Abstract
Tuberculosis (TB) remains one of the most important infectious diseases of humans and animals. Mycobacterium bovis BCG, the only currently available TB vaccine, demonstrates variable levels of efficacy; therefore, a replacement or supplement to BCG is required. Protein subunit vaccines have shown promise but require the use of adjuvants to enhance their immunogenicity. Using the protective mycobacterial antigen Rv3019c, we have evaluated the induction of relevant immune responses by adjuvant formulations directly in the target species for bovine TB vaccines and compared these to responses induced by BCG. We demonstrate that two classes of adjuvant induce distinct immune phenotypes in cattle, a fact not previously reported for mice. A water/oil emulsion induced both an effector cell and a central memory response. A cationic-liposome adjuvant induced a central memory response alone, similar to that induced by BCG. This suggests that water/oil emulsions may be the most promising formulations. These results demonstrate the importance of testing adjuvant formulations directly in the target species and the necessity of measuring different types of immune response when evaluating immune responses.
Tuberculosis (TB) caused by infection with Mycobacterium tuberculosis or M. bovis remains one of the most important infectious diseases of humans or animals, respectively, and continues to inflict a huge cost in both health and financial terms (3). The only currently available human TB vaccine, M. bovis bacillus Calmette-Guérin (BCG), demonstrates variable levels of efficacy in humans and cattle (4, 9); therefore, there is an urgent need for a new vaccine to replace or supplement BCG. In various models, subunit vaccines against TB have demonstrated promising efficacy when used alone but especially when used in conjunction with BCG in a “prime-boost” strategy (12, 17, 21, 29).
One requirement for the development of novel protein subunit vaccines is the need for the antigen to be administered as an adjuvant to elicit a suitable protective immune response, as purified protein or peptide antigens are poorly immunogenic when administered on their own (19). In particular, in view of the predominant protective role of CD4+ Th1 responses in TB (21), any prophylactic vaccine needs to induce cellular immunity driving these responses.
Another consideration in the rational design and formulation of adjuvants is that of which particular immune parameters (correlates) are predictive of protective vaccination against a particular disease. In some cases this is known, e.g., with bacterial meningitis, where production of a certain titer of bactericidal antibodies directed against the antigen is sufficient (2). In the case of more complex infections, such as TB, these protective immune parameters are unknown, although, as mentioned, evidence is clear that at the very least cellular Th1 responses are essential (21). Recent evidence from studies of experimental Leishmania major vaccines suggests that correlates to predict protective immunity are very complex (6). Data from our laboratory have demonstrated that the induction and measurement of central memory responses in cattle may be a potential correlate of protective immunity in cattle.
The great majority of research into novel adjuvant formulations and their mode of action is conducted with laboratory animal species, notably mice. However, research in our laboratory has shown that immunization of cattle with adjuvant formulations optimized for mice does not always translate to the same results (P. J. Hogarth and H. M. Vordermeier, unpublished). Here we sought to evaluate the efficacies of a number of adjuvants to induce relevant cellular immune responses to Rv3019c, a protective TB antigen (10), directly in the desired target species of cattle and compare these to responses induced by the only currently available TB vaccine, BCG.
MATERIALS AND METHODS
Animals.
Holstein-Friesian calves were obtained from herds free of bovine TB in areas where TB is not endemic and were used at 6 months of age. Disease-free status was confirmed, and calves were selected on the basis of low-level reactivity to M. bovis and M. avium purified protein derivative (PPD) by use of a gamma interferon (IFN-γ) Bovigam test (Prionics, Switzerland), as described previously (26).
Animals were housed in appropriate biological containment facilities at VLA, and work was carried out in accordance with the Animals (Scientific Procedures) Act 1986 (Home Office, London, United Kingdom), following local ethical review.
Adjuvants and immunization.
