Abstract
Having reported that pretreatment of serum samples with EDTA at 100°C improved the sensitivity for the detection of Histoplasma antigenemia, we have evaluated this method for the detection of Coccidioides antigenemia. Urine and serum samples from patients with coccidioidomycosis were tested using the MVista Coccidioides enzyme immunoassay, and serum samples with and without EDTA-heat treatment were tested. Antigenemia was detected in 28.6% of patients whose samples were not EDTA-heat treated and in 73.1% of those whose samples were treated. Antigenuria was detected in 50% of patients. Specificity of 100% was obtained in healthy subjects, but cross-reactions were seen in 22.2% of patients with histoplasmosis or blastomycosis. EDTA-heat treatment improves the sensitivity for the detection of Coccidioides antigenemia.
Antigen detection could be useful for diagnosis of coccidioidomycosis. We reported detection of antigenuria in 70.8% of cases using the MVista Coccidioides antigen enzyme immunoassay (EIA), which detects Coccidioides galactomannan (2). When combined with serology, antigen or seropositivity was detected in 88% of cases of moderate to severe coccidioidomycosis, equaling the rate of isolation of the organism in cultures (2).
Others have reported the detection of antigenemia in patients with coccidioidomycosis (4, 5, 8). In our earlier studies, antigenemia was not detected in the few cases that were tested (L. J. Wheat, unpublished observations). More recently we noted that pretreatment of serum at 100°C in the presence of EDTA, by dissociating immune complexes and destroying the freed antibody, markedly improved the detection of antigenemia in histoplasmosis (7). Among AIDS patients with histoplasmosis and undetectable antigenemia, antigen was detected in 95% of samples following EDTA-heat treatment. Herein we describe the effect of EDTA-heat treatment on the detection of antigenemia in the Coccidioides antigen EIA.
MATERIALS AND METHODS
EIA.
The MVista Coccidioides antigen EIA was performed as previously reported (2), using microplates coated with anti-Coccidioides antibodies. Following incubation of the test specimen in the precoated microplate, antigen that had attached to the capture antibody was detected with biotinylated rabbit anti-Coccidioides detector antibody. Results greater than or equal to the 0.07 ng/ml urinary galactomannan calibrator were considered positive. A single urine or serum specimen was tested for each patient.
Clinical specimens.
Urine and serum specimens were available from 16 patients diagnosed with coccidioidomycosis who were entered into approved clinical studies at three institutions (El Rio Special Immunology, Tucson, AZ; Brook Army Medical Center, San Antonio, TX; Naval Medical Center, San Diego, CA) and 12 additional patients diagnosed with coccidioidomycosis whose sera were tested at MiraVista Diagnostics, for whom clinical information was incomplete; a study with 3 of these 12 patients was previously reported (2). The criteria for diagnosis included a compatible clinical illness accompanied by laboratory evidence for coccidioidomycosis, including a positive culture (proven), histopathology (proven), serology (probable), or antigen (probable) (2). Serologic criteria for positivity included immunoglobulin G (IgG) or IgM positivity by EIA, precipitin bands by immunodiffusion, or complement fixation titers of 1:2 or more. Controls included serum specimens that were positive for Histoplasma (n = 13) (1) or Blastomyces antigen (n = 5) (3) and that were from healthy subjects from Kern County, CA (n = 69), and Memphis, TN (n = 25), purchased from SeraCare (Oceanside, CA). Specimens were stored frozen at −70°C until they were tested together as a batch.
Pretreatment of sera.
A total of 200 μl of 4% EDTA at pH 4.6 was added to 600 μl of sera, and the mixture was vortexed and then placed in a heat block (Fisher Scientific) at 100°C for 6 minutes, followed by centrifugation and collection of the supernatant (7).
Statistical analysis.
The respective proportions of patients with positive results were compared using the Fisher exact test.
RESULTS
Immunosuppressive conditions were present in 11 of 18 (61.1%) patients for whom information was available, with human immunodeficiency virus (HIV) infection or AIDS accounting for 9 (Table 1) . For patients with the available information, cultures were positive for 9 of 22 (40.9%) patients, and serology was positive for 19 of 19 (100%) patients. IgM and/or IgG antibodies detected by EIA alone were the basis for seropositivity in 7 of 19 (36.8%) patients.
TABLE 1.
