Abstract
In previous studies, we have demonstrated that liposomes with differential lipid components display differential adjuvant effects when antigens (Ags) are chemically coupled to their surfaces. When ovalbumin was coupled to liposomes made by using unsaturated fatty acids, it was found to be presented not only to CD4+ T cells but also to CD8+ T cells and induced cytotoxic T lymphocytes (CTLs) which effectively eradicated the tumor from mice. In this study, we coupled liposomes to immunodominant CTL epitope peptides derived from lymphocytic choriomeningitis virus (LCMV) and evaluated its potency as an antiviral vaccine. The intramuscular immunization of mice with the peptide-liposome conjugates along with CpG resulted in the efficient induction of antiviral CD8+ T-cell responses which conferred complete protection against not only LCMV Armstrong but also a highly virulent mutant strain, clone 13, that establishes persistent infections in immunocompetent mice. The intranasal vaccination induced mucosal immunity effective enough to protect mice from the virus challenge via the same route. Complete protection was achieved in mice even when the Ag dose was reduced to as low as 280 ng of liposomal peptide. This form of vaccination with a single CTL epitope induced Ag-specific memory CD8+ T cells in the absence of CD4+ T-cell help, which could be shown by the complete protection of CD4-knockout mice in 10 weeks as well as by the analysis of recall responses. Thus, surface-linked liposomal peptide might have a potential advantage for the induction of antiviral immunity.
The development of practical vaccines has been greatly potentiated by the availability of synthetic antigens (Ags), but progress has been hampered by the poor immunogenicity of Ags. Liposomes have successfully been used as drug carriers (35) and have also been proposed to be carriers of Ags and adjuvants to induce immune responses (33). Most of the liposomal vaccines proposed have been prepared by Ag encapsulation within the aqueous lumen of liposomes. However, it is known that the immune responses induced by encapsulated liposomal Ags are different from those induced by surface-linked liposomal Ags.
We have demonstrated that Ags chemically coupled to the surfaces of liposomes induce Ag-specific immunoglobulin G (IgG) but not IgE antibody production (28). The inducibility of Ag-specific IgG production was found to vary among liposome preparations: the greater that the membrane mobility in the liposomes is, the greater that the antibody production induced by Ag-liposome conjugates is (29). In our previous study, we have reported that ovalbumin (OVA) coupled to liposomes made with unsaturated fatty acids was presented to both CD4+ and CD8+ T cells, whereas OVA coupled to liposomes made with saturated fatty acids was presented only to CD4+ T cells. Furthermore, the cross-presentation of OVA coupled to liposomes consisting of unsaturated fatty acids was further confirmed by the in vivo induction of cytotoxic T lymphocytes (CTLs) which conferred tumor eradication (42) and protection against influenza virus (27) in mice. However, the advantages of the surface-linked liposomal peptides for other forms of vaccines, especially regarding the efficiency of effector CD8+ T cells and the inducibility of long-term memory CD8+ T cells, have not been demonstrated.
In the present study, we evaluated the potency of surface-coupled liposomal peptides as an antiviral vaccine using the infection of mice with lymphocytic choriomeningitis virus (LCMV) as a model. Use of that model enabled us to compare the effectiveness of this system with that of various vaccine formulas prepared with the same epitope peptide as the Ag reported elsewhere. We also elucidate the induction and maintenance of memory CD8+ T cells by a minimum CTL epitope peptide which does not seem to stimulate the help of CD4 cells.
MATERIALS AND METHODS
Mice and viruses.
Female C57BL/6 mice (age, 6 weeks) were purchased from Clea Japan, Inc. (Tokyo, Japan), and Tokyo Laboratory Animal Science Co. Ltd. (Tokyo, Japan). CD4-knockout (KO) mice of a C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). After they were bred, 6-week-old male CD4-KO mice were used for the experiments. The mice were housed in appropriate animal care facilities at Saitama Medical University (Saitama, Japan) and were handled according to international guidelines. The experimental protocols were approved by the Animal Research Committee of Saitama Medical School (approval number 634). The mice received 2 × 105 PFU of LCMV Armstrong (Arm) intraperitoneally (i.p.) or intranasally (i.n.) to initiate acute infection or 2 × 106 PFU of the mutant strain of LCMV Arm, clone 13 (Cl.13), intravenously to initiate chronic infection (45). The virus titers were determined by plaque assay on Vero cells, as described elsewhere (2). Vaccinia virus recombinant VVGP33 (30), which expresses the LCMV GP33-41 epitope, was provided by J. Lindsay Whitton (Scripps Research Institute, La Jolla, CA).
Reagents.
Synthetic CpG ODN (5002: TCCATGACGTTCTTGATGTT) was purchased from Hokkaido System Science (Sapporo, Japan) and was protected with phosphorothioate to avoid nuclease-dependent degradation. The LCMV peptides used were GP33-41 (KAVYNFATC, H-2Db), NP396-404 (FQPQNGQFI, H-2Db), GP283-291 (GYCLTKWMI, H-2Kd), GP61-80 (GLKGPDIYKGVYQFKSVEFD, I-Ab), and NP309-328 (SGEGWPYIACRTSIVGRAWE, I-Ab). They were synthesized by Operon Biotechnology (Tokyo, Japan) by using 9-fluorenylmethoxy carbonyl chemistry. The peptides were desalted and then analyzed by high-performance liquid chromatography. Mouse fibrosarcoma cell line MC57G (H-2b) was obtained from ATCC and was maintained in Eagle's minimal essential medium containing 10% fetal calf serum (FCS) until use.
Coupling of peptides to liposomes.
The oleoyl liposomes consisted of dioleoyl phosphatidylcholine, dioleoyl phosphatidylethanolamine, dioleoyl phosphatidylglycerol acid, and cholesterol in a 4:3:2:7 molar ratio (29). Each peptide was coupled to the surfaces of the liposomes by the use of disuccinimidyl suberate, as described previously (42). The final preparations contained 0.7 mg of the peptide and 10 mg of the liposome per ml. Liposomes conjugated with the GP33-41 peptide and NP396 (Lip-GP33/NP396), Lip-GP33/GP61, and Lip-GP33/NP309 were made by mixing peptide GP33-41 with peptide NP396-404, peptide GP61-80, and peptide NP309-328, respectively, in equal amounts before conjugation.
Immunization.
