Abstract
Invasive aspergillosis is a significant cause of morbidity and mortality in patients after stem cell transplantation, in solid organ transplant recipients, and in patients with hematological malignancies. The interactions between human immature dendritic cells (iDCs) and Aspergillus fumigatus antigens are widely uncharacterized. We analyzed the immune response of iDCs to different recombinant A. fumigatus antigens (Aspf1 and Crf1). One of these antigens, the 18-kDa RNase Aspf1, triggered the increased level of expression of genes encoding proinflammatory cytokines and chemokines, and augmented the activation of NFκB and the apoptosis of iDCs. Furthermore, by fluorescence microscopy, we could demonstrate that in the first 3 h a major portion of Aspf1 accumulates on the cell surface. Finally, we could show an increased segregation of cytokines and chemokines after the stimulation of iDCs by an Aspf1 deletion mutant strain of A. fumigatus.
Over the past decade, invasive aspergillosis (IA) has emerged as the most serious life-threatening infectious complication of intensive remission-induction chemotherapy and allogeneic hematopoietic stem cell transplantation. Aspergillus fumigatus is the species most commonly isolated from cases of IA. Despite improvements by the use of preventative strategies and the development of new antifungal drugs, IA has an incidence of 10 to 30% and is still associated with mortality rates as high as 90% in some surveys (4). In 2008, over 10,000 patients were hospitalized in the United States due to IA, resulting in 17.7 additional hospital days and $96,000 in additional costs per patient (32).
In addition to neutrophils and macrophages, dendritic cells (DCs) play a major role in the defense against IA. DCs can transport antigens from the site of infection to the lymph nodes (18). Exposure to this pathogen leads to changes in the expression of their characteristic surface markers (CD40, CD80, CD83, and CD86) and induces the secretion of proinflammatory cytokines and chemokines (9, 10).
There are few data regarding immunotherapy in patients with invasive fungal infections, and in particular, there are few data related to IA. This may be due, at least in part, to the complex antigenic properties of A. fumigatus, which have not yet been well characterized. However, several A. fumigatus proteins have been identified as immunogenic antigens. Among these are two proteins that have been used in this study: the 18-kDa immunoglobulin E (IgE)-binding protein Aspf1 and the glycosylphosphatidylinositol-anchored extracellular cell wall glucanase Crf1 (2). Aspf1 is a member of a family of conserved RNases that cleave defined phosphodiester bonds of the 28S rRNA of eukaryotic ribosomes (3, 16). In this study, we analyzed different aspects of the response of human monocyte-derived DCs to Crf1 and, especially, Aspf1.
MATERIALS AND METHODS
Generation of iDCs.
Peripheral blood mononuclear cells were separated from 50 ml buffy coat blood of healthy donors by Ficoll-Hypaque density gradient centrifugation (Biochrom AG, Berlin, Germany). Monocytes were isolated by magnetism-associated cell sorting with paramagnetic microbeads conjugated to anti-human CD14 monoclonal antibodies (Miltenyi Biotech, Bergisch Gladbach, Germany). Differentiation into immature DCs (iDCs) was achieved by treatment with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), as described previously (12). Flow cytometry (FACS Calibur flow cytometer; Becton Dickinson) revealed that more than 95% of the iDC population was positive for CD1a-fluorescein isothiocyanate (FITC), CD40-phycoerythrin (PE), CD80-PE, CD86-PE, and HLA-DR-FITC and negative for CD14-FITC.
Expression and purification of A. fumigatus antigens.
The antigens Aspf1 (M83781) and Crf1 (AY169706) were expressed as described previously (2, 28). The absence of lipopolysaccharide (LPS) from all antigen preparations was verified by high-pressure liquid chromatography.
For purification of hemagglutinin (HA)-tagged Aspf1 (for the microscopic studies), the corresponding gene was amplified from the cDNA of the A. fumigatus strain 46645 with oligonucleotides Mito-for (GCTCCCTCGCCCCTCGAC) and Mito-rev-HA (CTAGGCGTAGTCGGGCACGTCGTAGGGGTAATGAGAACACAGTCTCAAGTC). The resulting PCR product was cloned into the pQE30-UA expression vector (Qiagen, Hilden, Germany) and transformed into Escherichia coli strain M15(pREP4). The recombinant Aspf1 protein, which comprised an N-terminal His tag and a C-terminal HA tag, was purified from the lysate of isopropyl-β-d-thiogalactopyranoside-induced bacterial cells with a talon metal affinity resin (Clontech, Saint-Germain-en-Laye, France).
Determination of Aspf1 localization in human iDCs.
After exposure of the DCs to HA-tagged Aspf1 (5 μg/ml) for 15 min, 30 min, or 3 h, the cells were fixed with 3.7% formaldehyde for 5 min. If required, the cells were permeabilized with 0.2% Triton X-100 for 1 min. Staining with an HA-specific rat monoclonal antibody (Roche Applied Science, Mannheim, Germany) and an appropriate Cy3-labeled secondary antibody was performed. Samples were analyzed with an SP5 confocal laser scanning microscope (Leica Microsystems, Heidelberg, Germany).
