Abstract
Deer are acknowledged as hosts of Mycobacterium bovis, the causative agent of bovine tuberculosis (bTB), and determining the prevalence of infection in deer species is one of the key steps in understanding the epidemiological role played by cervids in the transmission and maintenance of bTB in the United Kingdom. This study evaluated a rapid lateral-flow test for the detection of bTB in samples from wild deer species in the United Kingdom. Fallow deer (Dama dama), roe deer (Capreolus capreolus), and red deer (Cervus elaphus) from areas in Wales, the Cotswolds, and southwestern England were necropsied for a bTB survey. Serum samples from individual deer were tested with the CervidTB STAT-PAK, and the results were evaluated against the culture of M. bovis from tissues (n = 432). Sensitivity and specificity were 85.7% (95% confidence interval [CI], 42.1 to 99.6%) and 94.8% (95% CI, 92.3 to 96.7%), respectively, with an odds ratio of 109.9 (95% CI, 12.7 to 953.6%) for a positive STAT-PAK result among culture-positive deer. The low prevalence of infection (3.8%, n = 860) affected the confidence of the sensitivity estimate of the test, but all culture-positive fallow deer (n = 6) were detected by the test. In addition, antibodies to M. bovis could be detected in poor-quality serum samples. The results suggest that the CervidTB STAT-PAK could be deployed as a field test for further evaluation.
In the United Kingdom, the involvement of wildlife species in the disease dynamics of bovine tuberculosis (bTB) has fueled research into their potential role in the maintenance and transmission of the disease. Badgers (Meles meles) are thought to be the main wildlife reservoir of bTB, but the presence of Mycobacterium bovis in United Kingdom deer species (1, 4) and the risk of spillover posed by deer in other countries (7, 18) have driven the need to evaluate the risk posed to cattle by the spillover of M. bovis from cervids in the United Kingdom.
A semiquantitative risk assessment indicated that fallow deer (Dama dama dama) in particular posed a relatively high, though localized, risk of transmission to cattle in areas with a high incidence of bTB (5). However, model predictions were constrained by the lack of adequate data on the abundance and distribution of the six United Kingdom deer species and the prevalence and distribution of M. bovis infection in these species. To address this issue, the Department for the Environment, Food and Rural Affairs (DEFRA) commissioned additional surveys of M. bovis in deer (6).
Obtaining statistically robust prevalence estimates from large wildlife populations poses a number of challenges, particularly when the prevalence of infection is low. In New Zealand, for example, control policies aimed at both domestic (cattle and deer) and wildlife (possums) species have reduced the herd prevalence of bTB in cattle and deer from 2.4% in 1993 to 0.35% in 2004 (21), but the assays used for whole-herd diagnostic testing include an antibody enzyme-linked immunosorbent assay and a lymphocyte transformation assay (9), both of which require specialized laboratory equipment and trained staff. The alternative is mycobacterial culture for the diagnosis of M. bovis infection, and although this is the “gold standard,” it is a labor-intensive, slow, and costly process which cannot be performed animal side. The sensitivity of culture depends on the thoroughness of the postmortem examination (the range of tissue types and the quantity and area of each tissue sampled), and it can take up to 12 weeks to obtain a result.
The availability of accurate, more rapid, less labor-intensive, and less expensive diagnostic assays which could be applied to deer under field conditions would be of great advantage. Serological assays, which are rapid and only require a blood sample, obtainable from a single live sampling event, lend themselves to the screening of large numbers of wild animals (2). Changes to the antigen cocktails utilized for the detection of specific antibody responses have improved sensitivity while maintaining specificity (10), and STAT-PAK (Chembio Diagnostic Systems Inc., Medford, NY), a lateral-flow system for the detection of immunoglobulin A (IgA), IgM, and IgG antibodies to M. bovis and M. tuberculosis, has shown good potential in a number of species (13, 14, 15), including badgers (3). The STAT-PAK test format has also proven particularly sensitive in detecting individuals with advanced disease caused by M. bovis (13), and given that individuals with advanced disease are also more likely to be excreting, they are important targets for control programs. Chronically infected deer are more likely to be skin test negative, and therefore antibody tests play an important role in identifying these animals (9, 17).