A total of six different adjuvants were evaluated. ISA50, ISA70, and ISA206 mineral oil-based adjuvants were obtained from Seppic (France) and formulated according to manufacturer instructions. Briefly, antigen was added to the recommended volume of phosphate-buffered saline (PBS) and adjuvant. This formulation was then passed 10 times through a syringe fitted with a 21-gauge needle. “Triple” adjuvant was a formulation of 70% ISA70, 12 mg/ml Quil-A (Brenntag Biosector, Denmark), and 120 mg/ml DEAE-dextran (Amersham, United Kingdom) (P. Wood, personal communication). The cationic-liposome adjuvants used were CAF01 low (dimethyldioctadecyl bromide [DDA], 2,500 μg/ml, and trehalose 6,6′-dibehenate [TDB], 500 μg/ml) and CAF01 high (DDA, 5,000 μg/ml, and TDB, 1,000 μg/ml); these adjuvants were obtained from the Statens Serum Institut (SSI, Demark) and prepared as previously described (7). All adjuvants were administered in a 1-ml dose containing 100 μg of recombinant Rv3019c antigen (Lionex, Germany).
Two immunization trials were performed as described in Table 1. Calves (n = 5 or 6) were immunized with either PBS or an adjuvant/antigen formulation subcutaneously at week 0 and week 4. An additional group was immunized with a single dose of 1 × 106 CFU of BCG Danish 1331 (SSI, Denmark) intradermally at week 0. In the first experiment, the BCG-immunized group was boosted with ISA70/antigen 14 weeks following the initial vaccination.
TABLE 1.
Design of adjuvant screening experiments
| Expt no. and groupa | Immunization formulation | Immunization schedule (wk) |
|---|---|---|
| Expt 1 | ||
| A | None/Rv3019c | 0 and 4 |
| B | ISA50/Rv3019c | 0 and 4 |
| C | ISA70/Rv3019c | 0 and 4 |
| D | ISA206/Rv3019c | 0 and 4 |
| E | Triple/Rv3019c | 0 and 4 |
| F | BCG | 0b |
| Expt 2 | ||
| A | None/Rv3019c | 0 and 4 |
| B | CAF01 low/Rv3019c | 0 and 4 |
| C | CAF01 high/Rv3019c | 0 and 4 |
| D | ISA70/Rv3019c | 0 and 4 |
| E | BCG | 0 |
For experiment 1, each group consisted of six calves; for experiment 2, each group consisted of five calves.
ISA70/Rv3019c boost at week 14.
Cell isolation.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized cattle blood before vaccination and at the time points postvaccination indicated in the figures by Histopaque-1077 (Sigma) gradient centrifugation and cultured in RPMI 1640 (Life Technologies, United Kingdom) supplemented with 5% fetal bovine serum (Sigma), nonessential amino acids (Sigma), 5 × 10−5 M 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (Gibco, United Kingdom).
Whole-blood IFN-γ ELISA.
Measurement of antigen-stimulated IFN-γ in whole-blood cultures was determined by culturing duplicate 250-μl aliquots of blood in the presence of 10 μg/ml of Rv3019c, bovine or avian PPD (VLA-Weybridge, United Kingdom), or RPMI 1640 medium alone. Plasma supernatants were harvested following a 20-h incubation at 37°C and 5% CO2 and tested for IFN-γ by use of a Bovigam bovine IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (Prionics, Switzerland). For reporting, IFN-γ optical density values at 450 nm were transformed into ng/ml by generating a standard curve using recombinant bovine IFN-γ (Endogen).
Antigen-specific IgG ELISA.
Serum levels of Rv3019c-specific immunoglobulin G (IgG) were measured by ELISA as previously described (32), with the minor modification that the plates were coated with 2 μg/ml recombinant Rv3019c.
Ex vivo IFN-γ ELISPOT assay.