Exposure of iDCs to A. fumigatus antigens and analysis of DC response.
The cocultivation of iDCs (1 × 106 cells) and the Aspergillus antigens (each at 5 μg/ml, as determined in preexperiments) was carried out for 6 h, 24 h, and 48 h. LPS (1 μg/ml; Sigma-Aldrich, Steinheim, Germany) was used as a positive control. RNA was extracted by the use of RNeasy spin columns (Qiagen), according to the instructions of the manufacturer. Quantitative real-time reverse transcription-PCR for the expression profiling of tumor necrosis factor alpha (TNF-α), IL-8, IL-10, IL-23, chemokine (C-X-C motif) ligand 10 (CXCL10), chemokine (C-C motif) ligand 20 (CCL20), Toll-like receptor 2 (TLR2), and TLR4 was performed as described previously (23); and the profiles were normalized against that for the housekeeping gene h-ALAS (Roche Diagnostics, Mannheim, Germany). Furthermore, the secretion of IL-10, IL-23, and CCL20 was analyzed by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN), according to the instructions of the manufacturer (lowest detection thresholds in the ELISA analysis, 10 pg/ml for CCL20 and 9.8 pg/ml for IL-10 and IL-23).
NFκB translocation assay.
Assessment of nuclear factor kappa B (NFκB) binding activity in nuclear extracts was achieved with Trans-AM p65 transcription factor assay kits (Active Motife, Rixensart, Belgium), according to the protocol of the supplier. Five micrograms of nuclear extracts was incubated with plate-coated NFκB consensus oligonucleotides. Anti-p65 antibody binding was detected with a secondary horseradish peroxidase-conjugated antibody, and the reaction was developed with 3,3′,5,5′-tetramethylbenzidine as the substrate. The intensity of the reaction was measured at 450 nm. DCs were stimulated with either Aspf1 or LPS, as described above, and the results were compared to those for unstimulated DCs.
Analysis of maturation marker upregulation by flow cytometry.
At 48 h after treatment of the iDCs (1 × 106 cells) with Aspf1 or Crf1 (5 μg/ml each), the cells were stained with FITC- or PE-labeled antibodies (BD Biosciences-Pharmingen) against CD1a, CD40, CD80, CD83, CD86, and HLA class II. Flow cytometric data were compensated for by using the FlowJo program (version 8.8.6; Trustees of Leland Stanford, Jr., University).
Cocultivation of the Δaspf1 mutant strain with human iDCs.
Monocyte-derived DCs (1 × 106 cells; multiplicity of infection, 1) were cocultivated for 6 h with either living or ethanol-inactivated (70% ethanol, 30 min) germ tubes of wild-type strain ATCC46645 and a Δaspf1 mutant strain (26). For further analysis, the supernatants were frozen at −20°C and the RNA was immediately extracted from the iDC pellets with an RNeasy mini kit (Qiagen).
Mixed lymphocyte reaction (MLR).
Allogeneic T cells were enriched from purified peripheral blood mononuclear cell fractions by the depletion of CD8− and CD69+ cells with a mixture of biotinylated monoclonal antibodies (CD4, CD15, CD16, CD19, CD34, CD36, CD56, CD69, CD123, T-cell receptor-γ/δ, CD235a) and microbead-conjugated antibiotin monoclonal antibodies (Miltenyi Biotech). T-cell proliferation was assessed by flow cytometry with carboxyfluorescein succinimidyl ester labeling, as recommended by the manufacturer (Invitrogen).
At 48 h prior to T-cell isolation, the iDCs were exposed either to A. fumigatus antigen Aspf1 (5 μg/ml) or to human cytomegalovirus antigen pp65 (100 μg/ml; Miltenyi Biotech). The cells were then washed three times in Hanks balanced salt solution to minimize the transfer of soluble factors or cytokines before they were used as stimulators in the iDC MLR. DCs were added to responder T cells (2.5 × 105) in 1 ml AIMV medium (which included 10% allogeneic human serum, 10 IU/ml IL-2, 75 ng/ml GM-CSF, and 15 ng/ml IL-4) per well at a stimulator cell/responder cell ratio of 1:10 for 9 days, until the measurement of proliferation by flow cytometry (FACS Calibur flow cytometer; Becton Dickinson). As a positive control for T-cell proliferation without DCs, M-type phytohemagglutinin (2.25%, vol/vol; Invitrogen, Karlsruhe, Germany) was used in all experiments. After 2 days, 1 ml AIMV medium (which included 10% allogeneic human serum and cytokines) was added to achieve additional final concentrations of 5 IU/ml IL-2, 37.5 ng/ml GM-CSF, and 7.5 ng/ml IL-4.
Quantification of apoptosis after incubation with Aspf1.