The mice received the liposomal peptides at the indicated amounts together with 5 μg of CpG in 100 μl of phosphate-buffered saline (PBS) by one of the three different routes: subcutaneously (s.c.), intramuscularly (i.m.), or i.p. The i.n. immunization was performed by introducing 20 μl of a liposome solution (14 μg of peptide) and 5 μg of CpG (5 μl) into the nasal cavity. Mice that had recovered from an acute infection of LCMV Arm served as positive controls.
51Cr-release assay.
Spleen cells were prepared 7 days after immunization to obtain effector cells. The cytotoxic activities of the GP33-specific CTLs were measured by standard 4-h 51Cr-release assays. For peptide-pulsed target cells, 1 × 106 MC57G cells were pulsed with 10 μM of peptide GP33 for 2 h and then labeled with 100 μCi of Na251CrO4 for 1 h. For virus-infected targets, the MC57G cells were infected with VVGP33 or wild-type vaccinia virus at a multiplicity of infection of 5. After 1 h of infection, the cells were washed, resuspended in medium, and incubated overnight. The infected targets were then labeled with 51Cr, as described above. The labeled target cells were plated in wells of a round-bottom 96-well plate at 2 × 103 cells/well with effector cells at various effector-cell-to-target-cell (E/T) ratios. Blocking by antibodies was performed by adding anti-CD4 or anti-CD8 (5 μg/ml; eBioscience, San Diego, CA) to the mixture. Percent lysis was calculated as [(cpmsample − cpmspontaneous)/(cpmmaximum − cpmspontaneous)] × 100. The maximum amount of 51Cr released was determined with the supernatants of cells that were lysed by addition of 2% Nonidet P-40. The spontaneous release of 51Cr from target cells incubated without effector cells was measured in the supernatants. Percent specific lysis was calculated by subtracting the percent lysis with unpulsed targets from that with peptide-pulsed targets.
In vivo CTL assay.
The in vivo CTL assay was carried out as reported elsewhere (42). Briefly, spleen cells from naive C57BL/6 mice were split into two equal populations. One population was pulsed with a peptide at a final concentration of 10 μM for 1 h at 37°C and then labeled with a high concentration (2.5 μM) of carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 10 min at 37°C. The other was unpulsed and labeled with a lower concentration (0.25 μM) of CFSE. An equal number of cells (1 × 107) from each population was mixed and transferred into immunized mice intravenously. Twelve hours later, spleen cells were prepared and analyzed by flow cytometry. Percent specific lysis was calculated as 1 − [(number of CFSElow cells in healthy mice/number of CFSEhigh cells in healthy mice)/(number of CFSElow cells in immunized mice/number of CFSEhigh cells in immunized mice)] × 100, where CFSElow and CFSEhigh represent low and high CFSE concentrations, respectively.
IFN-γ ELISPOT assay.
The detection of gamma interferon (IFN-γ)-secreting cells was performed by using a mouse IFN-γ enzyme-linked immunospot (ELISPOT) assay set (BD Biosciences-Pharmingen, San Diego, CA). Each well of a sterile 96-well ImmunoSpot ELISPOT assay plate (BD Biosciences-Pharmingen) was precoated with 0.5 μg of unlabeled anti-IFN-γ capture antibody (clone R4-6A2; BD Biosciences-Pharmingen) per well at 4°C overnight. The plates were washed and then blocked with RPMI 1640 containing 10% FCS for 2 h at room temperature. Spleen cells from individual immunized mice were prepared, and red blood cells were removed by treatment with ammonium chloride. After the blocking solution was discarded, effector spleen cells were added to the wells of the plates at two cell densities (105 and 106 cells/well), along with 106 gamma-irradiated (40 Gy) syngeneic spleen cells. The cells were incubated in the presence or the absence of an appropriate peptide at a final concentration of 10 μM at 37°C for 2 days. The cells were then removed by five washes with PBS containing 0.05% Tween 20, followed by the addition of biotinylated anti-mouse IFN-γ detection antibody (clone SMG1.2; BD Biosciences-Pharmingen) at a concentration of 0.5 μg/well. After a 2-h incubation at room temperature, the detection antibody was removed by three washes with PBS containing 0.05% Tween 20. Avidin-horseradish peroxidase was added to the wells. After the unbound avidin-horseradish peroxidase was washed, the spots were developed by using freshly prepared substrate buffer (0.3 mg of 3-amino-9-ethylcarbazole/ml and 0.015% H2O2 in 0.1 M sodium acetate).
Intracellular IFN-γ staining.
Intracellular cytokine staining was performed as described previously (26). Briefly, the spleen cells of four mice per group were pooled and resuspended in RPMI 1640 containing 10% FCS. In each well of a 96-well round-bottom plate, 2 × 106 spleen cells were incubated with 1 × 105 cells of syngeneic spleen cells which had been pulsed with an appropriate peptide for 3 h in the presence of 0.2 μl/well brefeldin A (GolgiPlug; BD Biosciences) for 5 h at 37°C. The cells were then washed once and incubated for 10 min at 4°C with a rat anti-mouse CD16/CD32 monoclonal antibody (MAb; Fc Block; BD Biosciences) at a concentration of 1 μg/well. Following incubation, the cells were stained with a fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8α MAb (clone 53-6.7; BD Biosciences) at a concentration of 0.5 μg/well for 30 min at 4°C. After the cells were washed twice, they were fixed and permeabilized by using a Cytofix/Cytoperm kit (BD Biosciences) and stained with a phycoerythrin (PE)-conjugated rat anti-mouse IFN-γ MAb (clone XMG1.2; BD Biosciences). After the cells were washed, flow cytometric analyses were performed with a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ), and the data were analyzed with CellQuest software (Becton Dickinson). The experiment was repeated three times.
Tetramer and CD62L staining.
Spleen cells were prepared and treated with an anti-mouse CD16/CD32 MAb as described above for intracellular IFN-γ staining and were then stained with a PE-conjugated GP33 H-2Db tetramer (Medical and Biological Laboratories, Nagoya, Japan), peridinin chlorophyll protein-conjugated anti-CD8a (BD Bioscience), and FITC-conjugated anti-CD62L (BD Bioscience) for 30 min at 4°C. After the cells were washed twice, they were fixed with PBS containing 1% formaldehyde and 2% FCS and analyzed by flow cytometry.
Statistical analysis.
Statistical analyses were performed by Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
Induction of LCMV-specific CD8+ T-cell response in mice immunized with liposome-coupled GP33 peptide.