Ex vivo enzyme-linked immunospot (ELISPOT) assays to enumerate effector T cells were performed according to manufacturer instructions (Mabtech, Sweden), with minor modifications. Briefly, ELISPOT plates (MAIP S4510; Millipore) were coated overnight at 4°C with a bovine IFN-γ-specific monoclonal antibody. Wells were blocked with 10% fetal calf serum in RPMI 1640 medium, and PBMC suspended in supplemented RPMI were added (2 × 105 PBMC/well) and cultured in the presence of bovine PPD or Rv3019c at 10 μg/ml for 24 h in a humidified 37°C, 5% CO2 incubator. Plates were washed once with distilled H2O and three times with PBS-0.05% Tween 20 (PBS-T). A pan-biotin IFN-γ monoclonal antibody was added (1 h at room temperature), and plates were washed three times with PBS-T. This was followed by incubation with streptavidin-horseradish peroxidase (1 h at room temperature) and three washes with PBS-T. Spots were visualized using a 3-amino-9-ethylcarbazole substrate (Sigma).
Cultured IFN-γ ELISPOT assay.
Cultured ELISPOT assays to enumerate memory T cells were performed using a modification of a previously described method (32). Briefly, short-term-cultured T-cell lines were generated by stimulating 2 × 106 PBMC/ml with antigen (Rv3019c, 5 μg/ml). Recombinant human interleukin-2 (IL-2) (10 U/ml; Sigma, United Kingdom) was added to the PBMC cultures at days 5 and 8. Fresh medium without IL-2 was added to the cultures at days 10 and 12. The total culture period was 13 days.
On day 12, ELISPOT plates (Millipore) were coated with anti-IFN-γ (Mabtech, Sweden) as described above. The following day, wells were blocked with 10% fetal calf serum in RPMI 1640 medium (2 h), and autologous PBMC suspended in supplemented RPMI were added (2 × 105 PBMC/well) for 2 h (37°C, 5% CO2). Nonadherent cells were aspirated, autologous cells were added from the short-term culture (2 × 104 cells/well), and the mixture was incubated overnight (37°C, 5% CO2). The following day, the ELISPOT plate was processed as described above.
Cellular proliferation assay.
Cellular proliferation to Rv3019c was measured by a [3H]thymidine incorporation assay, as described previously (28).
Statistical analysis.
Statistical analysis was performed by nonparametric analysis of variance (ANOVA) with a Tukey-Kramer multiple comparison test, using the statistical package Prism InStat 3.
RESULTS
Water-in-oil emulsions induce rapid and sustained effector responses in cattle.
IFN-γ is an essential component of the protective immune response against TB. To analyze the antigen-specific component of the effector responses induced by vaccination, we measured the ex vivo frequencies of IFN-γ-producing cells induced by vaccination with (i) water/oil emulsion-based adjuvants or (ii) cationic-liposome-based adjuvants.
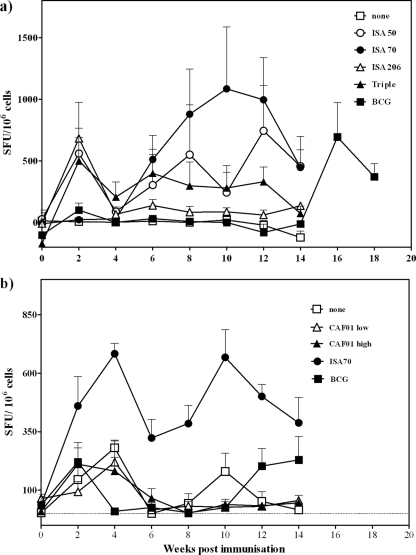
Vaccination with protein and adjuvants based on water-in-oil emulsions (ISA50 and ISA70) but not water/oil/water emulsions (ISA206) induced a statistically significant, rapid, sustained induction of effector cells, as assessed by ex vivo antigen-induced IFN-γ ELISPOT assay responses in PBMC (Fig. 1a). ISA70-induced responses showed a noticeably more pronounced boosting effect than ISA50-induced responses, indicating a clear anamnestic immune response. ISA70-induced responses were significantly stronger than control-induced responses from week 8 (P < 0.05, ANOVA) and ISA50-induced responses from week 12 (P < 0.05, ANOVA).There were no other statistically significant differences between the groups.
