Abstract
The aim of the study was to evaluate the influence of treatment with spiramycin on the increase of immunoglobulin G (IgG) titers and IgG avidity indexes (AI) in pregnant women with seroconversion from the beginning of therapy until delivery and after delivery. This group was compared with adult patients with recently acquired untreated toxoplasmosis. One hundred four samples from 32 pregnant women with seroconversion for toxoplasmosis and/or very low IgG AI were followed from the beginning of therapy with spiramycin until delivery. Twenty-nine women were further followed some months after delivery and interruption of therapy. Thirty-eight samples from 16 untreated, nonpregnant patients were evaluated as the control group. The Toxoplasma gondii-specific IgG antibody and the T. gondii-specific IgG AI were significantly delayed in pregnant women receiving therapy compared to nonpregnant, untreated controls, and the findings were consistent with the results of assays from two different manufacturers. The T. gondii-specific IgG AI increased in pregnant women after they gave birth. Avidity maturation is delayed during pregnancy and treatment, and low-avidity antibodies in pregnant women within 3 to 4 months cannot be taken as a sign of infection.
After infection, the specific immunoglobulin G (IgG) antibody response matures by the selection of clones of B cells producing antibodies with increasingly higher avidities against a specific antigen from the invading microorganism. In Toxoplasma gondii infections, specific IgM may be present for a long time (14-17), and measurement of the T. gondii-specific IgG avidity index (AI) is the best method to determine the time of infection (24) and is a further development of the differential agglutination assay (3, 27).
The original method developed by Hedman et al. (4, 5) used serial dilutions tested in enzyme immunoassays with and without 6 M urea, but automated assays calculate the IgG AI from two single measurements with and without urea (22). This introduces uncertainty, although experiments with only two serum sample dilutions showed excellent agreement with IgG AI measurements obtained with four serial serum sample dilutions (9).
A persistent, low IgG AI poses a diagnostic problem, at least in some pregnant women receiving treatment during pregnancy (21).
The observation that Toxoplasma gondii-specific IgG maturation is delayed in treated pregnant women compared to nontreated, nonpregnant individuals has been reported in two previous studies, which found significantly delayed IgG maturation in treated individuals (13, 26).
The maturation of the IgG response varies between individuals and may take months in pregnant, treated women, for whom one study found that a low IgG AI persisted up to 9 months postinfection (20). In a study of T. gondii-infected pregnant women identified prospectively through prenatal screening, one study found that 2 out of 73 women had IgG AI above 0.2 before 20 weeks of gestation, but many continued to have low IgG AI even a year after infection. It is assumed that all women were treated during pregnancy (7).
One problem with different findings in different studies is the lack of standardization of the T. gondii-specific IgG AI assay (12).
We report here that treatment of T. gondii infection may influence IgG production and avidity maturation in pregnant women, which was evaluated by two commercial methods.
MATERIALS AND METHODS
Patients and samples.
One hundred four samples from 25 pregnant women (median, 4 samples per patient; range, 3 to 7 samples per patient) with seroconversion for toxoplasmosis and/or very low IgG AI (<0.2 by the Vidas assay; <0.350 by the Liaison assay) were followed from the beginning of therapy with spiramycin until delivery (median follow-up time, 161 days; range, 38 to 218 days), and 20 women (total number of samples, 92; median, 3 samples per patient; range, 1 to 5 samples per patient) were also followed for 1 to 12 months after delivery (median, 152 days; range, 21 to 377 days). The women were referred to the outpatient service of the Infectious Diseases Department of the IRCCS Policlinico San Matteo Foundation because of suspected primary infection with T. gondii during pregnancy.
Control group.
The IgG antibody response and IgG AI were also evaluated in a control group of 16 untreated adult patients (total number of samples, 38; median, 2 samples per patient; range, 2 to 4 samples per patient) with seroconversion or very recent infection and lymphadenopathy and followed after diagnosis for 2 up to 15 months (median, 102 days; range, 35 to 102 days).
Antibody analysis.