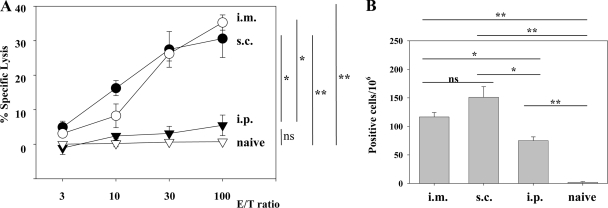
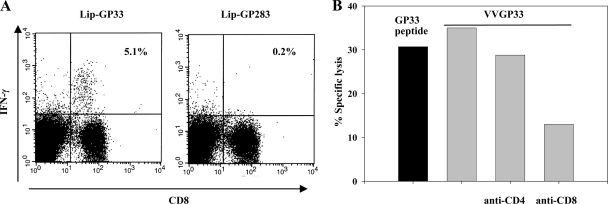
Ag chemically coupled to the surfaces of liposomes composed of unsaturated fatty acids has been shown to be presented to CD8+ T cells (42) and to induce protective immunity against influenza virus (27). For further evaluation of the use of surface-linked liposomal peptide as an antiviral vaccine, we chose an immunodominant CTL epitope, GP33-41, of LCMV, which is presented by the H-2Db molecule (10, 16, 36). H-2Kd-restricted epitope GP283-292 (10) is not presented in C57BL/6 mice (44) and served as the negative control in our experiments. We conjugated liposomes with GP33-41 peptide (Lip-GP33) or GP283-292 (Lip-GP283) and immunized C57BL/6 mice by one of three different routes (s.c., i.m., or i.p.) together with CpG. The immune responses were evaluated by 51Cr-release assays and ELISPOT assays. As shown in Fig. 1A and B, single i.m. and s.c. immunizations induced substantial levels of CTL activity and IFN-γ-producing cells, whereas i.p. immunizations induced much lower responses, and the unconjugated peptide induced no detectable response in either assay (see Fig. 6 for data only for the ELISPOT assay). In the absence of CpG, the responses were very low (data not shown), in accordance with the findings presented in a previous report (27). Therefore, in the following experiments, the immunizations with CpG were done mainly by the i.m. route. We confirmed the specificities of the reactions observed in the experiments described above as follows. Intracellular IFN-γ staining experiments revealed the peptide-specific IFN-γ production by CD8+ T cells (Fig. 2A). 51Cr-release assays with target cells expressing the GP33 epitope by infection with recombinant vaccinia virus VVGP33 demonstrated that the effector CTLs induced by the GP33-liposome conjugates recognized the epitope, which was endogenously processed and presented by major histocompatibility complex class I molecules (Fig. 2B). Blocking experiments with anti-CD4 and anti-CD8 antibodies demonstrated that the CTL activities were exerted by CD8+ T cells (Fig. 2B). The CTL activities induced by the liposome conjugate were also confirmed by in vivo CTL assays (see Fig. 4B).
FIG. 1.
Comparison of three different routes of immunization with Lip-GP33. Each C57BL/6 mouse received 20 μl of Lip-GP33 diluted in 100 μl of PBS containing 5 μg of CpG. Spleen cells were prepared 7 days after immunization for the 51Cr-release assays (A) and ELISPOT assays (B). (A) 51Cr-release assays were performed to detect GP33-specific CTL activity at various E/T ratios by using MC57G cells pulsed with peptide GP33 as targets. (B) IFN-γ-producing cells responding to peptide GP33 were detected by ELISPOT assays, as described in Materials and Methods. Spleen cells from nonimmunized (naive) mice were used as a negative control. The data are representative of those from two independent and reproducible experiments. The results are shown as the means of four mice per group ± standard errors of the means. *, P < 0.05; **, P < 0.01; ns, not statistically significant.
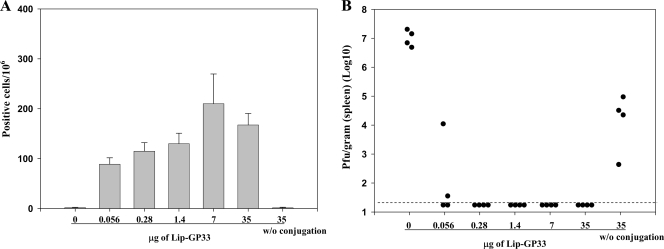
FIG. 6.
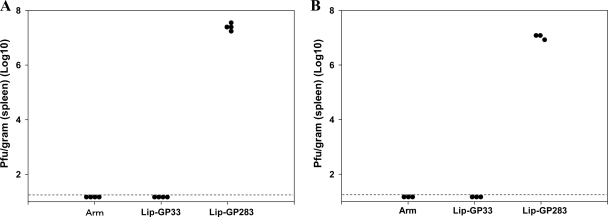
Dose-response experiments. Serial fivefold dilutions of Lip-GP33 starting at 50 μl were prepared in 100 μl of PBS containing 5 μg of CpG and injected i.m. to each C57BL/6 mouse. Seven days later, ELISPOT (IFN-γ) assays (A) and challenge experiments with LCMV Arm (B) were performed. (A) ELISPOT assays were performed with spleen cells from immunized mice. One group of mice received peptide GP33 (35 μg) without (w/o) conjugation, and one group received GP33 at the same dose conjugated with 5 μg of CpG as one of the Lip-GP33 immunizations. The data are representative of those from three independent and reproducible experiments. The results are shown as the means for four mice per group ± standard errors of the means. (B) The immunized mice received 2 × 105 PFU of LCMV Arm i.p., and the virus titers in the spleens were quantitated by plaque assay on Vero cells at day 4 postchallenge. Virus titers are indicated for each animal in the study. The dotted line represents the lower limit of detection (2 × 101 PFU/g [spleen]). The data are representative of those from two independent and reproducible experiments.
FIG. 2.