Clinical and laboratory findings in patients with proven or probable coccidioidomycosisa
| Patient no. | Underlying condition | Presentation | Clinical and laboratory findings
|
|||||
|---|---|---|---|---|---|---|---|---|
| Positive cultureb | Pathology | Serology | Urine antigen testing (ng/ml) | Serum antigen testing (ng/ml) | Serum antigen EDTA testing (ng/ml) | |||
| 1 | Unk | CNS | Unk | Unk | Unk | 0.14 | 0.09 | 4.42 |
| 2 | Unk | CNS | Unk | Unk | EIA IgM Pos, IgG Pos | Neg | Neg | Neg |
| 3 | Cirrhosis corticosteroid | Pulmonary, fatal | BAL specimen | Unk | EIA IgM Pos, IgG Neg | Neg | Neg | Neg |
| 4 | Unk | Unk | Unk | Unk | Unk | Not done | 0.09 | 3.28 |
| 5 | None | Pulmonary, chronic cavitary | BAL specimen | Neg | CF of 16 | Neg | Neg | Neg |
| 6 | Unk | Disseminated | Ear | Unk | Unk | 3.13 | 3.82 | >8.2 |
| 7 | Unk | Disseminated, fatal | Unk | Unk | Unk | 3.43 | 8.03 | >8.2 |
| 8 | Heart transplant | Pulmonary, fungemia, fatal | Blood sample | Not done | EIA IgM Neg, IgG Pos | 3.42 | Neg | 0.07 |
| 9 | None | Disseminated, bone, soft tissuec | Soft tissue | Unk | Unk | 5.06 | >8.2 | >8.2 |
| 10 | Unk | Unk | Unk | Unk | Unk | 0.48 | 0.18 | 0.28 |
| 11 | Unk | Unk | Unk | Unk | Unk | 0.15 | Neg | 0.73 |
| 12 | Unk | Unk | Unk | Unk | Unk | Not done | Neg | 0.77 |
| 13 | Metabolic syndrome | Pulmonary | Sputum, BAL specimen | Not done | EIA IgM Pos, IgG Pos, CF of 8 | Neg | Neg | 1.33 |
| 14 | HIV | Asymptomatic | Not done | Not done | IgM Neg, IgG Pos, CF Neg | Not done | Neg | 0.25 |
| 15 | Unk | Pulmonary, disseminated | Skin | Skin | EIA IgM Pos, IgG Pos, CF of 256 | 0.80 | Neg | 0.65 |
| 16 | Unk | Pulmonary, disseminated, bone | Not done | Not done | EIA IgM Neg, IgG Pos, CF of 128 | Neg | Neg | Neg |
| 17 | Diabetes, hypertension | Pulmonary, disseminated, prostatec | Seminal fluid | Not done | CF of 4 | Neg | Neg | Neg |
| 18 | None | Disseminated, soft tissue | Not done | Not done | EIA IgM Neg, IgG Pos, IDCF Pos, IDTP Neg, CF of 256, CSF CF Neg | Not done | Neg | 0.29 |
| 19 | None | Pulmonary, disseminated, bonec | Not done | Not done | CF of 4 | Neg | Neg | 0.20 |
| 20 | None | Disseminated, fatal | CSF, bone | Not done | CF of 128, CSF CF of 8 | 5.68 | 6.99 | >8.2 |
| 21 | AIDS | Pulmonary | Not done | Not done | CF of 128 | 0.88 | 0.26 | 0.88 |
| 22 | AIDS | Pulmonaryd | Not done | Not done | EIA IgM Neg, IgG Pos | Neg | Neg | Neg |
| 23 | HIV | Pulmonarye | Not done | Not done | EIA IgM Neg, IgG Pos | Neg | Neg | Neg |
| 24 | AIDS | Pulmonaryf | Not done | Not done | Not done | 0.11 | Neg | Neg |
| 25 | AIDS | Pulmonary, disseminated, CNS | Not done | Not done | EIA IgM Neg, IgG Pos, CF of 256, CSF CF of 128 | 0.08 | Neg | 0.21 |
| 26 | HIV | Pulmonary | Not done | Not done | EIA IgM Neg, IgG Pos, CF Neg | Neg | Neg | Neg |
| 27 | AIDS | Pulmonary | Not done | Not done | CF of 128 | Neg | Neg | 0.66 |
| 28 | AIDS | Pulmonary, disseminated, lymph nodes | Not done | Not done | EIA IgM Neg, IgG Pos, CF of 2 | Neg | Neg | 0.16 |
Patients with proven coccidioidomycosis, n = 9; patients with probable coccidioidomycosis, n = 19. Abbreviations: CNS, central nervous system; BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid; Neg, negative; Pos, positive; Unk, unknown; CF, reciprocal complement fixation titer; IDCF, immunodiffusion assay for complement-fixing antibody; IDTP, immunodiffusion assay for tube precipitin antibody.
Indication of a specimen type denotes that Coccidioides was isolated from that specimen.
Relapse, culture, sputum, bone, and lymph node samples were positive during initial infection.
Diagnosis of coccidioidomycosis as the cause of lung nodules was uncertain because of melanoma.
Culture proven to be pulmonary coccidioidomycosis 5 years earlier.