All samples were positive for IgM antibodies by the Toxo-ISAGA (bioMérieux, Marcy l'Etoile, France) and Liaison Toxo IgM (Diasorin, Saluggia, Italy) tests. T. gondii-specific IgG antibodies were measured by the Vidas systems Toxo IgG II and Toxo IgG avidity (BioMérieux, Marcy l'Etoile, France) and the Liaison systems Toxo IgGII and Toxo IgG avidity II (Diasorin, Saluggia, Italy).
Treatment.
The women received treatment with spiramycin in the standard dose of 9 × 106 units per day until delivery.
Statistical analysis.
The Shapiro-Wilks test was used to test the normal distribution of quantitative variables, and parametric or nonparametric tests were used to compare differences between times or groups, as appropriate. Repeated-measure multiple analysis of variance models (after log transformation for non-normally distributed data) were used to test for statistically significant changes over time and between groups. The χ2 test or Fisher's exact test, as appropriate, was used to test qualitative variables for differences. A P value of <0.05 was regarded as statistically significant. Analyses were performed with Stata statistical software (release 9.0, 2000; StataCorp, College Station, TX).
RESULTS
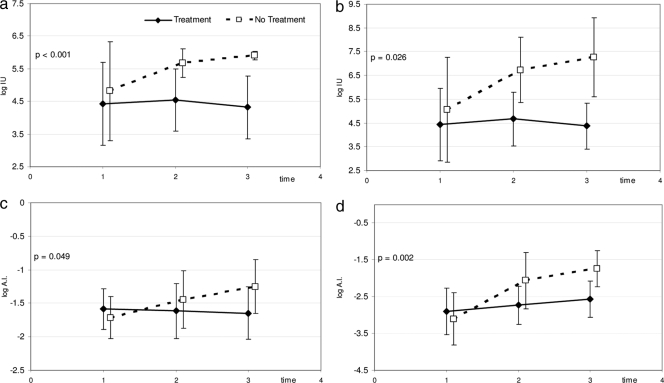
The T. gondii-specific IgG antibody level reached more than 100 IU/ml in all untreated patients, as measured by both methods, within a month after diagnosis of T. gondii infection (Fig. 1a and b). In contrast, the T. gondii-specific IgG antibody levels were lower in pregnant women, with half of the samples showing IgG levels between 10 IU/ml and 100 IU/ml (Fig. 1a and b). The difference in the increases of IgG specific antibodies between treated pregnant women and untreated patients is highly statistically significant (P < 0.0001).
FIG. 1.
T. gondii-specific IgG antibodies in 32 pregnant women treated with spiramycin from the time of diagnosis until delivery and in 16 nonpregnant patients with acute T. gondii infection and not receiving any treatment. 0, time of diagnosis; 1, 2, and 3, trimesters of pregnancy. Toxoplasma gondii-specific IgG antibodies (log IU/ml) tested with the Liaison assay (Diasorin, Saluggia, Italy) (a) and the enzyme-linked fluorescent assay (bioMérieux, Marcy l'Etoile, France) (b). T. gondii-specific IgG AI (log A.I.) measured with the Liaison assay (Diasorin, Saluggia, Italy) (c) and the Vidas assay (bioMérieux, Marcy l'Etoile, France) (d).
Both assays showed that the maturation of the T. gondii-specific IgG response was delayed in treated pregnant women compared to untreated, nonpregnant controls (Fig. 1c and d).
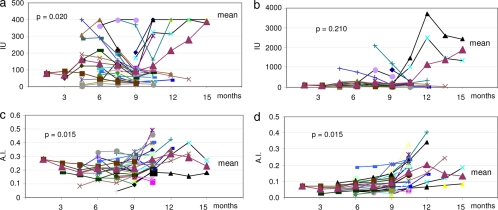
In some pregnant patients, the T. gondii-specific IgG AI increased after delivery (Fig. 2a and b). The difference between the IgG AI measured by the Liaison Toxo IgG avidity assay and that measured by the Vidas Toxo IgG assay was significant only in the later phases of infection. In many cases, the IgG AI increased after delivery and the termination of therapy; the increase in AI before and after delivery was statistically significant for both tests (P = 0.015, Liaison Toxo IgG AI; P = 0.015, Vidas Toxo IgG AI) (Fig. 2c and d).