FIG. 1.
Kinetics of IFN-γ-producing cells following immunization with water-in-oil emulsion-based (a) or cationic-liposome-based (b) adjuvant formulations. Animals were immunized at weeks 0 and 4. At week 14, the group immunized with BCG was boosted with an immunization of antigen formulated in ISA70. Points represent the mean (±standard error) spot-forming units (SFU) induced by stimulation with Rv3019c, normalized to SFC/106 cells.
In a separate experiment (Fig. 1b), vaccination with protein in adjuvants based on cationic liposomes did not induce a measurable effector cell response, whereas a positive-control adjuvant (ISA70) induced significantly stronger responses than the controls did from week 4 (P < 0.05, week 4; P < 0.0005, weeks 6 to 12; P < 0.005, week 14; ANOVA) in this experiment. There were no other statistically significant differences between the groups.
BCG vaccination induced a weak and transient effector cell response in both experiments shown (Fig. 1a and b).
Levels of IFN-γ produced in antigen-specific in vitro recall assays were also determined in both experiments, and results showed the same patterns as for the ELISPOT assays (data not shown).
Encouragingly, the best-performing adjuvant (based on effector responses), ISA70, was able to significantly boost responses induced by BCG vaccination, reaffirming the potential for BCG prime-protein boost vaccination protocols (P < 0.05, weeks 16 and 18 compared to preboost week 14; ANOVA) (Fig. 1a).
Specific antibody levels mirror IFN-γ-based effector cell responses.
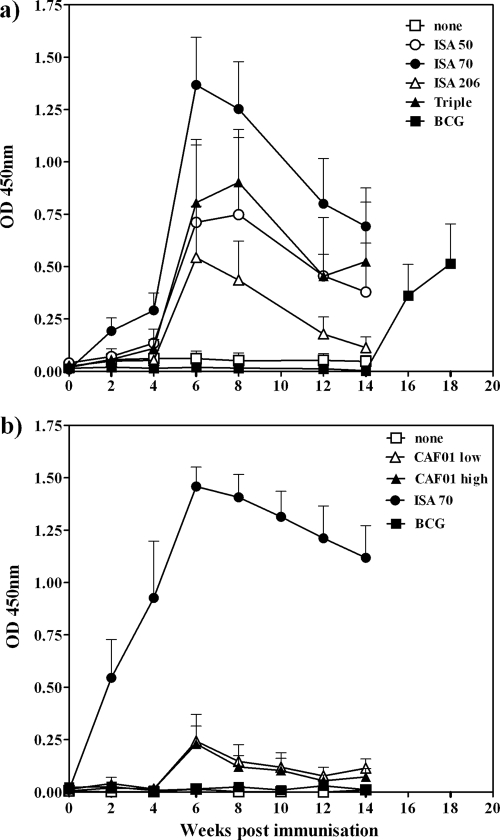
The antigen-specific IgG levels in the sera from immunized cattle throughout both experiments were also measured. As can be seen in Fig. 2, these IgG levels mirror the responses measured by the ELISPOT readout. In the first experiment, ISA70-induced responses were significantly stronger than responses for the control group (P < 0.05) and the group receiving BCG (P < 0.01) at weeks 4 to 12 (Fig. 2a). In the second experiment, only ISA70 induced significantly stronger responses than the controls did (P < 0.01 versus all other groups at weeks 2 and 4; P < 0.001 versus other groups at all other time points) (Fig. 2b).
FIG. 2.