Original diagnosis 2 years earlier; presented with presumed pulmonary relapse, which responded to fluconazole.
The infection was classified as pulmonary for nine patients, disseminated for eight, pulmonary and disseminated for six, asymptomatic for one, and unknown for four. Nine (32.1%) of the cases were classified as proven based upon isolation of Coccidioides from tissue or body fluid samples, and 19 (67.9%) as probable based upon positive serology (n = 12) or antigen testing (n = 7). Twelve patients were hospitalized, eleven were not, and the hospitalization status was unknown for five. Twelve of 17 (70.6%) patients for whom information was available had received antifungal therapy for 5 days or more, including eight (47.1%) who had received therapy for 3 weeks or more at the time the specimens were obtained for antigen testing. Overall, 15 of 17 (88.2%) patients for whom information was available were treated for coccidioidomycosis, and 4 of 21 (19.0%) for whom survival information was available died of coccidioidomycosis.
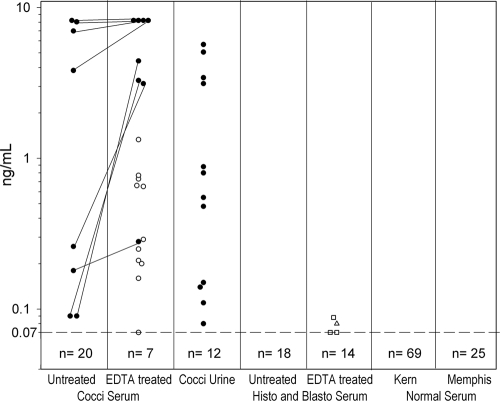
Antigenemia was detected in 8 of 28 (28.6%) patients whose samples were not EDTA-heat treated and in 19 of 26 (73.1%) of those whose samples were treated (P = 0.0024) (Table 2). Sufficient serum samples for EDTA-heat treatment were unavailable for two patients. Among the eight patients whose samples were both untreated and EDTA-heat treated with positive results, concentrations increased following treatment for seven and could not be evaluated for one because the concentration was above the upper limit of quantification (8.2 ng/ml) (Fig. 1). Antigenemia was detected in six of nine (66.7%) proven cases and 13 of 19 (68.4%) probable cases. Antigenuria was detected in 12 of 24 (50%) patients from whom urine was available (Table 2). Antigenuria was detected in five of nine (55.6%) proven cases and 7 of 19 (36.8%) probable cases.
TABLE 2.
Sensitivity of antigen testing for coccidioidomycosis
| Parameter | No. of positive cases/total no. of cases (%) |
|---|---|
| Antigenuria | 12/24 (50.0) |
| Antigenemia without EDTA-heat treatment | 8/28 (28.6) |
| Antigenemia with EDTA-heat treatment | 19/26 (73.1) |
| Antigenuria or antigenemia | 20/28 (71.4) |
FIG. 1.
Effect of EDTA-heat pretreatment on the detection of Coccidioides antigenemia. Results are expressed in ng/ml and depicted on the vertical axis. Results above the dashed horizontal line at 0.07 ng/ml are positive. Each circle represents a single patient, and positive results are connected with diagonal lines. Open circles represent samples from patients with coccidioidomycosis which had positive results with EDTA-heat treatment but negative results without treatment. The open triangle represents samples from a patient with histoplasmosis, and the open squares represent three patients with blastomycosis who had positive results only for EDTA-heat-treated serum samples. The number indicated below the cutoff line represents specimens that were negative. Abbreviations: Cocci, coccidioidomycosis; Histo, histoplasmosis; Blasto, blastomycosis; Kern, Kern County, CA; Memphis, Memphis, TN.
Among controls with histoplasmosis, positive results were not observed in any of the 13 patients whose samples were not EDTA-heat treated compared to a positive result observed in 1 of 13 patients whose samples were treated, with a level of 0.08 ng/ml. The Histoplasma antigen concentration was 28.35 ng/ml in the specimen that cross-reacted in the Coccidioides assay. Among those with blastomycosis, positive results were not observed in any of the five patients whose samples were not EDTA-heat treated, and positive results were observed in three of five patients whose samples were EDTA-heat treated, each at a low level between 0.07 and 0.09 ng/ml. The Blastomyces antigen levels obtained were 2.29, 8.16, and 19.67 units in the three cross-reactive specimens. With both groups combined, cross-reactions were present in samples from 4 of 18 (22.2%) patients. False-positive results, with or without EDTA-heat treatment, were not observed in samples obtained from healthy subjects from an area of disease endemicity (n = 69; Kern County, CA) or nonendemicity (n = 25; Memphis, TN).