FIG. 2.
T. gondii-specific IgG antibodies in 29 pregnant women treated with spiramycin from the time of diagnosis until delivery and followed after delivery. Month 9 is the time of delivery. T. gondii-specific IgG antibodies (IU/ml) tested with the Liaison assay (Diasorin, Saluggia, Italy) (a) and the enzyme-linked fluorescent assay (IU/ml) (bioMérieux, Marcy l'Etoile, France) (b). T. gondii-specific IgG AI tested with the Liaison assay (Diasorin, Saluggia, Italy) (c) and the Vidas assay (bioMérieux, Marcy l'Etoile, France) (d). Each symbol connected by a line represents data from a single patient.
The increase of T. gondii-specific IgG antibodies and the maturation of the IgG AI were delayed after therapy in pregnant women, as measured by both tests (Fig. 2). The increase in T. gondii-specific IgG antibodies over time was significantly higher in untreated patients than in treated patients. The two tests showed similar patterns, although we could not compare very high titers due to the different measurement ranges of the two assays (the maximum value for the Liaison Toxo IgG avidity assay is 400 IU; we could perform dilutions and could also obtain a more precise IgG quantitation for higher antibody titers with the Vidas Toxo IgG assay). Moreover, early therapy seems to affect IgG production even after interruption: in the treated pregnant population after delivery when treatment stopped, the increase in IgG antibody titers was highly variable depending on the test employed (data not shown). The delay was more evident for the AI maturation when we compared treated and untreated patients with the Vidas Toxo IgG avidity assay (P = 0.002) than when we compared them with the Liaison Toxo IgG avidity assay (P = 0.049).
DISCUSSION
The maturation of the T. gondii-specific IgG response varies considerably between individuals. In one study, two seroconverting mothers already had IgG AI above 20% at the time of diagnosis, but most patients had developed an IgG AI above 15% after 180 days (10). The cutoff value defining a low IgG AI differs markedly between different studies and different methods (6). A study from France found an average IgG AI of 0.2 in pregnant women infected within the past 5 months (11).
Up to half of the patients with acute infections may show a low or borderline IgG AI at 6 months after the infection (19, 25), which is in concordance with the results reported in our previous study (21). A comparison between the Vidas and the LabSystems (Helsinki, Finland) IgG AI showed correlation coefficients of 0.6 for pregnant women and 0.88 for other patients (1), but the difference was not further discussed. Improvement of the IgG avidity assay using recombinant antigens (2) or the Western blot technique has been attempted and revealed differences in the maturation of the IgG antibodies specific to different antigens (18, 28).
One study evaluated the IgG AI using single-dilution assays with and without urea and showed that because the signal obtained in an enzyme immunoassay system is not linear, it makes a difference whether the Toxoplasma IgG AI is calculated from the optical density values or from the activity of Toxoplasma-specific IgG antibodies measured in international units per ml (23).
We found that treated pregnant women developed a lower total T. gondii-specific IgG antibody level than nonpregnant, untreated adults with acute toxoplasmosis. We also found that even after birth, T. gondii-specific IgG AI increased slowly in many previously treated pregnant women infected with T. gondii during pregnancy. The study was performed with pregnant women, and the observed delay in T. gondii-specific IgG AI maturation could be due to either treatment or pregnancy. We believe that the delay in AI maturation most probably is due to treatment and is not an effect of pregnancy.
In newborn children, reduced production of T. gondii-specific IgG antibodies has been observed during treatment during the first year of life (8, 29). A generally reduced antibody response to pathogens could be due to either pregnancy or the drug (13).
The delay found in our study could be due to a reduced parasite load. An increase in parasite replication after treatment is stopped could also explain the antibody rebound.
