 1997. Inhibition of intracellular multiplication of human strains of Chlamydia trachomatis by nitric oxide. Biochem. Biophys. Res. Commun. 232:595-601. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, P., D. Radzioch, and M. M. Stevenson. 1996. In vivo regulation of nitric oxide production by tumor necrosis factor alpha and gamma interferon, but not by interleukin-4, during blood stage malaria in mice. Infect. Immun. 64:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, K. L., J. N. Brauman, N. P. Groome, D. M. de Kretser, and D. J. Phillips. 2000. Activin A release into the circulation is an early event in systemic inflammation and precedes the release of follistatin. Endocrinology 141:1905-1908. [DOI] [PubMed] [Google Scholar]
- 29.Jones, K. L., D. M. de Kretser, I. J. Clarke, J. P. Scheerlinck, and D. J. Phillips. 2004. Characterisation of the rapid release of activin A following acute lipopolysaccharide challenge in the ewe. J. Endocrinol. 182:69-80. [DOI] [PubMed] [Google Scholar]
- 30.Jones, K. L., A. Mansell, S. Patella, B. J. Scott, M. P. Hedger, D. M. de Kretser, and D. J. Phillips. 2007. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc. Natl. Acad. Sci. USA 104:16239-16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagiannidis, C., G. Hense, C. Martin, M. Epstein, B. Ruckert, P. Y. Mantel, G. Menz, S. Uhlig, K. Blaser, and C. B. Schmidt-Weber. 2006. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J. Allergy Clin. Immunol. 117:111-118. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann, S. H. 1990. Heat shock proteins and the immune response. Immunol. Today 11:129-136. [DOI] [PubMed] [Google Scholar]
- 33.Lan, J., A. J. van den Brule, D. J. Hemrika, E. K. Risse, J. M. Walboomers, M. E. Schipper, and C. J. Meijer. 1995. Chlamydia trachomatis and ectopic pregnancy: retrospective analysis of salpingectomy specimens, endometrial biopsies, and cervical smears. J. Clin. Pathol. 48:815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Land, J. A., A. P. Gijsen, A. G. Kessels, M. E. Slobbe, and C. A. Bruggeman. 2003. Performance of five serological chlamydia antibody tests in subfertile women. Hum. Reprod. 18:2621-2627. [DOI] [PubMed] [Google Scholar]
- 35.Letterio, J. J., and A. B. Roberts. 1998. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16:137-161. [DOI] [PubMed] [Google Scholar]
- 36.Lindquist, S., and E. A. Craig. 1988. The heat-shock proteins. Annu. Rev. Genet. 22:631-677. [DOI] [PubMed] [Google Scholar]
- 37.Maccato, M., R. Estrada, H. Hammill, and S. Faro. 1992. Prevalence of active Chlamydia trachomatis infection at the time of exploratory laparotomy for ectopic pregnancy. Obstet. Gynecol. 79:211-213. [PubMed] [Google Scholar]
- 38.Manavi, K. 2006. A review on infection with Chlamydia trachomatis. Best Pract. Res. Clin. Obstet. Gynaecol. 20:941-951. [DOI] [PubMed] [Google Scholar]
- 39.Matsuse, T., A. Ikegami, E. Ohga, T. Hosoi, T. Oka, K. Kida, M. Fukayama, S. Inoue, T. Nagase, Y. Ouchi, and Y. Fukuchi. 1996. Expression of immunoreactive activin A protein in remodeling lesions associated with interstitial pulmonary fibrosis. Am. J. Pathol. 148:707-713. [PMC free article] [PubMed] [Google Scholar]
- 40.McLean, C. A., H. Cleland, N. J. Moncrieff, R. J. Barton, D. M. de Kretser, and D. J. Phillips. 2008. Temporal expression of activin in acute burn wounds—from inflammatory cells to fibroblasts. Burns 34:50-55. [DOI] [PubMed] [Google Scholar]
- 41.Michel, U., S. Ebert, D. Phillips, and R. Nau. 2003. Serum concentrations of activin and follistatin are elevated and run in parallel in patients with septicemia. Eur. J. Endocrinol. 148:559-564. [DOI] [PubMed] [Google Scholar]
- 42.Michel, U., J. Gerber, A. E. O'Connor, S. Bunkowski, W. Bruck, R. Nau, and D. J. Phillips. 2003. Increased activin levels in cerebrospinal fluid of rabbits with bacterial meningitis are associated with activation of microglia. J. Neurochem. 86:238-245. [DOI] [PubMed] [Google Scholar]
- 43.Money, D. M., S. E. Hawes, D. A. Eschenbach, R. W. Peeling, R. Brunham, P. Wolner-Hanssen, and W. E. Stamm. 1997. Antibodies to the chlamydial 60 kd heat-shock protein are associated with laparoscopically confirmed perihepatitis. Am. J. Obstet. Gynecol. 176:870-877. [DOI] [PubMed] [Google Scholar]