DISCUSSION
EDTA-heat treatment improved the sensitivity for the detection of antigenemia in samples from patients with coccidioidomycosis. Antigenemia was detected in EDTA-heat-treated serum samples for 73.1% of patients compared to untreated samples for 28.6% of patients. Antigenuria was present in 50% of patients. Antigen was detected in the urine specimen but not the serum specimen for a single patient. In previous studies, EDTA-heat treatment decreased Histoplasma antigen levels in urine specimens, probably because of the dilution that occurs when 200 μl EDTA is added to 600 μl of the specimen (7). Those findings suggested that Histoplasma antigen in urine specimens was not bound to antibodies and that dissociation of immune complexes did not increase the sensitivity for the detection of antigenuria. Consequently, we did not study the effect of EDTA-heat treatment on the detection of Coccidioides antigenuria. Antigenuria was present in a higher proportion of patients (71%) in our earlier study (2). One possible reason for the lower positivity for antigenuria in this report is the inclusion of milder cases of coccidioidomycosis. In support of this possibility are the higher rates of culture positivity (2.7-fold higher [87.5% and 32.1%]), immunosuppression (2.0-fold higher [79.2% and 39.3%]), and mortality (1.5-fold higher [29.2% and 19.0%]) in the earlier study (2). Prior therapy may also have contributed to the lower rate of antigenuria in this study, as follows: 47.1% of patients (8 of 17) in this study had received treatment for 3 weeks or more before specimens were obtained compared to 4.2% (1 of 24) in the earlier study (2). As most of the patients in this and our earlier study (2) had progressive coccidioidomycosis, the role of antigen testing for diagnosis of the more common self-limited forms of coccidioidomycosis remains unknown.
Specificity was excellent in healthy subjects. Earlier studies demonstrated positive results in urine specimens from 10.7% of patients with histoplasmosis or blastomycosis and 1% with nonfungal infections, but none were positive in healthy subjects. Cross-reactions were detected in 22.2% of patients with histoplasmosis or blastomycosis, but at very low concentrations (0.07 to 0.09 ng/ml).
Serologic tests were positive for all patients for whom information was available. However, of those with positive results, EIA was the sole basis for seropositivity in 36.8% of patients. In comparison to immunodiffusion, the EIA is less specific (6), and the role of EIA seropositivity as the sole laboratory basis for diagnosis of coccidioidomycosis remains unclear (9).
In summary, EDTA-heat treatment markedly improved the sensitivity for the detection of Coccidioides antigenemia. Additional studies are needed to fully assess the added benefit of testing urine specimens as well, but until such studies are completed, we encourage combined testing.
Acknowledgments
L.J.W., S.S., M.D., and E.H. are employees of MiraVista Diagnostics, the company performing the MVista Coccidioides antigen EIA, and MiraBella Technologies, the company that produces the MVista Coccidioides antigen EIA.
Footnotes
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Connolly, P. A., M. M. Durkin, A. M. LeMonte, E. J. Hackett, and L. J. Wheat. 2007. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 14:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durkin, M., P. Connolly, T. Kuberski, R. Myers, B. M. Kubak, D. Bruckner, D. Pegues, and L. J. Wheat. 2008. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin. Infect. Dis. 47:e69-e73. [DOI] [PubMed] [Google Scholar]
- 3.Durkin, M., J. Witt, A. LeMonte, B. Wheat, and P. Connolly. 2004. Antigen assay with the potential to aid in diagnosis of blastomycosis. J. Clin. Microbiol. 42:4873-4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galgiani, J. N., K. O. Dugger, J. I. Ito, and M. A. Wieden. 1984. Antigenemia in primary coccidioidomycosis. Am. J. Trop. Med. Hyg. 33:645-649. [DOI] [PubMed] [Google Scholar]
- 5.Galgiani, J. N., G. M. Grace, and L. L. Lundergan. 1991. New serologic tests for early detection of coccidioidomycosis. J. Infect. Dis. 163:671-674. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman, L., A. S. Sekhon, N. Moledina, M. Jalbert, and D. Pappagianis. 1995. Comparative evaluation of commercial Premier EIA and microimmunodiffusion and complement fixation tests for Coccidioides immitis antibodies. J. Clin. Microbiol. 33:618-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swartzentruber, S., A. LeMonte, J. Witt, D. Fuller, T. Davis, C. Hage, P. Connolly, M. Durkin, and L. J. Wheat. 2009. Improved detection of Histoplasma antigenemia following dissociation of immune complexes. Clin. Vaccine Immunol. 16:320-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner, M. H. 1983. Antigenemia detected in human coccidioidomycosis. J. Clin. Microbiol. 18:136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheat, L. J. 2009. Approach to the diagnosis of the endemic mycoses. Clin. Chest Med. 30:379-389. [DOI] [PubMed] [Google Scholar]