The CervidTB STAT-PAK, which utilizes a range of antigens, including MPB83, ESAT-6, and CFP10, has been developed for the detection of antibodies to M. bovis and M. tuberculosis in deer. As an easy-to-use test which can be performed in the field with only a small volume of blood, serum, or plasma, the CervidTB STAT-PAK is potentially a valuable alternative to mycobacterial culture and cell-based assays currently available for use in cervids (23). This study aimed to evaluate the sensitivity, specificity, and accuracy of the CervidTB STAT-PAK on serum from deer against the culture of M. bovis from deer tissues obtained during DEFRA's survey of tuberculosis in deer in southwestern England and the Cotswolds and a similar survey of deer in Wales.
MATERIALS AND METHODS
The deer survey.
The survey focused on areas of high bTB prevalence in cattle in the United Kingdom and was designed for a 95% certainty of detecting disease at a prevalence of ≥5% (20). Running between December 2006 and February 2008, the survey aimed to sample 700 deer across 10 sites in the southwestern peninsula and the Cotswolds; for this study, additional samples were obtained from the Wales deer survey (22) between November 2007 and January 2008 inclusive.
In the southwestern peninsula, samples were obtained from all areas of the public forest estate (Forestry Commission land) in Somerset, Devon, and Cornwall. In the Cotswolds, deer were sampled from three large private estates: the Barnsley Wold deer management area (32 km2), Chedworth (143 km2), and the Cirencester Park area (253 km2). In Wales, deer were sampled from Forestry Commission land in the Lower Wye Valley forest district and the Coed-y-Cymoedd forest district. These areas were selected based on the high frequency of cattle herd breakdowns and a high prevalence of bTB in badgers.
Tissue sampling.
All deer shot for routine management and sporting purposes during each species' open season were sampled. All carcasses were subjected to a routine postmortem meat inspection as required by European Union food hygiene regulations. Stalkers and Forestry Commission officials were trained and instructed to collect tissues by a standard protocol, thereby ensuring that all postmortems were performed to the same standard; the stalkers' knowledge was valuable, as it enabled them to better detect the lesions. Tissues were collected at the time of carcass inspection or gutting (removal of offal), and a maximum of 20 g of tissue from each individual was submitted. From carcasses with visible lesions (VL) typical of bTB infection, the two most characteristic lesions with a small circumference of normal tissue were submitted, for a total of no more than 20 g of tissue. Samples from deer with no VL (classified as those with no VL or lesions atypical of bTB) comprised a predefined list of lymph node tissue samples: paired bronchial samples, mediastinal samples, paired retropharyngeal samples, a sample of mesenteric lymph nodes, and samples of any other abnormal lymph nodes. Other lymph nodes, known to harbor bTB lesions, were only included if they looked abnormal, i.e., paired prescapular, supramammary, hepatic, and head nodes other than the retropharyngeal nodes.
Culture of M. bovis from tissue samples.
Tissue samples were submitted by first class mail to the TB Diagnostics Department of the Veterinary Laboratories Agency at Weybridge. Tissues were trimmed of fat, any VL were described, and then the tissues were ground in a stomacher with 15 ml 0.85% saline. The tissue was decontaminated with 15 ml 10% oxalic acid and washed in 30 ml 0.85% physiological saline, and then 0.3 ml of inoculum preparation was sown onto the following solid-medium slopes: three slopes each of Stonebrinks medium, modified 7H11 medium, and Dubos medium with or without mycobactin and two slopes each of Lowenstein-Jensen (LJ) medium base, LJ medium base with pyruvate, and LJ medium base with glycerol. The remaining inoculum was frozen for reculture and utilized after being retreated with oxalic acid if more than one of the LJ medium base or 7H11 medium cultures were contaminated. Single liquid cultures were prepared with 3 and 10 ml of 7H9 medium and double-strength Dubos broth, respectively, both contained in screw-cap glass vials. The slopes were read after 6 weeks of incubation at 37°C. In cases with no evidence of growth on the solid medium, a Ziehl-Neelsen stain was made from both types of Dubos medium, and if it was clear of acid-fast bacteria, the sample was declared negative. Any growths characteristic of mycobacteria or any identified as acid-fast bacteria were identified as M. bovis by spoligotyping (11, 12).
Blood collection and processing.