Therapy with spiramycin in the early phase of infection also modifies the IgG antibody response in pregnant women. These results are evident, albeit at different degrees with all the tests employed in this study. The effect of spiramycin on the trophozoites, with the ensuing reduction of parasitic load, might explain the delay in serological response. Such an effect must be taken into account by those involved in the management of the health of pregnant women. In this respect, the influence of immunosuppression from pregnancy seems to be irrelevant; in fact, in some treated, nonpregnant patients, the antibody increase and avidity maturation were similar to those observed in the pregnant ones (data not shown). The increase in AI observed after delivery may also be explained by therapy interruption.
Our results emphasize the need for further studies to clarify the avidity maturation process in pregnant women under drug therapy.
Footnotes
Published ahead of print on 19 August 2009.
REFERENCES
- 1.Alvarado-Esquivel, C., S. Sethi, K. Janitschke, H. Hahn, and O. Liesenfeld. 2002. Comparison of two commercially available avidity tests for Toxoplasma-specific IgG antibodies. Arch. Med. Res. 33:520-523. [DOI] [PubMed] [Google Scholar]
- 2.Beghetto, E., W. Buffolano, A. Spadoni, M. Del Pezzo, M. Di Cristina, O. Minenkova, E. Petersen, F. Felici, and N. Gargano. 2003. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 41:5414-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannemann, B. R., W. C. Vaughan, P. Thulliez, and J. S. Remington. 1990. Differential agglutination test for diagnosis of recently acquired infection with Toxoplasma gondii. J. Clin. Microbiol. 28:1928-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedman, K., M. Lappalainen, I. Seppala, and O. Makela. 1989. Recent primary Toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159:726-779. [DOI] [PubMed] [Google Scholar]
- 5.Hedman, K., M. Lappalainen, M. Söderlund, and L. Hedman. 1993. Avidity of IgG in serodiagnosis of infectious diseases. Rev. Med. Microbiol. 4:123-129. [Google Scholar]
- 6.Holliman, R. E., R. Raymond, N. Renton, and J. D. Johnson. 1994. The diagnosis of toxoplasmosis using IgG avidity. Epidemiol. Infect. 112:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenum, P. A., B. Stray-Pedersen, and A.-G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahi, S., G. J. Cozon, J. M. Pinon, T. Greenland, M. Wallon, M. Al Kurdi, J. Ferrandiz, and F. Peyron. 1999. A switch towards Th2 during serological rebound in children with congenital toxoplasmosis. Clin. Exp. Immunol. 117:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korhonen, M. H., J. Brunstein, H. Haario, A. Katnikov, R. Rescaldani, and K. Hedman. 1999. A new method with general diagnostic utility for the calculation of immuglobulin G avidity. Clin. Diagn. Lab. Immunol. 6:725-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lappalainen, M., P. Koskela, M. Koskiniemi, P. Ämmälä, V. Hiilesmaa, K. Teramo, K. O. Raivio, J. S. Remington, and K. Hedman. 1993. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J. Infect. Dis. 167:691-697. [DOI] [PubMed] [Google Scholar]
- 11.Lecolier, B., and B. Pucheu. 1993. Intérêt de l'étude de l'avidité des IgG pour le diagnostic de la toxoplasmose. Pathol. Biol. (Paris) 41:155-158. [PubMed] [Google Scholar]
- 12.Lefevre-Pettazzoni, M., S. Le Cam, M. Wallon, and F. Peyron. 2006. Delayed maturation of immunoglobulin G avidity: implication for the diagnosis of toxoplasmosis in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 25:687-693. [DOI] [PubMed] [Google Scholar]