Confirmation of GP33-specific CD8+ T-cell responses of immunized mice. C57BL/6 mice were injected i.m. with 20 μl of Lip-GP33 or Lip-GP283 diluted in 100 μl of PBS containing 5 μg of CpG, and spleen cells were prepared 7 days later for analysis. (A) The numbers of IFN-γ-producing CD8+ T cells specific to GP33 were determined by intracellular IFN-γ staining. Spleen cells from mice immunized with Lip-GP33 or Lip-GP283 were stimulated with either GP33-pulsed or unpulsed syngeneic spleen cells for 5 h and were then stained to detect their surface expression of CD8 (x axis) with FITC-conjugated MAb and their intracellular expression of IFN-γ (y axis) with PE-conjugated MAb. All lymphocytes were gated and analyzed on a FACScan flow cytometer by the use of CellQuest software (BD Biosciences). The values shown in the upper right quadrants indicate the percentage of CD8+ cells that are positive for intracellular IFN-γ after stimulation with GP33-pulsed spleen cells. The results of stimulation with unpulsed spleen cells were almost zero (data not shown). Each experiment used four mice per group, and the spleen cells of the mice in each group were pooled. The data shown are representative of those from three independent and reproducible experiments. (B) 51Cr-release assays with MC57G cells infected with VVGP33 as targets were performed to detect CTL activity against the endogenously expressed GP33 epitope (gray bars). As control targets, MC57G cells were infected with wild-type vaccinia virus. The same effector cells were used as GP33 peptide-pulsed and unpulsed targets as well for comparison (black bar). Lysis was also tested in the presence of an anti-CD4 or an anti-CD8 MAb (5 μg/ml) in the CTL assay. Specific lysis is demonstrated at an E/T ratio of 50. Each experiment used three mice, and the spleen cells of the three mice were pooled. The data are representative of those from two independent and reproducible experiments.
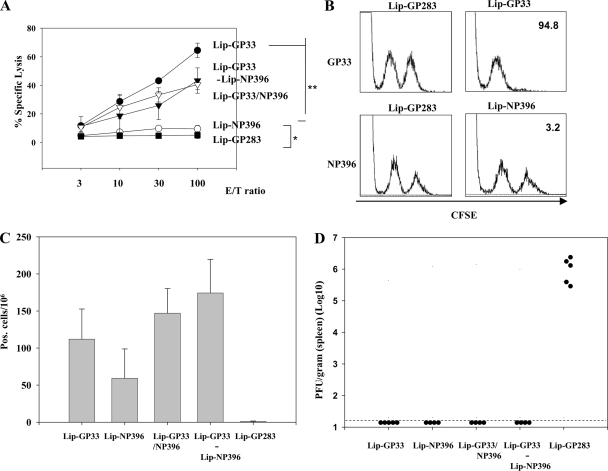
FIG. 4.
Comparison of GP33 and NP396 peptides as vaccine components. C57BL/6 mice were immunized i.m. with either Lip-GP33, Lip-NP396, Lip-GP283, or Lip-GP33/NP396 (20 μl each) or with Lip-GP33-Lip-NP396 (10 μl each) in 100 μl of PBS containing 5 μg of CpG. Seven days later, 51Cr-release assays (A), in vivo CTL assays (B), ELISPOT assays (C), and challenge experiments with LCMV Arm (D) were performed. The 51Cr-release assays (A) and ELISPOT assays (C) were done as described in the legend to Fig. 1. The in vivo CTL assays (B) were performed only for the mice immunized with either Lip-GP33, Lip-NP396, or Lip-GP283. One week after immunization, the mice received an equal number of a relevant peptide (GP33 or NP396)-pulsed CFSEhigh targets and unpulsed CFSElow targets. The numbers show the percentages of specific lysis. The experiment was repeated twice. The challenge experiments with LCMV Arm (D) were performed by the methods described in the legend to Fig. 3A. *, P < 0.05; **, P < 0.01. No statistically significant difference was observed between the four groups by the ELISPOT assays (C).
Experimental challenge of immunized mice with LCMV.
To examine the protective efficacy of Lip-GP33, the immunized mice were challenged with LCMV Arm. Four days after the infection, the virus titers in the spleens of the immunized mice were determined. As LCMV Arm is cleared from all tissues during the first week of infection and infection with this virus results in the formation of functional and protective memory CD8+ T cells (2), the mice which recovered from the LCMV Arm infection were used as positive controls. As shown in Fig. 3A, immunization with Lip-GP33 as well as LCMV Arm infection conferred complete protection to the mice, and the titers in the spleen were below the detection limit (2.0 × 101 PFU/g), whereas the mice immunized with the negative control peptide (Lip-GP283) showed titers of (1.69 ± 0.40) × 107 PFU/g (Fig. 3A). Again, in the absence of CpG, the immunized mice were not protected at all (data not shown). The protective efficacy of Lip-G33 with CpG was further tested by challenging the immunized mice with LCMV Cl.13, a mutant strain of LCMV Arm which is known to induce viremia for ∼3 months, with the virus persisting in some tissues for life (1). Eight days after infection, the mice immunized with Lip-GP33 or LCMV Arm completely cleared the Cl.13 virus, whereas those which received the negative control peptide (Lip-GP283) had a high titer of the virus in the spleen (Fig. 3B), and the persistence of the virus was confirmed 4 weeks later (data not shown).
FIG. 3.
Challenge experiments with vaccinated and control mice. C57BL/6 mice were immunized by injecting Lip-GP33 or Lip-GP283, as described in the legends to Fig. 1 and 2, or by i.p. inoculation of 2 × 105 PFU of LCMV Arm. After 2 weeks, the mice received 2 × 105 PFU of LCMV Arm i.p. to initiate acute infection (A) or 2 × 106 PFU of Cl.13 intravenously to initiate chronic infection (B). The virus titers in the spleens were quantitated by plaque assay on Vero cells at day 4 (A) or day 8 (B) postchallenge. Virus titers are indicated for each animal in the study. A dotted line represents the lower limit of detection (2 × 101 PFU/g [spleen]). The data are representative of those from three independent and reproducible experiments.
Liposome-coupled peptide NP396 induces low CTL responses but complete protection.
The bulk of the CTL response to LCMV in H-2b mice is directed against three dominant epitopes, GP33-41, GP276-286, and NP396-404, which are presented by major histocompatibility complex class I H-2Db molecules (10, 16, 36). We investigated whether peptides other than GP33 were able to induce antiviral T-cell responses when they were coupled to the surfaces of liposomes. Peptide NP396-404 was coupled to liposomes by itself (Lip-NP396) or with peptide GP33 (Lip-GP33/NP396). Mice were immunized with either Lip-GP33, Lip-NP396, Lip-GP33/NP396, or a mixture of Lip-GP33 and Lip-NP396 (Lip-GP33-Lip-NP396), and the responses were compared by ex vivo CTL assays (Fig. 4A), in vivo CTL assays (Fig. 4B), ELISPOT assays (Fig. 4C), and challenge experiments with LCMV Arm (Fig. 4D).