Samplers were instructed to collect fresh nonclotted blood from the heart or jugular vein as soon as possible after death. Blood from each individual was collected into one 8.5-ml SST Vacutainer tube (BD, Plymouth, United Kingdom) which was submitted, by post, with the individual's tissue samples. In order to avoid a prolonged time in transit, samples were not posted on Fridays, Saturdays, or Bank Holidays but stored in approved larder chillers (4 to 6°C) until the next working day. Upon receipt, the blood tubes were stored between 4 and 8°C for a maximum of 8 h, after which the blood was centrifuged and the serum tested. Some serum samples had to be frozen prior to testing, but unless a test had to be repeated, no serum samples went through more than one freeze-thaw cycle prior to testing. Serum samples were stored frozen at −80°C. Blood sample quality was assessed by subjective scoring of the degree of hemolysis and a record of the length of time spent in transit. Samples with no visible hemolysis were given a score of 0; the maximum hemolysis score was 4 for highly hemolyzed and/or decomposed samples, all of which were tested. The length of time spent in transit was calculated as the time between slaughter and sample receipt.
CervidTB STAT-PAK.
The serum samples were tested according to the manufacturer's instructions. Briefly, 30 μl of serum was added to the test strip, followed by 3 drops of diluent; the results were read visually after 20 min. The test results were only considered valid if a blue band was visible in the control area of the test strip. If a blue band also developed in the test area, the sample was considered antibody positive; if the test area remained clear, the sample was considered antibody negative. Any tests which failed to develop a line in the control area were repeated with a new kit.
Statistical analyses.
The correlation between the time spent in transit and the degree of hemolysis was tested with the Spearman rank analysis tool of the GraphPad Prism 5.02 software (GraphPad Software, Inc., La Jolla, CA). The Fisher exact test analysis tool of the same software was utilized to test for a difference in the sensitivity of STAT-PAK as applied to good (low hemolysis score)- and poor (high hemolysis score)-quality samples. In both analyses, significance was taken as a P value of <0.05.
RESULTS
Prevalence of M. bovis and characterization of serum samples.
A total of 860 deer were tissue sampled for the culture of M. bovis; not all of the 860 deer could be STAT-PAK tested, as blood sampling was initiated 4 months after the start of the survey. For the STAT-PAK evaluation, 439 (86.1%) of the 512 tissue samples submitted during this evaluation were accompanied by blood samples. Of the 439 samples received, 7 (1.6%) failed to yield any serum and therefore 432 deer were tested for M. bovis infection by both STAT-PAK and tissue culture. The culture prevalence of the survey as a whole was low (3.8%, n = 860), and only 1.6% (n = 432) of the deer used in the STAT-PAK evaluation were culture positive.
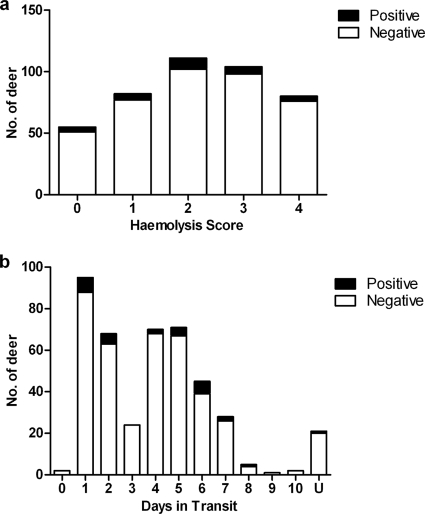
The blood samples showed a great variation in quality, as indicated by the degree of hemolysis (Fig. 1a), and spent up to 10 days (in storage and) in transit (Fig. 1b). More than half (57%) of the serum samples spent longer than 3 days in transit, and 87% (n = 432) were classed as hemolyzed (scores of 1 to 4); there was a weak association between the time spent in transit and the degree of hemolysis (Spearman rank, R = 0.62, P = 0.048). Nonetheless, antibodies to M. bovis were detected not only in good-quality serum samples but also in hemolyzed samples from culture-positive deer which had not been separated for as long as 6 days postslaughter.
FIG. 1.
Distribution of STAT-PAK-positive deer across the samples' hemolysis scores (a) and the times samples spent in transit (b). n = 432.
CervidTB STAT-PAK performance.