To analyze the viability of the DCs after treatment with either Aspf1 (5 μg/ml), α-sarcin (10 μg/ml; which was used as a positive control and which originated from Aspergillus giganteus; Sigma-Aldrich), or camptothecin (12 μM; which was used as a specific positive control for the induction of apoptosis by the inhibition of topoisomerase I; Sigma-Aldrich), the rates of apoptosis and necrosis were determined by flow cytometry (FACS Calibur flow cytometer; Becton Dickinson). A dual-color protocol that used annexin V-FITC to quantify phosphatidylserine and propidium iodide to quantify the exposed DNA of dead cells was used (BD Biosciences-Pharmingen).
Statistical analyses.
For statistical analysis, two-sided Student's t tests were performed by pairing sample data with data for the unstimulated control.
All experiments were repeated with blood from at least three independent blood donors.
RESULTS
Immune response of iDCs toward A. fumigatus antigens.
As an initial experiment, iDCs were stimulated with LPS and the levels of cytokine expression were compared to those by unstimulated iDCs. They showed differential levels of expression of the genes for TNF-α (at 6 h, mean of 25 times higher levels of expression; at 24 h, no change; at 48 h, mean of 3 times higher levels of expression), IL-8 (at 6 h, mean of 102 times higher levels of expression; at 24 h, mean of 15 times higher levels of expression; 48 h, at mean of 21 times higher levels of expression), IL-10 (at 6 h, mean of 8 times higher levels of expression; at 24 h, no change; at 48 h, no change), CXCL10 (at 6 h, mean of 2,600 times higher levels of expression; at 24 h, mean of 53 times higher levels of expression; at 48 h, mean of 4 times higher levels of expression), and CCL20 (at 6 h, mean of 1,150 times higher levels of expression; at 24 h, mean of 22 times higher levels of expression; at 48 h, mean of 5 times higher levels of expression). The increased levels of gene expression could be confirmed by ELISA analyses (data not shown).
In parallel with LPS, both recombinant antigens (Aspf1, Crf1) led to the differential expression of genes encoding the proinflammatory cytokines TNF-α, IL-1ß, IL-8, IL-12p35, and IL-23p19, as well as those encoding the chemokines CXCL10 and CCL20, compared to their levels of expression by unstimulated iDCs. However, the differential expression varied widely between the two antigens and the different cytokines and chemokines (Table 1).
TABLE 1.
Differential expression of genes for selected cytokines and chemokines after stimulation with the recombinant A. fumigatus antigens Aspf1 and Crf1a
| Gene | Differential expression (fold) at the indicated times after stimulation with:
|
|||||
|---|---|---|---|---|---|---|
| Aspf1
|
Crf1
|
|||||
| 6 h | 24 h | 48 h | 6 h | 24 h | 48 h | |
| TNF-α | 8.2 | 12 | 22 | — | — | 3 |
| IL-8 | 30 | 72 | 275 | 2.1 | 2.8 | 2.9 |
| IL-23p19 | 7.5 | 2.2 | — | ND | ND | ND |
| CXCL10 | 39 | 60 | 27 | 2.3 | 2.9 | 3.1 |
| CCL20 | 247 | 299 | 314 | — | 3.1 | — |
| IL-12p35 | ND | 14 | ND | ND | ND | ND |
| IL-10 | 0.6 | 0.8 | — | ND | ND | ND |
| IL-1β | ND | 7.4 | ND | ND | ND | ND |
DCs were stimulated for 6, 24, or 48 h with A. fumigatus antigens; and the levels of expression of the genes for TNF-α, IL-8, IL-23, CXCL10, and CCL20 were determined by quantitative reverse transcription-PCR. The level of gene expression was normalized against the level of expression of h-ALAS by evaluating the crossing point values for each sample by using the equation 2(ΔCP for target gene − ΔCP for h-ALAS), where ΔCP is the change in the crossing point (CP) value (7, 22). For each time point, the levels of gene expression by DCs cocultivated with A. fumigatus antigens were compared to the levels of gene expression by unstimulated cells (and are given as ratios). All experiments were repeated with blood from at least three independent blood donors, and the mean level of upregulation is indicated. —, unchanged expression, defined as a <2-fold change and a >0.8-fold change; ND, not determined.
After stimulation with the antigen Crf1, only slightly increased levels of expression (2.1 to 2.9 times) of the genes for IL-8, CXCL10, and CCL20 could be observed, whereas the level of expression of the gene for TNF-α remained unchanged. In contrast, the recombinant protein Aspf1 induced marked upregulation, especially of the genes for CXCL10, CCL20, IL-8, and, initially, IL-23 (Table 1).
After culture of iDCs with Aspf1, the level of gene expression usually increased at 6 h of cocultivation, with further increases occurring after 24 h and 48 h, whereas gene expression after LPS treatment of iDCs was maximal after 6 h and decreased after 24 h and 48 h (see above).