Kinetics of antigen-specific serum IgG production following immunization with water-in-oil emulsion-based (a) or cationic-liposome-based (b) adjuvant formulations. Animals were immunized at weeks 0 and 4. At week 14, the group immunized with BCG was boosted with an immunization of antigen formulated in ISA70. Points represent the mean (±standard error) Rv3019c-specific serum IgG levels, as measured by optical density at 450 nm (OD 450nm).
It is also interesting to note that, despite failing to induce an IFN-γ response, the emulsion formulation ISA206 did induce an IgG response, although not a significant one. Antibody responses were observed only with BCG-immunized cattle following a boosting immunization with ISA70 at week 14 (Fig. 2a).
Cationic-liposome-formulated adjuvants are able to induce central memory responses.
In light of the weak effector responses induced by the cationic-liposome formulations, we sought to investigate whether these vaccines induced other, immunologically relevant responses. This was determined by measuring antigen-specific T-cell proliferation as a surrogate for IL-2 production, as no bovine-specific IL-2 reagents are available. Central T-cell memory responses were measured using a cultured IFN-γ ELISPOT assay.
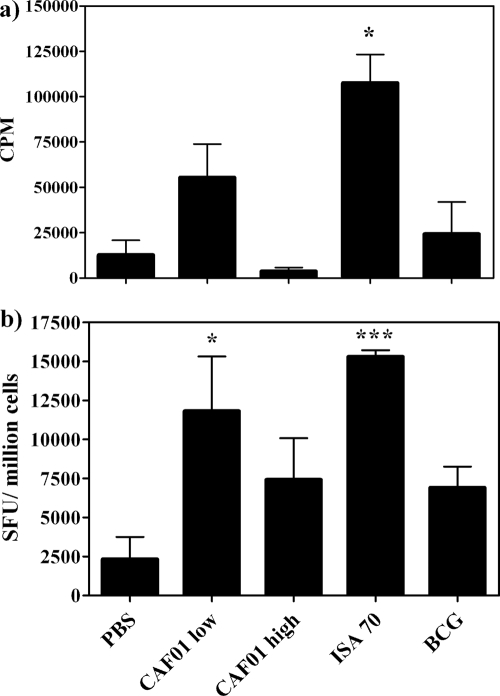
The assessment of T-cell proliferation revealed that the cationic-liposome formulation CAF01 induced a moderate response at the highest dose, approximately half of the magnitude of the ISA70 formulation, while BCG immunization did not induce a T-cell proliferative response (Fig. 3a). Only responses induced by ISA70 were statistically significantly different from control responses (P < 0.05, ANOVA).
FIG. 3.
Assessment of T-cell proliferation (a) and assessment of central memory function by cultured ELISPOT assay (b) in PBMC 12 weeks following vaccination and boost with different adjuvant formulations. (a) Rv3019c-induced proliferation, expressed as counts per minute (CPM), as determined by [3H]thymidine incorporation. Bars represent means ± standard errors (SE). (b) IFN-γ production following cultured ELISPOT assay, expressed as spot-forming units (SFU) induced by stimulation with Rv3019c, normalized to SFC/106 cells. Bars represent means ± SE. *, P < 0.05; ***, P < 0.0005 (versus PBS controls; ANOVA).
The measurement of central memory responses, however, showed that CAF01 induced statistically significant robust responses above the level for the control (P < 0.05, ANOVA), approaching the level for the reference formulation ISA70 (P < 0.0005, ANOVA), in a dose-dependent manner. BCG induced moderate central memory responses (Fig. 3b).
DISCUSSION
We demonstrate that immunization of cattle with different formulations of adjuvant induces two immunologically distinct phenotypes of measured responses. Mineral oil-based formulations induced both effector and central memory responses, while cationic-liposome-based formulations induced weak effector responses but strong central memory responses, similarly to the live attenuated BCG vaccine, which is currently the “gold standard” for animal models.
The mechanisms by which water-in-oil emulsions operate are still not fully defined, but it is likely that these distinct phenotypes are due to differing mechanisms. It is becoming clear, however, that the formulations also express similarities; for example, both formulations used in this study can act via Toll-like receptor-independent mechanisms (8, 24).