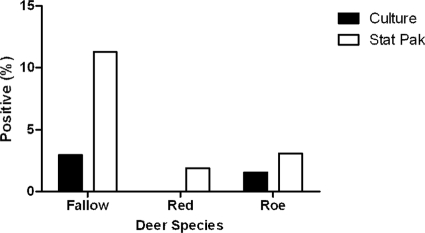
In this study, estimates of the sensitivity and positive predictive value (PPV; Table 1) of STAT-PAK had broad confidence intervals (CIs), as the prevalence of M. bovis was low. The proportion of positive STAT-PAK results (28/432, 6.5%) was higher than the culture prevalence (7/432, 1.6%) for each species (Fig. 2). Of the 7 culture-positive deer that were STAT-PAK tested, 6 (85.7%) were STAT-PAK reactive; of the 425 culture-negative deer, 403 (94.8%) were STAT-PAK nonreactive (Table 2). The odds ratio was 109.9 (95% CI, 12.7 to 953.6) for a positive STAT-PAK result among culture-confirmed cases. In other words, culture-positive deer were 110 times more likely to yield a positive STAT-PAK result than were culture-negative deer. The condition of the samples has been shown to negatively affect the sensitivity of STAT-PAK for the detection of bTB in badgers (3), but it was not possible to detect such an effect in this study (Fisher exact test, P > 0.05). Only two (0.5%) of the STAT-PAK tests failed to produce a line in the control section of the strip, and these culture-negative samples were retested and were both STAT-PAK negative.
TABLE 1.
Performance of CervidTB STAT-PAK
| Parametera | Value (%) | 95% CI |
|---|---|---|
| Sensitivity | 85.7 | 42.1-99.6 |
| Specificity | 94.8 | 92.3-96.7 |
| Accuracy | 94.7 | 92.1-96.6 |
| PPV | 21.4 | 8.3-41.0 |
| NPV | 99.8 | 98.6-100.0 |
The accuracy is the percentage of STAT-PAK results which were in agreement with bTB culture results. NPV, negative predictive value. n = 432.
FIG. 2.
Prevalence of M. bovis in the different deer species as determined by culture and STAT-PAK.
TABLE 2.
Numbers of deer positive and negative by STAT-PAK and culturea
| STAT-PAK result | No of cultures
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 6 | 22 | 28 |
| Negative | 1 | 403 | 404 |
| Total | 7 | 425 | 432 |
Culture-positive deer were those positive for M. bovis, while culture-negative deer included those positive for M. avium and other, unclassified, mycobacteria. Of those culture negative for M. bovis (n = 425), 6 were positive for M. avium and 2 for unclassified mycobacteria. STAT-PAK was specific, as only one of the M. avium-positive deer was STAT-PAK positive.
DISCUSSION
The performance of the CervidTB STAT-PAK on United Kingdom deer (Table 1) compared well to a previous evaluation with white-tailed deer (Odocoileus virginianus) in the United States in which the sensitivity, specificity, and accuracy were 75.0% (95% CI, 54.8 to 88.6%), 98.9% (95% CI, 97.2 to 99.6%), and 97.4% (95% CI, 95.4 to 98.6%), respectively (13). The CervidTB STAT-PAK performed similarly well (sensitivity, 73%; specificity, 100%) with experimentally infected C. elaphus (10). However, comparisons between different deer species must be interpreted with caution, as different species vary in susceptibility to bTB (16) and experimentally infected animals may yield a higher test sensitivity than naturally infected free-ranging animals (13).
Although the CervidTB STAT-PAK was sensitive, the low prevalence of bTB among deer in this survey resulted in a low PPV and broad CIs for the sensitivity estimate (85.7%, 42.1 to 99.6%). Only a small subset of all culture-positive deer were STAT-PAK tested (7/33 culture positives out of 860 deer sampled in the survey as a whole). This is, in part, due to the fact that the blood sampling started 4 months after the survey started and because samplers failed to collect blood from 73 animals. This meant that five additional culture-positive deer could not be STAT-PAK tested. A lower sensitivity has also been observed elsewhere for this assay (19), and this has implications for the use of the test on United Kingdom deer if further evaluations on the United Kingdom deer species again result in a low sensitivity estimate.
A relatively high proportion of positive CervidTB STAT-PAK results remained unconfirmed by culture, as STAT-PAK positivity was higher than the culture prevalence and more than 70% of the STAT-PAK-positive deer were culture negative. This may be due to the fact that culture underestimates the true prevalence of infection. The probability of isolating M. bovis by culture is higher for samples taken from a whole (and thoroughly inspected) carcass than it is from gutting alone (5). Culture from selected lymph nodes may not be sensitive enough to detect those with very low-grade infections. However, the prevalence estimate for the southwest in the most recent survey (3.4%, 22/655; 95% CI, 2.1 to 5.4%) (20) was similar to that of a previous survey of the same region (2.2%, 36/1646; 95% CI, 1.5 to 3.0%) which utilized more-thorough sampling techniques (5).