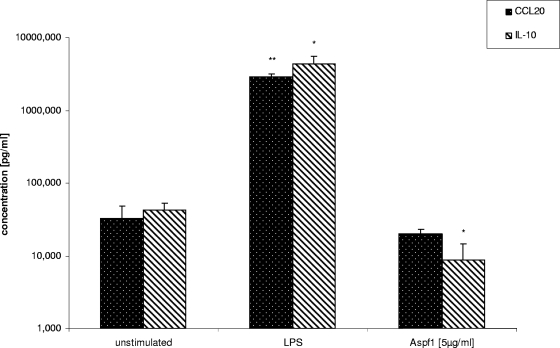
In contrast, the anti-inflammatory cytokine IL-10 showed slightly decreased levels of gene expression (as shown for Aspf1; at 6 h, mean of a 0.6-fold decreased level of expression; at 24 h, mean of a 0.8-fold decreased level of expression; at 48 h, no change) compared to the levels of expression by unstimulated cells. Modified gene expression profiles after Aspf1 stimulation could exemplarily be confirmed by ELISAs for CCL20 and IL-10 (Fig. 1).
FIG. 1.
Quantification of CCL20 and IL-10 in culture supernatants after 24 h of cocultivation by ELISA. The results for Aspf1- or LPS-treated samples were compared to those for unstimulated controls. *, P < 0.1; **, P < 0.05.
Analyses of the expression of the genes for the pattern recognition receptors TLR2 and TLR4 revealed that none of the applied antigens triggered significantly differential expression (data not shown), whereas LPS has been shown to reduce the level of expression of the gene for TLR4 (23).
NFκB translocation assay.
Quantification of activated NFκB p65, localized in the nucleolus, was performed. We could demonstrate that Aspf1 (in parallel with LPS) induced the activation of NFκB by the detection of NFκB translocation into the nucleus (for LPS, at 24 h, 3.2 times higher levels of expression; at 48 h, 2 times higher levels of expression; for Aspf1, at 24 h, 1.5 times higher levels of expression; at 48 h, 2.2 times higher levels of expression [the data are means compared to the results obtained for iDCs without addition of a stimulus]).
Analysis of interaction of DCs with A. fumigatus aspf1-knockout strain.
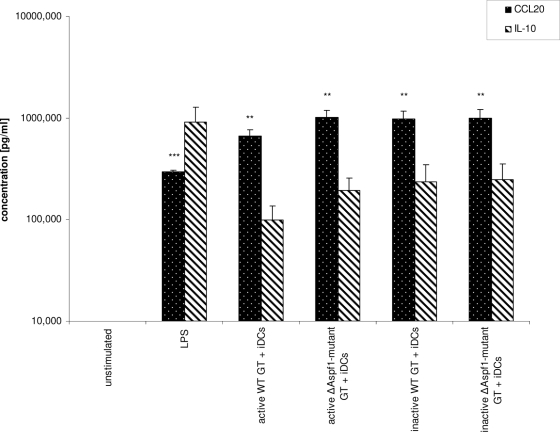
Analysis of cytokine (IL-10, CCL20) release by iDCs after stimulation with either LPS, the viable wild-type strain, or the corresponding Δaspf1 mutant strain revealed increased cytokine levels compared to the levels released by the unstimulated controls. Interestingly, after stimulation of the iDCs with the A. fumigatus aspf1-knockout strain, significantly higher levels of CCL20 (P = 0.05) and IL-10 (P = 0.09) could be observed. In contrast, comparison of ethanol-inactivated wild-type and mutant strains showed no significant difference in the levels of cytokine release (Fig. 2).
FIG. 2.
Coculturing of iDCs with wild-type and mutant strains of A. fumigatus, followed by ELISA analysis of cell culture supernatants. Student's t test by pairing of active wild-type (WT) germ tubes (GT) with active Δaspf1 mutant germ tubes samples revealed P values of 0.057 for CCL20 and 0.089 for IL-10. **, P < 0.05; ***, P < 0.01.
Uptake and antigen presentation of Aspf1 by DCs.
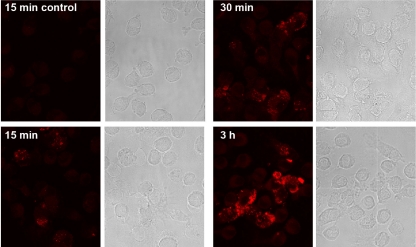
Our data demonstrated that, in contrast to the antigen Crf1, the ribotoxin Aspf1 markedly modified the levels of gene expression by DCs. Therefore, we investigated if DC activation depends on the cytoplasmic localization of the antigen or if activation occurs by attachment to the cell membrane only. Confocal microscopy revealed that Aspf1 was already detectable at the cell surface within 15 min after addition to iDCs and that the signal intensities strongly increased when coculturing was extended up to 3 h (Fig. 3). Comparison of the results for samples stained with or without permeabilization revealed no differences, suggesting that most of the bound Aspf1 remained on the cell surface.
FIG. 3.
Localization of Aspf1 antigen in iDCs. Confocal microscopy analysis was performed for determination of the localization of HA-tagged Aspf1 in iDCs after exposure of cells to Aspf1 for 15 min, 30 min, or 3 h.
Maturation of iDCs after contact with A. fumigatus antigens Aspf1 and Crf1.