- 44.Morrison, S. G., and R. P. Morrison. 2005. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 175:7536-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narvanen, A., M. Puolakkainen, W. Hao, K. Kino, and J. Suni. 1997. Detection of antibodies to Chlamydia trachomatis with peptide-based species-specific enzyme immunoassay. Infect. Dis. Obstet. Gynecol. 5:349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuer, A., S. D. Spandorfer, P. Giraldo, S. Dieterle, Z. Rosenwaks, and S. S. Witkin. 2000. The role of heat shock proteins in reproduction. Hum. Reprod. Update 6:149-159. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi, Y., H. Yabushita, M. Noguchi, M. Fujita, M. Asai, and C. A. Del Carpio. 2002. Detection of Chlamydia trachomatis infection with DNA extracted from formalin-fixed paraffin-embedded tissues. Diagn. Microbiol. Infect. Dis. 43:1-6. [DOI] [PubMed] [Google Scholar]
- 48.O'Connell, C. M., I. A. Ionova, A. J. Quayle, A. Visintin, and R. R. Ingalls. 2006. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J. Biol. Chem. 281:1652-1659. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa, K., M. Funaba, and M. Tsujimoto. 2008. A dual role of activin A in regulating immunoglobulin production of B cells. J. Leukoc. Biol. 83:1451-1458. [DOI] [PubMed] [Google Scholar]
- 50.Osser, S., and K. Persson. 1992. Chlamydial antibodies and deoxyribonucleic acid in patients with ectopic pregnancy. Fertil. Steril. 57:578-582. [DOI] [PubMed] [Google Scholar]
- 51.Packeisen, J., H. Buerger, R. Krech, and W. Boecker. 2002. Tissue microarrays: a new approach for quality control in immunohistochemistry. J. Clin. Pathol. 55:613-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patton, D. L., Y. T. Sweeney, and C. C. Kuo. 1994. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathogenic mechanism of tubal damage. J. Infect. Dis. 169:680-683. [DOI] [PubMed] [Google Scholar]
- 53.Phillips, D. J., K. L. Jones, J. Y. Scheerlinck, M. P. Hedger, and D. M. de Kretser. 2001. Evidence for activin A and follistatin involvement in the systemic inflammatory response. Mol. Cell. Endocrinol. 180:155-162. [DOI] [PubMed] [Google Scholar]
- 54.Piura, B., B. Sarov, and I. Sarov. 1993. Persistence of antichlamydial antibodies after treatment of acute salpingitis with doxycycline. Eur. J. Obstet. Gynecol. Reprod. Biol. 48:117-121. [DOI] [PubMed] [Google Scholar]
- 55.Prebeck, S., H. Brade, C. J. Kirschning, C. P. da Costa, S. Durr, H. Wagner, and T. Miethke. 2003. The Gram-negative bacterium Chlamydia trachomatis L2 stimulates tumor necrosis factor secretion by innate immune cells independently of its endotoxin. Microbes Infect. 5:463-470. [DOI] [PubMed] [Google Scholar]
- 56.Ramsey, K. H., G. S. Miranpuri, I. M. Sigar, S. Ouellette, and G. I. Byrne. 2001. Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome. Infect. Immun. 69:5131-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsey, K. H., I. M. Sigar, S. V. Rana, J. Gupta, S. M. Holland, and G. I. Byrne. 2001. Role for inducible nitric oxide synthase in protection from chronic Chlamydia trachomatis urogenital disease in mice and its regulation by oxygen free radicals. Infect. Immun. 69:7374-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramsey, K. H., I. M. Sigar, S. V. Rana, J. Gupta, S. M. Holland, G. I. Byrne, and J. D. Morrow. 2003. Inducible nitric oxide synthase regulates production of isoprostanes in vivo during chlamydial genital infection in mice. Infect. Immun. 71:7183-7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Refaat, B., S. Amer, B. Ola, N. Chapman, and W. Ledger. 2008. The expression of activin-betaA- and -betaB-subunits, follistatin, and activin type II receptors in fallopian tubes bearing an ectopic pregnancy. J. Clin. Endocrinol. Metab. 93:293-299. [DOI] [PubMed] [Google Scholar]
- 60.Refaat, B. A., A. O. Bahathiq, S. Sockanathan, R. L. Stewart, M. Wells, and W. L. Ledger. 2004. Production and localization of activins and activin type IIA and IIB receptors by the human endosalpinx. Reproduction 128:249-255. [DOI] [PubMed] [Google Scholar]
