Abstract
The ability of a recombinant Mycobacterium bovis BCG strain that secretes major membrane protein II (MMP-II) of Mycobacterium leprae (BCG-SM) to confer protection against leprosy was evaluated by use of a mouse footpad model. C57BL/6J mice intradermally inoculated with BCG-SM produced splenic T cells which secreted significant amounts of gamma interferon (IFN-γ) in response to either the recombinant MMP-II, the M. leprae-derived membrane fraction, or the BCG-derived cytosolic fraction in vitro more efficiently than those from the mice infected with the vector control BCG strain (BCG-pMV, a BCG strain containing pMV-261). A higher percentage of CD8+ T cells obtained from BCG-SM-inoculated mice than those obtained from BCG-pMV-inoculated mice produced intracellular IFN-γ on restimulation with the M. leprae antigens. BCG-SM inhibited the multiplication of M. leprae in the footpads of C57BL/6J mice more efficiently than BCG-pMV. These results indicate that a BCG strain that secretes MMP-II could be a better vaccine candidate for leprosy.
Leprosy, which is caused by Mycobacterium leprae, is an infectious disease that still affects thousands of people worldwide. According to WHO's weekly epidemiological report, 254,525 new cases were detected in 2007 (25). One reason why leprosy is still prevalent may be due to the inherent characteristics of M. leprae, i.e., slow growth and weak pathogenicity. It takes 12 to 14 days for M. leprae to replicate, so it is predicted that 2 to 5 years are necessary for the clinical manifestations to appear after an infection (1, 18). Likewise, it takes 6 to 8 months for the recognizable swelling of the footpad to appear in nude mice (22).
Leprosy is clinically divided into two major categories: multibacillary (MB) leprosy and paucibacillary (PB) leprosy. In the lesions of patients with PB leprosy, dendritic cells (DCs) and activated T cells are involved with confining M. leprae to a localized area. These pathological observations indicate that cell-mediated reactions are triggered and that the activation of both CD4+ and CD8+ T cells is closely associated with inhibition of the spread of the bacilli. In contrast, abundant foamy macrophages loaded with bacilli but not DCs appear in the lesions of MB patients (11). It can be speculated that antigen (Ag)-presenting cells such as DCs recognize the immunodominant Ags of M. leprae and express those derivatives on their surfaces, thereby activating T cells. Previously, using T cells from patients with PB leprosy, we have identified major membrane protein II (MMP-II), also known as bacterioferritin (ML2038), as one of the immunodominant Ags (8). We found that MMP-II activates DCs through Toll-like receptor 2, leading to higher levels of expression of major histocompatibility complex class I and class II, CD86, and CD83 Ags and increased levels of production of interleukin-12 p70. Furthermore, MMP-II-pulsed DCs derived from patients with PB leprosy activated both autologous CD4+ T cells and CD8+ T cells to produce gamma interferon (IFN-γ) in amounts larger than the amounts produced by T cells from patients with MB leprosy and M. bovis BCG-vaccinated healthy individuals, indicating that T cells from patients with PB leprosy may be primed with MMP-II in vivo.
The BCG vaccine has been used for the prevention of tuberculosis, although its role in the prevention of leprosy is still being debated. The protective efficacy of BCG against leprosy has been tested in several trials, including studies in the Karonga District of northern Malawi, in which 50% protection was observed (17). Through combined systematic analyses of experimental studies, Setia et al. found that the BCG vaccine had an overall level of protective efficacy of 26% against human leprosy (19). Their observational studies overestimated the protective effect at 61%. In another review of 29 studies, Zodpey reported that 44.8% of the reports indicated that the BCG vaccine had a level of efficacy of 50% or more (26). These observations indicate that improvements to the BCG vaccine are necessary to increase its protective effect. Recently, we produced a recombinant BCG strain that secretes MMP-II (strain BCG-SM, where SM indicates secreting MMP-II). Since MMP-II has the ability to ligate Toll-like receptor 2, we expected BCG-SM to highly activate human T cells. In fact, BCG-SM activated not only naïve CD4+ T cells but also naïve CD8+ T cells through DCs (9). The fact that BCG-SM was more efficient than the parental BCG strain at the activation of both subsets of naïve T cells led us to seek further insights into the protective activity of BCG-SM. In the present study, we investigated the effect of vaccination of BCG-SM on the multiplication of M. leprae in mice.