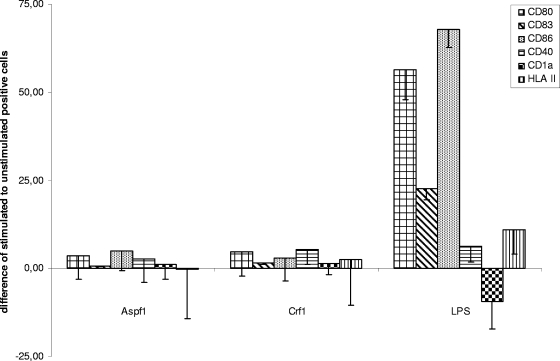
After the exposure of iDCs to the Aspf1 or Crf1 antigen for 48 h, the levels of expression of CD1a, CD40, CD80, CD83, CD86, and HLA class II were analyzed by flow cytometry. Analysis revealed that the surface markers remained unchanged after Aspf1 or Crf1 stimulation, which indicates that both proteins were unable to trigger maturation of DCs (Fig. 4).
FIG. 4.
Expression of selected DC markers after stimulation with Aspf1, Crf1, and LPS by flow cytometric analysis of selected DC surface markers (CD1a, CD40, CD80, CD83, CD86, HLA class II). DCs were stimulated for 48 h with Aspf1, Crf1, and LPS, and their expression (in percent) was compared to that of unstimulated DCs.
The T-cell stimulation capacity of iDCs after coculturing with Aspf1.
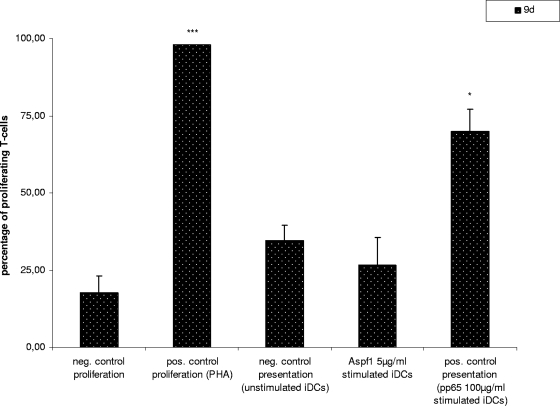
The capacity of DCs to stimulate T-cell proliferation was analyzed by MLR with Aspf1-loaded DCs. We could demonstrate that Aspf1-loaded DCs and unstimulated DCs triggered the proliferation of T cells to similar levels. In contrast, DCs loaded with pp65 induced a significantly higher percentage of proliferating CD8+ T cells (P = 0.099) (Fig. 5).
FIG. 5.
MLR. Student's t test was performed by pairing the results with those for the negative control for presentation by unstimulated iDCs. *, P < 0.1; ***, P < 0.01.
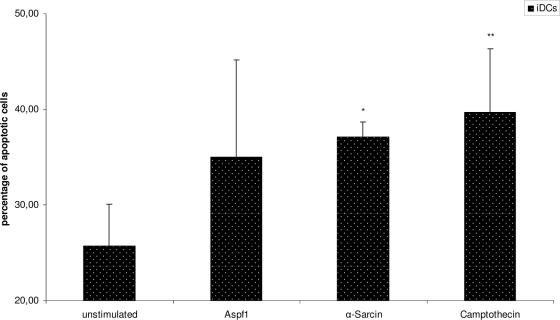
Aspf1 induces apoptosis in iDCs.
To quantify the apoptotic activity of Aspf1, this antigen was exposed to iDCs and the rates of early and late apoptosis were measured by the use of annexin V and propidium iodide staining. In parallel, α-sarcin and camptothecin were applied to the iDCs and the rates of apoptosis were compared to those induced by Aspf1. We found that Aspf1, α-sarcin, and camptothecin were able to induce increased levels of apoptosis of +9.3%, (P = 0.259), +11.3% (P = 0.099), and +13.93% (P = 0.034), respectively, after 24 h of exposure compared to the levels for untreated cells (Fig. 6).
FIG. 6.
Quantification of apoptosis of DCs after treatment with Aspf1, á-sarcin, or camptothecin. Percentage of apoptotic DCs was determined by flow cytometry after cocultivation of DCs for 24 h with Aspf1, á-sarcin, or camptothecin. *, P < 0.1; **, P < 0.05.
DISCUSSION
One of the major players of the immune defense against fungi are DCs, the sentries of innate immunity and the bridge to the adaptive immune system. They are strategically located at pathogen entry sites, such as the respiratory tract (14). DCs acquire antigens in the periphery, migrate into secondary lymphoid tissues, activate T cells, mature, and secrete cytokines and chemokines (21). In lymphoid organs, DCs present (i) antigen-specific, (ii) costimulatory, and (iii) Th1/Th2 polarizing signals to T-helper cells (29). The polarization of DCs is dependent on different factors, including DC subsets, pathogen recognition receptors (e.g., TLRs or dectin 1), and the morphology of the mold (1, 24).
DCs have previously been used as adjuvants and antigen carriers in various vaccination studies against malignancies and infectious agents, such as human cytomegalovirus and human immunodeficiency virus (8, 11, 35). Few data on DC vaccination against A. fumigatus in mice have been published (27). Svirshchevskaya et al. showed that the intravenous injection of Aspf1 peptides markedly inhibited the T-cell response induced by the exposure to crude A. fumigatus extract in mice (31). In addition, Bozza et al. showed that fungal RNA acts as a potent activator of murine DCs (6).