The level of induction of CTL activities by Lip-NP396 was very low in both the ex vivo and the in vivo assays, and Lip-GP33/NP396 and Lip-GP33-Lip-NP396 induced intermediate levels of CTL activity compared to the levels induced by Lip-GP33 and Lip-NP396 (Fig. 4A and B). On the other hand, Lip-NP396 induced substantial levels of IFN-γ-producing cells, and Lip-GP33/NP396 and Lip-GP33-Lip-NP396 induced even higher, although not significantly higher, numbers of IFN-γ-producing cells compared with the numbers induced by Lip-GP33 and Lip-NP396 (Fig. 4C). When these immunized mice were challenged with LCMV Arm, all the liposomal conjugates except Lip-GP283 were found to have the potency necessary to confer complete protection to mice (Fig. 4D).
i.n. immunization of mice with liposome-coupled peptide.
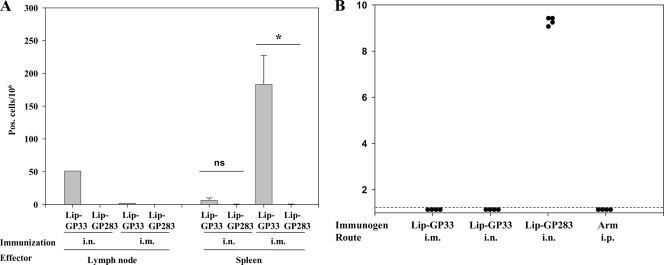
LCMV is transmitted through contact with secretions from infected animals or by inhalation of dried particles from them. Therefore, LCMV infection is also suitable for the study of the capacity of liposomal peptides to induce mucosal immunity and protect mice from infection by viruses through the mode of transmission described above. We introduced Lip-GP33 and CpG by the i.n. or i.m. route, and 1 week later, the numbers of IFN-γ-producing CD8+ T cells in draining cervical lymph nodes and spleens were measured (Fig. 5A). The i.n. immunizations resulted in the induction of IFN-γ-producing CD8+ T cells at high levels in the cervical lymph nodes but at only low levels in the spleens, indicating that effector CD8+ T cells were induced locally near the nasal cavity. On the other hand, the i.m. immunizations induced high numbers of IFN-γ-producing CD8+ T cells in the spleens but only small numbers in the cervical lymph nodes (Fig. 5A). When the immunized mice were challenged with LCMV Arm via the i.n. route, both i.m. and i.n. immunizations with Lip-GP33 were found to provide complete protection to the mice (Fig. 5B).
FIG. 5.
i.n. immunizations with Lip-GP33. C57BL/6 mice were immunized by the i.n. or i.m. route with 20 μl of Lip-GP33 or Lip-GP283 and 5 μg of CpG. One week later, lymphocytes from cervical lymph nodes and spleens were prepared and subjected to ELISPOT (IFN-γ) assays (A). The immunized mice were also challenged with 2 × 105 PFU of LCMV Arm via the i.n. route, and the virus titers in the spleens were quantitated (B). (A) ELISPOT assays of IFN-γ-producing CD8+ T cells from cervical lymph nodes (left) and spleens (right) of the mice immunized via the i.n. or i.m. route with Lip-GP33 or Lip-GP283. The data are representative of those from two independent and reproducible experiments. The results for one pool of cells from the lymph nodes and the means for spleen cells from four mice per group ± standard errors of the means are shown. *, P < 0.05 compared with the results obtained with Lip-GP283 immunization; ns, not statistically significant. (B) One week after immunization, the mice received 2 × 105 PFU of LCMV Arm i.n., and the virus titers in the spleens were quantitated by plaque assay on Vero cells at day 4 postchallenge. The virus titers are indicated for each animal in the study. A dotted line represents the lower limit of detection (2 × 101 PFU/g [spleen]). The data are representative of those from three independent and reproducible experiments.
Dose-response study of liposome-coupled peptides.
To evaluate further the efficacies of the liposomal peptides, we made serial fivefold dilutions of Lip-GP33 and injected them into mice in the presence of CpG. To our surprise, as little as 0.08 μl of Lip-GP33, which contained only 56 ng of the peptide, induced a significant level of IFN-γ-producing CD8+ T cells (Fig. 6A); and half of the immunized mice were completely protected against LCMV challenge (Fig. 6B) in two independent experiments. Immunizations with 0.4 μl or more of Lip-GP33 (≥280 ng peptide) conferred complete protection to all the mice, but the peptide (35 μg) not conjugated to liposomes did not induce either IFN-γ-producing CD8+ T cells or protection against the virus challenge (Fig. 6), which suggests the essential role of the liposome on the induction of the antiviral responses. The results were confirmed in three independent and reproducible experiments.
Effector and memory CD8+ T-cell induction by Lip-GP33 without the help of CD4.
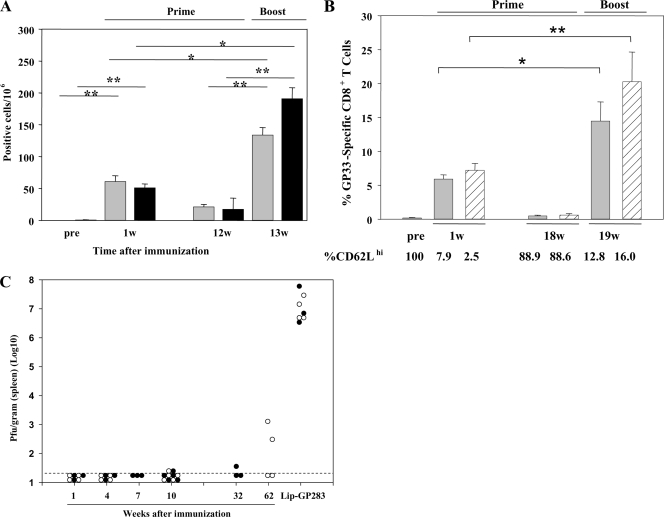
Several studies have demonstrated that CD4+ T cells play critical roles in generating primary CD8+ T cells or maintaining memory CD8+ T cells (5, 17, 19, 37, 39). When mice were immunized with minimal CTL epitope peptides, no functional CD8+ T cells could be detected past day 20 in an IFN-γ ELISPOT assay (8). Therefore, we wished to know how long the antiviral T-cell response persists and whether memory CD8+ T cells are established after immunization with Lip-GP33. We also wished to rule out the possibility that CD4+ T cells are involved in the induction of an antiviral T-cell response by Lip-GP33. We immunized both C57BL/6 and CD4-KO mice with a single dose of Lip-GP33 and compared the number of Ag-specific IFN-γ-producing T cells in their spleens by ELISPOT assay (Fig. 7A). When the primary CD8+ T-cell responses to GP33 were compared at 1 week postimmunization, there was no significant difference between the two groups. Twelve weeks after priming, when the Ag-specific IFN-γ-producing T cells in the spleen diminished, the mice were boosted in the same manner used to prime them. One week postboosting, clear recall responses of 6- and 10-fold expansions of Ag-specific IFN-γ-producing T cells could be seen in the two groups, respectively, and the difference was not significant between the two groups. The recall responses of the C57BL/6 mice were also assessed 18 weeks after priming by tetramer staining and compared with those of the mice which acquired sterile immunity by LCMV Arm infection (Fig. 7B). One week after the boost, 22- and 33-fold expansions of GP33-specific CD8+ T cells were observed in the two groups, respectively. We also analyzed the expression of CD62L, a surface molecule associated with T-cell memory function, of GP33-specific CD8+ T cells in both Lip-GP33- and LCMV Arm-immunized mice. As shown in Fig. 7B, CD62L was downregulated after the mice were primed. Eighteen weeks after the mice were primed, more than 88% of the GP33-specific CD8+ T cells recovered their ability to express CD62L and were again downregulated after the boost. To confirm the establishment of memory CD8+ T cells, the immunized mice were challenged with LCMV Arm at different times after vaccination. As shown in Fig. 7C, complete protection was seen even in the absence of CD4+ T cells at 10 weeks after priming. In the 62nd week, half of the C57BL/6 mice showed perfect protection, and on average, about 4-log-titer reductions were observed.