In this study, the CervidTB STAT-PAK showed a relatively low proportion of false positives (5.2%). A previous evaluation of this test with serum samples from animals infected with non-TB mycobacteria found no cross-reactions (13), and a specificity of 95% was also reported for badgers (8). In the present study, only one individual was STAT-PAK positive and M. avium culture positive, and it is possible that the serological response of this individual was due to a cryptic M. bovis infection or recovery from an M. bovis infection while still seropositive. In contrast, a recent evaluation of the CervidTB STAT-PAK in New Zealand demonstrated a lower specificity (83.8%) due to a partial cross-reactivity of the assay in deer infected with Johne's disease (B. Buddle, unpublished data); the prevalence of Johne's disease in United Kingdom deer is largely unknown.
In conclusion, this study showed the CervidTB STAT-PAK to be a potentially sensitive and practical assay for the detection of M. bovis infection in deer in Great Britain. It would be advantageous to deploy this test in the field for additional validation with fresh whole-blood samples to improve the confidence of the sensitivity estimate by eliminating postal delays and improving the quality of the blood samples tested.
Acknowledgments
We thank the staff of the Veterinary Laboratories Agency TB Diagnostic Department for their help with the samples and culture data. We also thank C. Griffiths of the Welsh Assembly Government's Department for Rural Affairs for her assistance in obtaining deer samples and S. Palmer and R. Ashford for their assistance with the sample processing.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Anonymous. 1997. Bovine tuberculosis in badgers. MAFF Publications, London, United Kingdom.
- 2.Buddle, B. M., M. A. Skinner, and M. A. Chambers. 2000. Immunological approaches to the control of tuberculosis in wildlife reservoirs. Vet. Immunol. Immunopathol. 74:1-16. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, M. A., T. Crawshaw, S. Waterhouse, R. Delahay, R. G. Hewinson, and K. P. Lyashchenko. 2008. Validation of the BrockTB Stat-Pak assay for detection of tuberculosis in Eurasian badgers (Meles meles) and influence of disease severity on diagnostic accuracy. J. Clin. Microbiol. 46:1498-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delahay, R. J., C. L. Cheeseman, and R. S. Clifton-Hadley. 2001. Wildlife disease reservoirs: the epidemiology of Mycobacterium bovis infection in the European badger (Meles meles) and other British mammals. Tuberculosis (Edinburgh) 81:43-49. [DOI] [PubMed] [Google Scholar]
- 5.Delahay, R. J., G. C. Smith, A. M. Barlow, N. Walker, A. Harris, R. S. Clifton-Hadley, and C. L. Cheeseman. 2007. Bovine tuberculosis infection in wild mammals in the South-West region of England: a survey of prevalence and a semi-quantitative assessment of the relative risks to cattle. Vet. J. 173:287-301. [DOI] [PubMed] [Google Scholar]
- 6.Department for Environment, Food, and Rural Affairs. 28 November 2008. Wild deer and bovine TB. Department for Environment, Food, and Rural Affairs, London, United Kingdom. http://www.defra.gov.uk/news/2008/081126b.htm.