However, to our knowledge, no data exist about the interaction of human DCs with recombinant antigenic structures of A. fumigatus. Within the last 15 years, Latgé and colleagues characterized several antigens of A. fumigatus (5). Those studies showed that the production of recombinant antigens of A. fumigatus in Pichia pastoris is extremely efficient. Large amounts of recombinant proteins (5 to 50 μg/ml) are produced extracellularly and easily purified on an Ni column. His tagging did not interfere with the antigenic capacity of the proteins, and unlike proteins derived from the commonly used E. coli expression systems, these antigens are endotoxin free.
Among the purified antigens, Aspf1 is a member of a family of conserved RNases that cleave a single phosphodiester bond of the 28S rRNA of eukaryotic ribosomes (16). The natural function of Aspf1 is still unknown. However, an indication that Aspf1 is produced during infection was provided by Lamy et al., who detected Aspf1 within the kidney cells of mice infected with A. fumigatus in regions of necrosis surrounding fungal colonies (20). Furthermore, Arruda et al. demonstrated that 85% of the patients with IgE antibodies to A. fumigatus also had IgE antibodies to Aspf1, which they defined as a major allergen of the fungus (3).
Our results show that Aspf1 is able to induce the apoptosis of human iDCs in vitro, as previously described for α-sarcin, an additional fungal ribotoxin (25). It could be speculated that the induction of apoptosis is a natural immune evasion mechanism of A. fumigatus, as DC apoptosis may limit the generation of an appropriate adaptive immune response during infection, including an impaired capacity to stimulate T lymphocytes. Additionally, Kim et al. have demonstrated that phosphatidylserine from apoptotic cells induces the regulation of DC activation. Downstream events following apoptotic cell contact or phosphatidylserine contact with DCs resulted in the inhibition of IL-12p35 transcription and, thus, IL-12p70 synthesis (17). Additional studies demonstrated that another molecule secreted by A. fumigatus, gliotoxin, inhibited antigen-presenting cell function and induced the preferential death of monocytes, leading to a marked decrease in the monocyte-lymphocyte ratio (30).
It was previously shown that ribotoxins cross lipid membranes in the absence of any known protein receptor, possibly by endocytosis and by interacting with negatively charged phospholipids vesicles. These extremely positively charged proteins also have a high affinity for RNA, which alleviates the localization of the α-sarcin/ricin loop of 28S rRNA. However, cells are killed only if ribotoxins cross their membrane, which is facilitated in cells showing altered membrane permeability, such as virus-infected or transformed cells (19). As Aspf1 was detectable on the DC surface 15 min postinfection and the signal markedly increased up to 3 h, only a minority of the protein might have been able to cross the cell membrane and to inhibit ribosomal activity.
Our results indicate that Aspf1 induces a proinflammatory cytokine response. The levels of expression of two distinct chemokines, CXCL10 and CCL20, were markedly increased compared to the levels expressed by unstimulated DCs. CXCL10 has been shown to have chemoattraction for monocytes and T cells and to promote T-cell adhesion to endothelial cells. CCL20 is strongly chemotactic for lymphocytes and weakly attracts neutrophils.
Interestingly, stimulation with LPS and also A. fumigatus germlings (23) resulted in maximum levels of gene expression and cytokine release after 6 h, whereas Aspf1 stimulation revealed the maximum levels of expression and secretion of the cytokine genes after 24 h and 48 h. TLR ligands, such as LPS, not only stimulate the transcription of cytokines and costimulatory molecules but also signal an array of responses that affect the membrane vacuolar system, the cytoskeleton, and the machinery of protein translation and degradation (34).
The cytokine release by iDCs was weaker if they were stimulated by wild-type germlings than if they were stimulated with germlings from the Δaspf1 mutant strain. This observation might indicate that Aspf1, which is secreted into the culture medium, could possibly impair in vitro the release of defined cytokines and chemokines.
Induction of the proinflammatory immune response by Aspf1 might potentially be regulated by the transcription factor NFκB. This DNA-binding protein exists in an inactive form bound to the inhibitory IκB proteins in the cytoplasm. LPS or Aspf1 treatment of DCs led to the release of NFκB dimers, which subsequently translocated to the nucleus, where they activated immune-relevant target genes. Careful regulation of the transcriptional responses to many different stimuli is crucial to the proper functioning of the mammalian immune system (13). Activation of NFκB as a result of the reduction of the level of protein synthesis due to ribosome inhibition by Aspf1 or other related ribotoxins is mediated by SAPK/JNK1 (stress-activated protein kinases and cJun NH2-terminal kinases, respectively) (25). Damage of the 28S rRNA at the cleavage site of ribotoxins causes the activation of SAPK/JNK1 (15). These members of the family of mitogen-activated protein kinases phosphorylate substrates such as the cAMP response binding protein and NFκB (33).