FIG. 7.
Analyses of effector and memory CD8+ T-cell induction in the absence of CD4 help. Each of C57BL/6 and CD4-KO mice received 20 μl of Lip-GP33 or Lip-GP283 diluted in 100 μl of PBS containing 5 μg of CpG; and ELISPOT assays (A), tetramer and CD62L staining (B), and challenge experiments with LCMV Arm (C) were performed at different times. (A) At 1 and 12 weeks postimmunization, IFN-γ-producing cells responding to peptide GP33 were detected by ELISPOT assays of spleen cells from C57BL/6 (gray bars) and CD4-KO (black bars) mice. At week (w) 12, the mice were boosted in the same manner in which they received the primary immunizations, and ELISPOT assays were performed 1 week later. (B) GP33 H-2Db tetramer staining was performed 1 and 18 weeks postimmunization for C57BL/6 mice (gray bars). For comparison, mice immunized by inoculating 2 × 105 PFU of LCMV Arm i.p. were included in these experiments (shaded bars). At week 18, both groups of mice were boosted with Lip-GP33 and were analyzed for their recall responses 1 week later. The expression of CD62L on gated GP33 H-2Db tetramer-positive CD8+ T cells was also determined by flow cytometry. (C) Challenge experiments were performed by the i.p. injection of 2 × 105 PFU of LCMV Arm at the indicated times. The virus titers are shown for each of the C57BL/6 (open circles) and the CD4-KO (closed circles) mice. The data are shown as the means for at least three and five mice per group ± standard errors of the means for the ELISPOT and tetramer assays, respectively. *, P < 0.05; **, P < 0.01.
We then examined if the presence of CD4+ T-cell help would augment the induction of effector and memory CD8+ cells by Lip-GP33. As helper T-cell epitopes, I-Ab-restricted LCMV GP61-80 and NP309-328 were chosen and conjugated to liposomes by themselves (Lip-GP61 and Lip-NP309) or with peptide GP33 (Lip-GP33/GP61 and Lip-GP33/NP309). Mice were immunized with either Lip-GP33, Lip-GP33/GP61, Lip-GP33/NP309, or a mixture of Lip-GP33 and either Lip-GP61 or Lip-NP309 (Lip-GP33-Lip-GP61 and Lip-GP33-Lip-NP309, respectively). One and 18 weeks later, IFN-γ ELISPOT assays and challenge experiments with LCMV Arm were performed, but the results of the three immunizations (four mice per group) did not show any significant differences (data not shown).
Taken together, these findings suggest that Lip-GP33 generated functional effector and memory CD8+ T cells without the evident help of T cells.
DISCUSSION
In the study described in this report, we demonstrate that antigenic peptides coupled to the surfaces of liposomes serve as efficient vaccine vehicles for the induction of antiviral immunity mediated by CD8+ T cells. We used LCMV infection of mice as a model system because LCMV has been extensively used to study CD8+ T-cell-mediated antiviral immunity (21, 25, 43). In addition, LCMV causes respiratory tract as well as percutaneous infections; and some LCMV variants, such as Cl. 13, WE, and Docile, induce persistent infections in immunocompetent mice. These features of LCMV enabled us to evaluate the efficacy of our vaccine preparation by comparing the findings obtained with this preparation with those obtained in many other studies in which the same epitope was presented as the peptide (3, 7, 18, 31) or in which liposome-encapsulated peptide (23), plasmid DNA (4, 15, 24, 34), recombinant vaccinia virus (14, 20, 30, 44), recombinant lentivirus (46), and recombinant Listeria (38) vaccines were used.
Lip-GP33 efficiently induced CTLs, IFN-γ-producing CD8+ T cells, and complete protection against LCMV challenge. Although the numbers of positive cells in the ELISPOT assays were low compared to the results of the intracellular cytokine staining (Fig. 2A), that result seems to be simply due to technical factors because spleen cells from the same immunized animal showed such differences, and these were also observed in mice immunized by LCMV Arm infection.
The minimum amount of GP33 peptide (0.28 μg) required to yield perfect protection in this study was extremely low. Peptide vaccines with adjuvants, cytokines, CpGs, etc., usually require multiple injections in amounts ranging from 10 to 500 μg (3, 7, 18, 31). When peptide GP33 was encapsulated within liposomes and injected with CpG, two immunizations with 80 μg were required to obtain perfect protection, while a smaller dose gave only partial protection (23). In distinction, we expressed Ags on the surfaces of liposomes and speculate that they might be more efficiently recognized by Ag-presenting cells, which resulted in an enhanced presentation to T cells. In direct comparisons, we have found that surface-linked liposomal Ags induced a significantly higher level of Ag-specific IgG production than that induced by liposome-encapsulated Ags in mice (our unpublished observation). In addition, a significant difference was observed between liposomes with different lipid components; more Ags coupled to the unsaturated liposomes were engulfed by macrophages in vitro, and a higher level of Ag-specific antibody production was induced in vivo when unsaturated liposomes were used than when saturated liposomes were used (29). These lines of evidence indicate that the adjuvant effects of liposomes would depend on both the antigenic topology to the liposomal membrane and the membrane mobility of the liposomes. The efficient induction of antiviral immunity with a very low dose of peptide demonstrated in the present study (Fig. 6) could be an advantage of the use of surface-coupled liposomal peptides for the development of peptide-based vaccines.