- 13.Lefevre-Pettazzoni, M., A. Bissery, M. Wallon, G. Cozon, F. Peyron, and M. Rabilloud. 2007. Impact of spiramycin treatment and gestational age on maturation of Toxoplasma gondii immunoglobulin G avidity in pregnant women. Clin. Vaccine Immunol. 14:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, S., G. Galvan, F. G. Araujo, Y. Suzuki, J. S. Remington, and S. Parmley. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 7:781-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L. Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesenfeld, O., J. G. Montoya, S. Kinney, C. Press, and J. S. Remington. 2001. Effect of testing for IgG avidity in the diagnosis of T. gondii infection in pregnant women: experience in a U.S. reference laboratory. J. Infect. Dis. 183:1248-1253. [DOI] [PubMed] [Google Scholar]
- 17.Liesenfeld, O., J. G. Montoya, S. Kinney, C. Press, and J. S. Remington. 2001. Confirmatory serological testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibody titers. Am. J. Obstet. Gynecol. 184:140-145. [DOI] [PubMed] [Google Scholar]
- 18.Marcolino, P. T., D. A. O. Silva, P. G. Leser, M. E. Camargo, and J. R. Mineo. 2000. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin. Diagn. Lab. Immunol. 7:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montoya, J. G., O. Liesenfeld, S. Kinney, C. Press, and J. S. Remington. 2002. VIDAS test for avidity of Toxoplasma-specific immunoglobulin G for confirmatory testing of pregnant women. J. Clin. Microbiol. 40:2504-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelloux, H., E. Brun, G. Vernet, S. Marcillat, M. Jolivet, D. Guergour, H. Fricker-Hidalgo, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1998. Determination of anti-Toxoplasma gondii immunoglobulin G avidity: adaptation to the Vidas system (bioMérieux). Diagn. Microbiol. Infect. Dis. 32:69-73. [DOI] [PubMed] [Google Scholar]
- 21.Petersen, E., M. V. Borobio, E. Guy, O. Liesenfeld, V. Meroni, A. Naessens, E. Spranzi, and P. Thulliez. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin (IgG) and IgM and the IgG avidity index. J. Clin. Microbiol. 43:1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Press, C., J. G. Montoya, and J. S. Remington. 2005. Use of a single serum sample for diagnosis of acute toxoplasmosis in pregnant women and other adults. J. Clin. Microbiol. 43:3481-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prince, H. E., and M. Wilson. 2001. Simplified assay for measuring T. gondii immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 8:904-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert, A., V. Luyasu, J. Zuffrey, K. Hedman, and E. Petersen. 2001. Potential of the specific markers in the early diagnosis of toxoplasma-infection: a multicentre study using combination of isotype IgG, IgM, IgA and IgE with values of avidity assay. Eur. J. Clin. Microbiol. Infect. Dis. 20:467-474.11561802 [Google Scholar]
- 25.Rossi, C. L. 1998. A simple, rapid enzyme-linked immunosorbent assay for evaluating immunoglobulin G antibody avidity in toxoplasmosis. Diagn. Microbiol. Infect. Dis. 30:25-30. [DOI] [PubMed] [Google Scholar]
- 26.Sensini, A., S. Pascoli, D. Marchetti, A. Castronari, M. Marangi, G. Sbaraglia, C. Cimmino, A. Favero, M. Castelletto, and A. Mottola. 1996. IgG avidity in the serodiagnosis of acute T. gondii infection: a multicentre study. Clin. Microbiol. Infect. 2:25-29. [DOI] [PubMed] [Google Scholar]
- 27.Thulliez, P., J. S. Remington, F. Santoro, G. Ovlaque, S. Sharma, and G. Desmonts. 1989. A new agglutination reaction for the diagnosis of the development stage of acquired toxoplasmosis. Pathol. Biol. (Paris) 34:173-177. [PubMed] [Google Scholar]
- 28.Villavedra, M., J. Battistoni, and A. Nieto. 1999. IgG recognizing 21-24 kDa and 30-33 kDa tachyzoite antigens show maximum avidity maturation during natural and accidental human toxoplasmosis. Rev. Inst. Med. Trop. Sao Paulo 41:297-303. [DOI] [PubMed] [Google Scholar]
- 29.Wallon, M., G. Cozon, R. Ecochard, P. Lewin, and F. Peyron. 2001. Serological rebound in congenital toxoplasmosis: long-term follow-up of 133 children. Eur. J. Pediatr. 160:534-540. [DOI] [PubMed] [Google Scholar]