By MLR, we were able to demonstrate that in contrast to the CMV antigen pp65, Aspf1 is not processed and presented by iDCs; the proliferation of T cells was not significantly altered compared to the level of proliferation achieved with unloaded iDCs. Similar results were obtained after the stimulation of iDCs with α-sarcin.
In conclusion, the induction of cytokine release and apoptosis by Aspf1 in human iDCs may constitute a novel immunomodulatory mechanism that results in the destruction and functional impairment of iDCs and, in parallel, the immune evasion of A. fumigatus.
Acknowledgments
This study was supported by research funding from Schwerpunktprogramm 1160 of the Deutsche Forschungsgemeinschaft, the EU project Development of Novel Management Strategies for Invasive Aspergillosis (MANASP, LSHE-CT-2006-037899), the IZKF Wuerzburg (project A50), and the Bayerische Immunnetzwerk (BayImmuNet).
We thank Oliver Morton, Trinity College, Dublin, Ireland, for correction of the English language and all volunteers for their generous blood donations.
We do not have any conflicts regarding financial interests.
Footnotes
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Akira, S., and H. Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo, J., J. Sarfati, M. T. Baixench, E. Ragni, M. Guillén, J. M. Rodriguez-Peña, L. Popolo, and J. P. Latgé. 2007. The GRI-anchored Gas and Crh families are fungal antigens. Yeast 4:289-296. [DOI] [PubMed] [Google Scholar]
- 3.Arruda, L. K., T. A. Platts-Mills, J. W. Fox, and M. D. Chapman. 1990. Aspergillus fumigatus allergen I, a major IgE-binding protein, is a member of the mitogillin family of cytotoxins. J. Exp. Med. 172:1529-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes, P. D., and K. A. Marr. 2007. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br. J. Haematol. 139:519-531. [DOI] [PubMed] [Google Scholar]
- 5.Beauvais, A., M. Monod, J. P. Debeaupuis, M. Diaquin, H. Kobayashi, and J. P. Latgé. 1997. Biochemical and antigenic characterization of a new dipeptidyl-peptidase isolated from Aspergillus fumigatus. J. Biol. Chem. 272:6238-6244. [DOI] [PubMed] [Google Scholar]
- 6.Bozza, S., K. Perruccio, C. Montagnoli, R. Gaziano, S. Bellocchio, E. Burchielli, G. Nkwanyuo, L. Pitzurra, A. Velardi, and L. Romani. 2003. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 102:3807-3814. [DOI] [PubMed] [Google Scholar]
- 7.Bustin, S. A., V. Benes, J. A. Garson, J. Hellemans, J. Huggett, M. Kubista, R. Mueller, T. Nolan, M. W. Pfaffl, G. L. Shipley, J. Vandesompele, and C. T. Wittwer. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611-622. [DOI] [PubMed] [Google Scholar]
- 8.Einsele, H., G. Rauser, U. Grigoleit, H. Hebart, C. Sinzger, S. Riegler, and G. Jahn. 2002. Induction of CMV-specific T-cell lines using Ag-presenting cells pulsed with CMV protein or peptide. Cytotherapy 4:49-54. [DOI] [PubMed] [Google Scholar]
- 9.Gafa, V., R. Lande, M. C. Gagliardi, M. Severa, E. Giacomini, M. E. Remoli, R. Nisini, C. Ramoni, P. Di Francesco, D. Aldebert, R. Grillot, and E. M. Coccia. 2006. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect. Immun. 74:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gafa, V., M. E. Remoli, E. Giacomini, M. C. Gagliardi, R. Lande, M. Severa, R. Grillot, and E. M. Coccia. 2007. In vitro infection of human dendritic cells by Aspergillus fumigatus conidia triggers the secretion of chemokines for neutrophil and Th1 lymphocyte recruitment. Microbes Infect. 9:971-980. [DOI] [PubMed] [Google Scholar]
- 11.Grigoleit, U., M. Kapp, H. Hebart, K. Fick, R. Beck, G. Jahn, and H. Einsele. 2007. Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. J. Infect. Dis. 196:699-704. [DOI] [PubMed] [Google Scholar]
- 12.Grigoleit, U., S. Riegler, H. Einsele, K. Laib Sampaio, G. Jahn, H. Hebart, P. Brossart, F. Frank, and C. Sinzger. 2002. Human cytomegalovirus induces a direct inhibitory effect on antigen presentation by monocyte-derived immature dendritic cells. Br. J. Haematol. 119:189-198. [DOI] [PubMed] [Google Scholar]
- 13.Hayden, M. S., and S. Ghosh. 2004. Signaling to NFκB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 14.Holt, P. G. 2000. Antigen presentation in the lung. Am. J. Respir. Crit. Care Med. 162:151-156. [DOI] [PubMed] [Google Scholar]