It has been shown that peptide vaccination of mice immune to LCMV or vaccinia virus causes tumor necrosis factor-dependent shock-like signs (22). This indicates the need for caution in the development of antiviral peptide vaccines. Antiviral vaccines are currently administered to millions of people, and preexisting immunity to viruses is common. These potential problems with peptide vaccines may be even more important in the application to immunotherapy of a persistent infection (6, 13). However, the induction of the shock-like signs by peptide vaccination is dose dependent; less than 4 μg of peptide showed no evident effects (22). Therefore, the high level of efficiency of surface-linked liposomal peptides may circumvent these issues.
Of note, Lip-NP396 also conferred perfect protection, although only low levels of cytolytic activity were detected in both the ex vivo and the in vivo CTL assays (Fig. 4). This is in contrast to the immunization with GP33 peptide encapsulated in liposomes, which induced very strong cytolytic activity by CD8+ T cells but very low protective immunity (23). This may be explained by the difference between GP33- and NP396-specific CTLs. NP396-spepcific CTLs have been found to be ∼1,000 times more sensitive to Ag and to have more protective capacity than GP33-specific CTLs (9). Moreover, LCMV and hepatitis B virus have been found to be susceptible to noncytopathic antiviral control mechanisms that depend on local IFN-γ and tumor necrosis factor alpha induction (11, 12, 32, 41). This noncytopathic mechanism may underlie the protective immunity exerted by Lip-NP396. It is unclear why the two immunodominant epitopes, GP33 and NP396, exhibited different CD8+ T-cell responses when they were coupled on the surfaces of liposomes. In our further study of liposomal vaccines with hepatitis C virus epitopes, some immunodominant CTL epitopes also showed such differences when they were used as conventional peptide vaccines and surface-linked liposomal peptide vaccines (unpublished data). Further elucidation of the mechanisms underlying the differences between the induction of CTL activity and cytokine production by surface-linked liposomal peptides may lead to the development of more beneficial vaccines.
Several studies have demonstrated that while the primary expansion of antiviral CD8+ T cells can occur independently of CD4+ T-cell help, CD4 help is required for the long-term (>2-month) survival of memory CD8+ T cells (17, 39, 40). Unhelped memory populations have been reported to be defective in their ability to generate a recall response following secondary challenge (39). LCMV peptide GP33 has been reported to induce effector CD8+ T cells by being emulsified in incomplete Freund adjuvant (3), mixed with CpG (18, 31), or encapsulated in liposomes (23); however, the induction and maintenance of memory CD8+ T cells by those immunizations have not been elucidated. In the present study, it was demonstrated that IFN-γ production and protective immunity were induced and maintained for more than 1 year after a single immunization with Lip-GP33 (Fig. 7). Significant recall responses were observed in ELISPOT assays and staining of the tetramer after the booster injection with Lip-GP33. These findings as well as the analysis of CD62L expression indicate that the immunization with Lip-GP33 and CpG induced the maturation and maintenance of Ag-specific memory CD8+ T cells. To our knowledge, this is the first time that long-lasting protective immunity against LCMV infection has been induced by the use of minimum CTL epitope peptides. These findings may be useful in the development of vaccines against human pathogens.
In summary, we report that the peptides coupled on the surfaces of liposomes consisting of unsaturated fatty acids very efficiently induce protective antiviral immunity in mice. The mode of action is still unclear, but it seems to be very unique on the basis of the differences in the responses depending on the epitope peptides used and the induction/maturation of memory CD8+ T cells without the help of CD4 cells.
Acknowledgments
This work was supported by a grant from the Ministry of Health, Labor, and Welfare of Japan and a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
We thank Hiroe Akatsuka for technical assistance. We are also grateful to J. Lindsay Whitton (The Scripps Research Institute) for providing us VVGP33 and technical advice, Rafi Ahmed (Emory University School of Medicine) for providing LCMV Arm and Cl.13, and Stephen M. Feinstone (CBER, FDA) for helpful discussions and preparing the manuscript.
Footnotes
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Ahmed, R., and M. B. Oldstone. 1988. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 167:1719-1724. (Erratum, 168:457.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aichele, P., K. Brduscha-Riem, R. M. Zinkernagel, H. Hengartner, and H. Pircher. 1995. T cell priming versus T cell tolerance induced by synthetic peptides. J. Exp. Med. 182:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartholdy, C., A. Stryhn, J. P. Christensen, and A. R. Thomsen. 2004. Single-epitope DNA vaccination prevents exhaustion and facilitates a broad antiviral CD8+ T cell response during chronic viral infection. J. Immunol. 173:6284-6293. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297:2060-2063. [DOI] [PubMed] [Google Scholar]
- 6.Brooks, D. G., A. M. Lee, H. Elsaesser, D. B. McGavern, and M. B. A. Oldstone. 2008. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 205:533-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciupitu, A. M., M. Petersson, C. L. O'Donnell, K. Williams, S. Jindal, R. Kiessling, and R. M. Welsh. 1998. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J. Exp. Med. 187:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernando, G. J. P., V. Khammanivong, G. R. Leggatt, W. J. Liu, and I. H. Frazer. 2002. The number of long-lasting functional memory CD8+ T cells generated depends on the nature of the initial nonspecific stimulation. Eur. J. Immunol. 32:1541-1549. [DOI] [PubMed] [Google Scholar]
- 9.Gallimore, A., T. Dumrese, H. Hengartner, R. M. Zinkernagel, and H. G. Rammensee. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187:1647-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallimore, A., H. Hengartner, and R. Zinkernagel. 1998. Hierarchies of antigen-specific cytotoxic T-cell responses. Immunol. Rev. 164:29-32. [DOI] [PubMed] [Google Scholar]