- 7.Gortázar, C., M. J. Torres, J. Vicente, P. Acevedo, M. Reglero, J. de la Fuente, J. J. Negro, and J. Aznar-Martin. 2008. Bovine tuberculosis in Donana Biosphere Reserve: the role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS ONE 3:e2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwald, R., J. Esfandiari, S. Lesellier, R. Houghton, J. Pollock, C. Aagaard, P. Andersen, R. G. Hewinson, M. Chambers, and K. Lyashchenko. 2003. Improved serodetection of Mycobacterium bovis infection in badgers (Meles meles) using multiantigen test formats. Diagn. Microbiol. Infect. Dis. 46:197-203. [DOI] [PubMed] [Google Scholar]
- 9.Griffin, J. F., and C. G. Mackintosh. 2000. Tuberculosis in deer: perceptions, problems and progress. Vet. J. 160:202-219. [DOI] [PubMed] [Google Scholar]
- 10.Harrington, N. P., O. P. Surujballi, J. F. Prescott, J. R. Duncan, W. R. Waters, K. Lyashchenko, and R. Greenwald. 2008. Antibody responses of cervids (Cervus elaphus) following experimental Mycobacterium bovis infection and the implications for immunodiagnosis. Clin. Vaccine Immunol. 15:1650-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewinson, R. G., H. M. Vordermeier, N. H. Smith, and S. V. Gordon. 2006. Recent advances in our knowledge of Mycobacterium bovis: a feeling for the organism. Vet. Microbiol. 112:127-139. [DOI] [PubMed] [Google Scholar]
- 12.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, M. A. Chambers, J. Vicente, C. Gortazar, N. Santos, M. Correia-Neves, B. M. Buddle, R. Jackson, D. J. O'Brien, S. Schmitt, M. V. Palmer, R. J. Delahay, and W. R. Waters. 2008. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 132:283-292. [DOI] [PubMed] [Google Scholar]
- 14.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, D. Greenwald, C. A. Nacy, S. Gibson, P. J. Didier, M. Washington, P. Szczerba, S. Motzel, L. Handt, J. M. Pollock, J. McNair, P. Andersen, J. A. Langermans, F. Verreck, S. Ervin, F. Ervin, and C. McCombs. 2007. PrimaTB STAT-PAK assay, a novel, rapid lateral-flow test for tuberculosis in nonhuman primates. Clin. Vaccine Immunol. 14:1158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, J. H. Olsen, R. Ball, G. Dumonceaux, F. Dunker, C. Buckley, M. Richard, S. Murray, J. B. Payeur, P. Andersen, J. M. Pollock, S. Mikota, M. Miller, D. Sofranko, and W. R. Waters. 2006. Tuberculosis in elephants: antibody responses to defined antigens of Mycobacterium tuberculosis, potential for early diagnosis, and monitoring of treatment. Clin. Vaccine Immunol. 13:722-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackintosh, C. G., T. Qureshi, K. Waldrup, R. E. Labes, K. G. Dodds, and J. F. Griffin. 2000. Genetic resistance to experimental infection with Mycobacterium bovis in red deer (Cervus elaphus). Infect. Immun. 68:1620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nol, P., K. P. Lyashchenko, R. Greenwald, J. Esfandiari, W. R. Waters, M. V. Palmer, B. J. Nonnecke, T. J. Keefe, T. C. Thacker, J. C. Rhyan, F. E. Aldwell, and M. D. Salman. 2009. Humoral immune responses of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis BCG vaccination and experimental challenge with M. bovis. Clin. Vaccine Immunol. 16:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien, D. J., S. M. Schmitt, J. S. Fierke, S. A. Hogle, S. R. Winterstein, T. M. Cooley, W. E. Moritz, K. L. Diegel, S. D. Fitzgerald, D. E. Berry, and J. B. Kaneene. 2002. Epidemiology of Mycobacterium bovis in free-ranging white-tailed deer, Michigan, USA, 1995-2000. Prev. Vet. Med. 54:47-63. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien, D. J., S. M. Schmitt, K. P. Lyashchenko, W. R. Waters, D. E. Berry, M. V. Palmer, J. McNair, R. Greenwald, J. Esfandiari, and M. K. Cosgrove. 2009. Evaluation of blood assays for detection of Mycobacterium bovis in white-tailed deer (Odocoileus virginianus) in Michigan. J. Wildl. Dis. 45:153-164. [DOI] [PubMed] [Google Scholar]
- 20.Paterson, A. 7 August 2008. Report of the 2006-2007 South-west England and Cotswolds survey of tuberculosis in deer, p. 36. Department for Environment, Food, and Rural Affairs, London, United Kingdom. http://www.defra.gov.uk/animalh/tb/pdf/deer-survey2008.pdf.
- 21.Ryan, T. J., P. G. Livingstone, D. S. Ramsey, G. W. de Lisle, G. Nugent, D. M. Collins, and B. M. Buddle. 2006. Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: the experience from New Zealand. Vet. Microbiol. 112:211-219. [DOI] [PubMed] [Google Scholar]
- 22.Symmons, J. November 2008. Deer and bovine tuberculosis in Wales: project report to Welsh Assembly Government, Cardiff, p. 7. Welsh Assembly Government, Cardiff, Wales. http://wales.gov.uk/docs/drah/publications/090311deerreporten.rtf.
- 23.Waters, W. R., M. V. Palmer, T. C. Thacker, K. Orloski, P. Nol, N. P. Harrington, S. C. Olsen, and B. J. Nonnecke. 2008. Blood culture and stimulation conditions for the diagnosis of tuberculosis in cervids by the Cervigam assay. Vet. Rec. 162:203-208. [DOI] [PubMed] [Google Scholar]