The modes of action of DDA-TDM formulations have been studied extensively (5, 14, 23). These formulations operate via a synergistic mechanism of a delivery vehicle (DDA liposomes) promoting antigen delivery and uptake to antigen-presenting cells, interacting with the immunomodulatory component (TDB). TDB acts in a MyD88-dependent but a Toll-like receptor 2, 3, 4, and 7-independent manner (1), with a defined molecular activation pathway (31). DDA-TDM formulations are potent Th1 and IFN-γ inducers in mice (1, 11, 23) and promote long-term protective immune responses, characterized by high levels of multifunctional T cells (16).
In light of the potent effector cellular immune response induced by cationic liposomes in mice and the protective effect observed to occur in mice, guinea pigs, and nonhuman primates (1, 15, 20), the weak discernible effector responses in cattle were unexpected. This could be due to a number of reasons. It is possible that the dose of the cationic-liposome formulation was not optimal for cattle, although the responses observed using the cultured ELISPOT assay as a marker of memory cell activation indicate that the dose used was capable of inducing relevant immune responses. These results also suggest that these formulations are not murine specific, thus discounting species specificity as a reason for the lack of effector cell responses. It is also possible that cattle require a particularly reactogenic adjuvant formulation. The cattle used in these studies are not housed under the usual specific-pathogen-free facilities in which laboratory animals are kept and are constantly under immunogenic exposure. Due to the potentially high level of continuing immune responses in cattle, perhaps preexisting regulatory mechanisms require a particularly reactive formulation to cross a threshold and instigate an effector response. We have previously observed immunization regimens which induce memory responses in the absence of demonstrable active ex vivo responses (27), but the mechanisms remain unclear.
The major caveat associated with the use of a subunit vaccine is the necessity for coadministration in an effective adjuvant formulation. Clearly, the most appropriate method of evaluating adjuvant formulations is that of assessing levels of protection induced, using known protective subunit antigens. The species specificity of adjuvants necessitates the use of the target species, and given the enormous costs involved in conducting these experiments with cattle, this becomes an unrealistic proposition. The challenge is then to determine on which immune parameter to base adjuvant evaluation in the absence of protection data.
In the case of TB, specific correlates of immunity are unknown. IFN-γ production is known to be an essential component, but its detection based on ex vivo responses does not reliably predict vaccine success whether total or individual T-cell IFN-γ responses are measured (18). Indeed, as demonstrated in previous studies, BCG immunization, which induces significant protection against bovine TB, often induces a weak and transient IFN-γ effector cell response (26).
It has been proposed that the evaluation of central memory responses may represent a better correlate of immunity. In malaria vaccination trials, the measurement of central memory T-cell responses by a cultured but not ex vivo IFN-γ ELISPOT assay represents a correlate of protection (13, 22). In our previous studies (25, 27, 30, 32), we have reported an association between protective immunity and cultured ELISPOT assay responses and so have measured these to elucidate whether vaccination with a protein subunit formulated with adjuvants induces these responses.
The established efficacy of BCG suggests that weak effector but strong central memory induces protection. As defined immune correlates are unknown, it is not known what the contribution of a concomitant strong effector response as induced by ISA70 may be and which one (or both) of these phenotypes would result in the most effective protective response.
This highlights several factors that are relevant in adjuvant screening regimens. First, there is a need to use relevant assays to screen the induction of different types of immune responses in adjuvant testing protocols. Second, there is a need for adjuvant screening of the target species (or those with proven comparability to the target species). Finally, it is important to establish reliable correlates of protection, such that promising vaccine formulations could be tested without the need to progress to challenge. This would greatly reduce the cost of such studies and accelerate vaccine discovery and evaluation.
Acknowledgments
We are grateful for the excellent services provided by the VLA Animal Services Unit.