- 15.Iordanov, M. S., D. Pribnow, J. L. Magun, T. H. Dinh, J. A. Pearson, S. L. Y. Chen, and B. E. Magun. 1997. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 17:3373-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao, R., and J. Davies. 1995. Fungal ribotoxins: a family of naturally engineered targeted toxins? Biochem. Cell Biol. 73:1151-1159. [DOI] [PubMed] [Google Scholar]
- 17.Kim, S., K. B. Elkon, and X. Ma. 2004. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21:643-653. [DOI] [PubMed] [Google Scholar]
- 18.Kubach, J., C. Becker, E. Schmitt, K. Steinbrink, E. Huter, A. Tuettenberg, and H. Jonuleit. 2005. Dendritic cells: sentinels of immunity and tolerance. Int. J. Hematol. 81:197-203. [DOI] [PubMed] [Google Scholar]
- 19.Lacadena, J., E. Álvarez-García, N. Carreras-Sangrà, E. Herrero-Galán, J. Alegre-Cebollada, L. García-Ortega, M. Onaderra, J. G. Gavilanes, and Á. M. del Pozo. 2007. Fungal ribotoxins: molecular dissection of a family of natural killers. FEMS Microbiol. Rev. 31:212-237. [DOI] [PubMed] [Google Scholar]
- 20.Lamy, B., M. Moutaouakil, J. P. Latgé, and J. Davies. 1991. Secretion of a potential virulence factor, a fungal ribonucleotoxin, during human aspergillosis infections. Mol. Microbiol. 5:1811-1815. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263-266. [DOI] [PubMed] [Google Scholar]
- 22.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 23.Mezger, M., S. Kneitz, I. Wozniok, O. Kurzai, H. Einsele, and J. Loeffler. 2008. Pro-inflammatory response of human immature dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J. Infect. Dis. 15:924-931. [DOI] [PubMed] [Google Scholar]
- 24.Netea, M. G., J. W. Van der Meer, and B. J. Kullberg. 2006. Role of the dual interaction of fungal pathogens with pattern recognition receptors in the activation and modulation of host defence. Clin. Microbiol. Infect. 12:404-409. [DOI] [PubMed] [Google Scholar]
- 25.Olmo, N., J. Turnay, G. González de Buitrago, I. López de Silames, J. G. Gavilanes, and M. A. Lizarbe. 2001. Cytotoxic mechanism of the ribotoxin α-sarcin. Eur. J. Biochem. 268:2113-2123. [DOI] [PubMed] [Google Scholar]
- 26.Paris, S., M. Monod, M. Diagiun, B. Lamy, L. K. Arruda, P. J. Punk, and J. P. Latgé. 1993. A transformant of Aspergillus fumigatus deficient in the antigenic cytotoxin ASPFI. FEMS Microbiol. Lett. 111:31-36. [DOI] [PubMed] [Google Scholar]
- 27.Perruccio, K., S. Bozza, C. Montagnoli, S. Bellocchio, F. Aversa, M. Martelli, F. Bistoni, A. Velardi, and L. Romani. 2004. Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol. Dis. 33:248-255. [DOI] [PubMed] [Google Scholar]
- 28.Sarfati, J., M. Monod, P. Recco, A. Sulahian, C. Pinel, E. Candolfi, T. Fontaine, J. P. Debeaupuis, M. Tabouret, and J. P. Latgé. 2006. Recombinant antigens as diagnostic markers for aspergillosis. Diagn. Microbiol. Infect. Dis. 55:279-291. [DOI] [PubMed] [Google Scholar]
- 29.Shortman, K., and W. R. Heath. 2001. Immunity or tolerance? That is the question for dendritic cells. Nat. Immunol. 2:988-989. [DOI] [PubMed] [Google Scholar]
- 30.Stanzani, M., E. Orciuolo, R. Lewis, D. P. Kontoyiannis, S. L. Martins, L. S. St. John, and K. V. Komanduri. 2005. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 105:2258-2265. [DOI] [PubMed] [Google Scholar]
- 31.Svirshchevskaya, E., E. Frolova, L. Alekseeva, O. Kotzareva, and V. P. Kurup. 2000. Intravenous injection of major and cryptic peptide epitopes of ribotoxin, Asp f 1 inhibits T cell response induced by crude Aspergillus fumigatus antigens in mice. Peptides 21:1-8. [DOI] [PubMed] [Google Scholar]
- 32.Tong, K. B., C. J. Lau, K. Murtagh, A. J. Layton, and R. Seifeldin. 2009. The economic impact of aspergillosis: analysis of hospital expenditures across patient subgroups. Int. J. Infect. Dis. 13:24-36. [DOI] [PubMed] [Google Scholar]
- 33.Tsatsanis, C., A. Androulidaki, M. Venihaki, and A. N. Margioris. 2006. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 38:1654-1661. [DOI] [PubMed] [Google Scholar]
- 34.Watts, C., R. Zaru, A. R. Prescott, R. P. Wallin, and M. A. West. 2007. Proximal effects of Toll-like receptor activation in dendritic cells. Curr. Opin. Immunol. 19:73-78. [DOI] [PubMed] [Google Scholar]
- 35.Wierecky, J., M. Mueller, and P. Brossart. 2006. Dendritic cell-based cancer immunotherapy targeting MUC-1. Cancer Immunol. Immunother. 55:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]