- 61.Roan, N. R., and M. N. Starnbach. 2008. Immune-mediated control of Chlamydia infection. Cell. Microbiol. 10:9-19. [DOI] [PubMed] [Google Scholar]
- 62.Saiki, R. K., S. Scharf, F. Faloona, K. B. Mullis, G. T. Horn, H. A. Erlich, and N. Arnheim. 1985. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350-1354. [DOI] [PubMed] [Google Scholar]
- 63.Seidal, T., A. J. Balaton, and H. Battifora. 2001. Interpretation and quantification of immunostains. Am. J. Surg. Pathol. 25:1204-1207. [DOI] [PubMed] [Google Scholar]
- 64.Simms, I., K. Eastick, H. Mallinson, K. Thomas, R. Gokhale, P. Hay, A. Herring, and P. A. Rogers. 2003. Associations between Mycoplasma genitalium, Chlamydia trachomatis and pelvic inflammatory disease. J. Clin. Pathol. 56:616-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soriano, D., D. Hugol, N. T. Quang, and E. Darai. 2003. Serum concentrations of interleukin-2R (IL-2R), IL-6, IL-8, and tumor necrosis factor alpha in patients with ectopic pregnancy. Fertil. Steril. 79:975-980. [DOI] [PubMed] [Google Scholar]
- 66.Srivastava, P., R. Jha, S. Bas, S. Salhan, and A. Mittal. 2008. In infertile women, cells from Chlamydia trachomatis infected sites release higher levels of interferon-gamma, interleukin-10 and tumor necrosis factor-alpha upon heat-shock-protein stimulation than fertile women. Reprod. Biol. Endocrinol. 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugiyama, M., T. Ichida, T. Sato, T. Ishikawa, Y. Matsuda, and H. Asakura. 1998. Expression of activin A is increased in cirrhotic and fibrotic rat livers. Gastroenterology 114:550-558. [DOI] [PubMed] [Google Scholar]
- 68.Sziller, I., S. S. Witkin, M. Ziegert, Z. Csapo, A. Ujhazy, and Z. Papp. 1998. Serological responses of patients with ectopic pregnancy to epitopes of the Chlamydia trachomatis 60 kDa heat shock protein. Hum. Reprod. 13:1088-1093. [DOI] [PubMed] [Google Scholar]
- 69.Tabona, P., K. Reddi, S. Khan, S. P. Nair, S. J. Crean, S. Meghji, M. Wilson, M. Preuss, A. D. Miller, S. Poole, S. Carne, and B. Henderson. 1998. Homogeneous Escherichia coli chaperonin 60 induces IL-1 beta and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J. Immunol. 161:1414-1421. [PubMed] [Google Scholar]
- 70.Toth, M., D. L. Patton, L. A. Campbell, E. I. Carretta, J. Mouradian, A. Toth, M. Shevchuk, R. Baergen, and W. Ledger. 2000. Detection of chlamydial antigenic material in ovarian, prostatic, ectopic pregnancy and semen samples of culture-negative subjects. Am. J. Reprod. Immunol. 43:218-222. [DOI] [PubMed] [Google Scholar]
- 71.Van Voorhis, W. C., L. K. Barrett, Y. T. Cosgrove Sweeney, C. C. Kuo, and D. L. Patton. 1997. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect. Immun. 65:2175-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verkooyen, R. P., A. Luijendijk, W. M. Huisman, W. H. F. Goessens, J. A. J. W. Kluytmans, J. H. van Rijsoort-Vos, and H. A. Verbrugh. 1996. Detection of PCR inhibitors in cervical specimens by using the AMPLICOR Chlamydia trachomatis assay. J. Clin. Microbiol. 34:3072-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker, R. A. 2006. Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment. I. Histopathology 49:406-410. [DOI] [PubMed] [Google Scholar]
- 73a.Wang, S. P., and J. T. Grayston. 1970. Immunologic relationship between genital TRIC, lymphogranuloma venereum and related organisms in a new microtiter indirect immunofluorescence test. Am. J. Ophthalmol. 70:367-374. [DOI] [PubMed] [Google Scholar]
- 74.Werner, S., and C. Alzheimer. 2006. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 17:157-171. [DOI] [PubMed] [Google Scholar]
- 75.WHO. 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. World Health Organization, Geneva, Switzerland.
- 76.Yi, Y., G. Zhong, and R. C. Brunham. 1993. Continuous B-cell epitopes in Chlamydia trachomatis heat shock protein 60. Infect. Immun. 61:1117-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zugel, U., and S. H. Kaufmann. 1999. Immune response against heat shock proteins in infectious diseases. Immunobiology 201:22-35. [DOI] [PubMed] [Google Scholar]