This work was funded by the Department for Environment, Food and Rural Affairs (Defra), United Kingdom, under contract SE3224.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Agger, E. M., I. Rosenkrands, J. Hansen, K. Brahimi, B. S. Vandahl, C. Aagaard, K. Werninghaus, C. Kirschning, R. Lang, D. Christensen, M. Theisen, F. Follmann, and P. Andersen. 2008. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS ONE 3:e3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashford, D. A., E. Whitney, P. Raghunathan, and O. Cosivi. 2001. Epidemiology of selected mycobacteria that infect humans and other animals. Rev. Sci. Tech. 20:325-337. [DOI] [PubMed] [Google Scholar]
- 4.Bloom, B. R., and P. E. M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis, p. 531-557. In B. H. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC.
- 5.Christensen, D., K. S. Korsholm, I. Rosenkrands, T. Lindenstrom, P. Andersen, and E. M. Agger. 2007. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 6:785-796. [DOI] [PubMed] [Google Scholar]
- 6.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843-850. [DOI] [PubMed] [Google Scholar]
- 7.Davidsen, J., I. Rosenkrands, D. Christensen, A. Vangala, D. Kirby, Y. Perrie, E. M. Agger, and P. Andersen. 2005. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—a novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta 1718:22-31. [DOI] [PubMed] [Google Scholar]
- 8.Gavin, A. L., K. Hoebe, B. Duong, T. Ota, C. Martin, B. Beutler, and D. Nemazee. 2006. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314:1936-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogarth, P. J., R. G. Hewinson, and H. M. Vordermeier. 2006. Development of vaccines against bovine tuberculosis. J. Pharm. Pharmacol. 58:749-757. [DOI] [PubMed] [Google Scholar]
- 10.Hogarth, P. J., K. E. Logan, H. M. Vordermeier, M. Singh, R. G. Hewinson, and M. A. Chambers. 2005. Protective immunity against Mycobacterium bovis induced by vaccination with Rv3019c-a member of the esat-6 gene family. Vaccine 23:2557-2564. [DOI] [PubMed] [Google Scholar]
- 11.Holten-Andersen, L., T. M. Doherty, K. S. Korsholm, and P. Andersen. 2004. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect. Immun. 72:1608-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann, S. H. 2006. Envisioning future strategies for vaccination against tuberculosis. Nat. Rev. Immunol. 6:699-704. [DOI] [PubMed] [Google Scholar]
- 13.Keating, S. M., P. Bejon, T. Berthoud, J. M. Vuola, S. Todryk, D. P. Webster, S. J. Dunachie, V. S. Moorthy, S. J. McConkey, S. C. Gilbert, and A. V. Hill. 2005. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J. Immunol. 175:5675-5680. [DOI] [PubMed] [Google Scholar]
- 14.Korsholm, K. S., E. M. Agger, C. Foged, D. Christensen, J. Dietrich, C. S. Andersen, C. Geisler, and P. Andersen. 2007. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology 121:216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langermans, J. A., T. M. Doherty, R. A. Vervenne, T. van der Laan, K. Lyashchenko, R. Greenwald, E. M. Agger, C. Aagaard, H. Weiler, D. van Soolingen, W. Dalemans, A. W. Thomas, and P. Andersen. 2005. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 23:2740-2750. [DOI] [PubMed] [Google Scholar]
- 16.Lindenstrom, T., E. M. Agger, K. S. Korsholm, P. A. Darrah, C. Aagaard, R. A. Seder, I. Rosenkrands, and P. Andersen. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182:8047-8055. [DOI] [PubMed] [Google Scholar]
- 17.Ly, L. H., and D. N. McMurray. 2008. Tuberculosis: vaccines in the pipeline. Expert Rev. Vaccines 7:635-650. [DOI] [PubMed] [Google Scholar]