- 11.Guidotti, L. G., P. Borrow, A. Brown, H. McClary, R. Koch, and F. V. Chisari. 1999. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J. Exp. Med. 189:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 13.Ha, S. J., S. N. Mueller, E. J. Wherry, D. L. Barber, R. D. Aubert, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2008. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J. Exp. Med. 205:543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington, L. E., R. van der Most, J. L. Whitton, and R. Ahmed. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 76:3329-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassett, D. E., J. Zhang, and J. L. Whitton. 1999. Plasmid DNA vaccines are effective in the absence of IFN-γ. Virology 263:175-183. [DOI] [PubMed] [Google Scholar]
- 16.Hudrisier, D., H. Mazarguil, F. Laval, M. B. A. Oldstone, and J. E. Gairin. 1996. Binding of viral antigens to major histocompatibility complex class I H-2Db molecules is controlled by dominant negative elements at peptide non-anchor residues. J. Biol. Chem. 271:17829-17836. [DOI] [PubMed] [Google Scholar]
- 17.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 18.Johansen, P., T. Storni, L. Rettig, Z. Qiu, A. Der-Sarkissian, K. A. Smith, V. Manolova, K. S. Lang, G. Senti, B. Mullhaupt, T. Gerlach, R. F. Speck, A. Bot, and T. M. Kundig. 2008. Antigen kinetics determines immune reactivity. Proc. Natl. Acad. Sci. USA 105:5189-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaech, S. M., and R. Ahmed. 2003. CD8 T cells remember with a little help. Science 300:263-265. [DOI] [PubMed] [Google Scholar]
- 20.Klavinskis, L. S., J. L. Whitton, and M. B. A. Oldstone. 1989. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J. Virol. 63:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau, L. L., B. D. Jamieson, T. Somasundaram, and R. Ahmed. 1994. Cytotoxic T-cell memory without antigen. Nature 369:648-652. [DOI] [PubMed] [Google Scholar]
- 22.Liu, F., R. Feuer, D. E. Hassett, and J. L. Whitton. 2006. Peptide vaccination of mice immune to LCMV or vaccinia virus causes serious CD8+ T cell-mediated, TNF-dependent immunopathology. J. Clin. Investig. 116:465-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludewig, B., F. Barchiesi, M. Pericin, R. M. Zinkernagel, H. Hengartner, and R. A. Schwendener. 2000. In vivo antigen loading and activation of dendritic cells via a liposomal peptide vaccine mediates protective antiviral and anti-tumour immunity. Vaccine 19:23-32. [DOI] [PubMed] [Google Scholar]
- 24.Martins, L. P., L. L. Lau, M. S. Asano, and R. Ahmed. 1995. DNA vaccination against persistent viral infection. J. Virol. 69:2574-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matloubian, M., S. R. Kolhekar, T. Somasundaram, and R. Ahmed. 1993. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 67:7340-7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui, M., O. Moriya, T. Yoshimoto, and T. Akatsuka. 2005. T-bet is required for protection against vaccinia virus infection. J. Virol. 79:12798-12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagata, T., T. Toyota, H. Ishigaki, T. Ichihashi, K. Kajino, Y. Kashima, Y. Itoh, M. Mori, H. Oda, H. Yamamura, M. Taneichi, T. Uchida, and K. Ogasawara. 2007. Peptides coupled to the surface of a kind of liposome protect infection of influenza viruses. Vaccine 25:4914-4921. [DOI] [PubMed] [Google Scholar]
- 28.Nakano, Y., M. Mori, S. Nishinohara, Y. Takita, S. Naito, A. Horino, H. Kato, M. Taneichi, Y. Ami, Y. Suzaki, K. Komuro, and T. Uchida. 1999. Antigen-specific, IgE-selective unresponsiveness induced by antigen-liposome conjugates. Comparison of four different conjugation methods for the coupling of antigen to liposome. Int. Arch. Allergy Immunol. 120:199-208. [DOI] [PubMed] [Google Scholar]
- 29.Nakano, Y., M. Mori, S. Nishinohara, Y. Takita, S. Naito, H. Kato, M. Taneichi, K. Komuro, and T. Uchida. 2001. Surface-linked liposomal antigen induces IgE-selective unresponsiveness regardless of the lipid components of liposomes. Bioconjug. Chem. 12:391-395. [DOI] [PubMed] [Google Scholar]
- 30.Oldstone, M. B., A. Tishon, M. Eddleston, J. C. de la Torre, T. McKee, and J. L. Whitton. 1993. Vaccination to prevent persistent viral infection. J. Virol. 67:4372-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oxenius, A., M. M. Martinic, H. Hengartner, and P. Klenerman. 1999. CpG-containing oligonucleotides are efficient adjuvants for induction of protective antiviral immune responses with T-cell peptide vaccines. J. Virol. 73:4120-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasquetto, V., S. F. Wieland, S. L. Uprichard, M. Tripodi, and F. V. Chisari. 2002. Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte cultures. J. Virol. 76:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peek, L. J., C. R. Middaugh, and C. Berkland. 2008. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 60:915-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez, F., S. Harkins, J. M. Redwine, J. M. de Pereda, and J. L. Whitton. 2001. CD4+ T cells induced by a DNA vaccine: immunological consequences of epitope-specific lysosomal targeting. J. Virol. 75:10421-10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samad, A., Y. Sultana, and M. Aqil. 2007. Liposomal drug delivery systems: an update review. Curr. Drug Deliv. 4:297-305. [DOI] [PubMed] [Google Scholar]
- 36.Selin, L. K., and R. M. Welsh. 1997. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J. Immunol. 158:5366-5373. [PubMed] [Google Scholar]
- 37.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 38.Shen, H., M. K. Slifka, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. USA 92:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suresh, M., X. Gao, C. Fischer, N. E. Miller, and K. Tewari. 2004. Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection. J. Virol. 78:3906-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taneichi, M., H. Ishida, K. Kajino, K. Ogasawara, Y. Tanaka, M. Kasai, M. Mori, M. Nishida, H. Yamamura, J. Mizuguchi, and T. Uchida. 2006. Antigen chemically coupled to the surface of liposomes are cross-presented to CD8+ T cells and induce potent antitumor immunity. J. Immunol. 177:2324-2330. [DOI] [PubMed] [Google Scholar]
- 43.von Herrath, M., and J. L. Whitton. 2001. Animal models using lymphocytic choriomeningitis virus. Curr. Protoc. Immunol. May, chapter 19, unit 19.10. [DOI] [PubMed]
- 44.Wherry, E. J., J. N. Blattman, and R. Ahmed. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. D. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarei, S., S. Abraham, J. F. Arrighi, O. Haller, T. Calzascia, P. R. Walker, T. M. Kundig, C. Hauser, and V. Piguet. 2004. Lentiviral transduction of dendritic cells confers protective antiviral immunity in vivo. J. Virol. 78:7843-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]