- 18.Mittrucker, H. W., U. Steinhoff, A. Kohler, M. Krause, D. Lazar, P. Mex, D. Miekley, and S. H. Kaufmann. 2007. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. USA 104:12434-12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Hagan, D. T., and R. Rappuoli. 2004. Novel approaches to vaccine delivery. Pharm. Res. 21:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen, A. W., A. Williams, L. M. Okkels, G. Hatch, and P. Andersen. 2004. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect. Immun. 72:6148-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orme, I. M. 2006. Preclinical testing of new vaccines for tuberculosis: a comprehensive review. Vaccine 24:2-19. [DOI] [PubMed] [Google Scholar]
- 22.Reece, W. H., M. Pinder, P. K. Gothard, P. Milligan, K. Bojang, T. Doherty, M. Plebanski, P. Akinwunmi, S. Everaere, K. R. Watkins, G. Voss, N. Tornieporth, A. Alloueche, B. M. Greenwood, K. E. Kester, K. P. McAdam, J. Cohen, and A. V. Hill. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406-410. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkrands, I., E. M. Agger, A. W. Olsen, K. S. Korsholm, C. S. Andersen, K. T. Jensen, and P. Andersen. 2005. Cationic liposomes containing mycobacterial lipids: a new powerful Th1 adjuvant system. Infect. Immun. 73:5817-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su, S. B., P. B. Silver, R. S. Grajewski, R. K. Agarwal, J. Tang, C. C. Chan, and R. R. Caspi. 2005. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 175:6303-6310. [DOI] [PubMed] [Google Scholar]
- 25.Vordermeier, H. M., B. Villarreal-Ramos, P. J. Cockle, M. McAulay, S. G. Rhodes, T. Thacker, S. C. Gilbert, H. McShane, A. V. S. Hill, Z. Xing, and R. G. Hewinson. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vordermeier, H. M., K. Huygen, M. Singh, R. G. Hewinson, and Z. Xing. 2006. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect. Immun. 74:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vordermeier, H. M., R. Pontarollo, B. Karvonen, P. Cockle, R. Hecker, M. Singh, L. A. Babiuk, R. G. Hewinson, and S. van Drunen Littel-van Den Hurk. 2005. Synthetic peptide vaccination in cattle: induction of strong cellular immune responses against peptides derived from the Mycobacterium bovis antigen Rv3019c. Vaccine 23:4375-4384. [DOI] [PubMed] [Google Scholar]
- 29.Wang, J., and Z. Xing. 2002. Tuberculosis vaccines: the past, present and future. Expert Rev. Vaccines 1:341-354. [DOI] [PubMed] [Google Scholar]
- 30.Waters, W. R., M. V. Palmer, B. J. Nonnecke, T. C. Thacker, C. F. Scherer, D. M. Estes, R. G. Hewinson, H. M. Vordermeier, S. W. Barnes, G. C. Federe, J. R. Walker, R. J. Glynne, T. Hsu, B. Weinrick, K. Biermann, M. H. Larsen, and W. R. Jacobs, Jr. 2009. Efficacy and immunogenicity of Mycobacterium bovis DeltaRD1 against aerosol M. bovis infection in neonatal calves. Vaccine 27:1201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werninghaus, K., A. Babiak, O. Gross, C. Holscher, H. Dietrich, E. M. Agger, J. Mages, A. Mocsai, H. Schoenen, K. Finger, F. Nimmerjahn, G. D. Brown, C. Kirschning, A. Heit, P. Andersen, H. Wagner, J. Ruland, and R. Lang. 2009. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ-Syk-Card9-dependent innate immune activation. J. Exp. Med. 206:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whelan, A. O., D. C. Wright, M. A. Chambers, M. Singh, R. G. Hewinson, and H. M. Vordermeier. 2008. Evidence for enhanced central memory priming by live Mycobacterium bovis BCG vaccine in comparison with killed BCG formulations. Vaccine 26:166-173. [DOI] [PubMed] [Google Scholar]