MATERIALS AND METHODS
Preparation of M. leprae, the recombinant BCG strain, and Ags.
M. leprae (strain Thai-53) was maintained by serial passage in athymic BALB/c nu/nu mice (Clea Japan, Inc., Tokyo, Japan) by inoculation of the bacilli into both hind footpads. At 8 months postinoculation, the footpads were processed to recover M. leprae bacilli by a previously described method (12, 22). The isolated bacteria were counted by a previously described method (10, 21). Nonfrozen, freshly prepared bacteria were used for inoculation of the mice.
A recombinant BCG strain that secretes M. leprae-derived MMP-II was constructed as described previously (9). In brief, a shuttle vector, pMV-261, was used to construct pMV-SM with the MMP-II cDNA fragment. BCG substrain Pasteur was cultured in vitro in Middlebrook 7H9 broth (BD Biosciences-Pharmingen, San Jose, CA) supplemented with 0.05% Tween 80 and 10% albumin-dextrose-catalase (BD Biosciences). Expression vectors were introduced into the BCG strain by electroporation. Transformants were selected on Middlebrook 7H10 agar (BD Biosciences) plates supplemented with 10% oleic acid-albumin-dextrose-catalase (BD Biosciences) and 25 μg/ml kanamycin. The mycobacteria were subsequently grown in Middlebrook 7H9 broth containing 25 μg/ml of kanamycin. The BCG strain containing pMV-SM as an extrachromosomal plasmid is referred to as BCG-SM, while the BCG strain containing pMV-261 is referred to as BCG-pMV. In terms of in vitro growth and infectivity, there was no difference between the two strains. The recombinant MMP-II, the M. leprae-derived membrane fraction (MLM), and the cytosolic fraction of BCG (BCC) were obtained as described previously (8, 13).
Animal studies.
For inoculation into mice, recombinant BCG strains were cultured in Middlebrook 7H9 medium to the log phase of growth and were stored at 108 CFU/ml at −80°C. Before the aliquots were used for inoculation, the concentration of viable bacilli was determined by plating the bacilli on Middlebrook 7H11 agar. The indicated numbers of 5-week-old C57BL/6J mice (Clea Japan, Inc.) per group were inoculated intradermally with 0.1 ml of phosphate-buffered saline (PBS) or PBS containing 1 × 103 or 1 × 104 CFU of recombinant BCG per mouse. The animals were kept under specific-pathogen-free conditions and were supplied with sterilized food and water. Four or 34 weeks after inoculation, the spleens were removed and the splenocytes were suspended at a concentration of 2 × 106 cells per ml in culture medium. The splenocytes were stimulated with the indicated concentration of recombinant MMP-II, MLM, or BCC in triplicate in 96-well round-bottom microplates (8). The individual culture supernatants were collected 3 to 4 days after stimulation, and the level of IFN-γ was measured with an Opt EIA mouse enzyme-linked immunosorbent assay (ELISA) set (BD Biosciences). For the recovery of BCG in the spleen 3 weeks after inoculation, the cells were lysed with 0.2% saponin and plated on Middlebrook 7H10 agar for colony counting.
The splenocytes obtained from C57BL/6J mice infected with the recombinant BCG strains were also subjected to the identification of the T-cell subsets responsible for IFN-γ production. The level of intracellular production of IFN-γ by CD4+ T cells and CD8+ T cells, which were restimulated for 3 days in vitro with recombinant MMP-II or MLM, was assessed as follows: cells were treated with Golgi Stop (BD Biosciences), and Golgi transport was inhibited for 4 h. The cells were then surface stained with an allophycocyanin-labeled monoclonal antibody (MAb) to CD4 (MAb RM4-5; BD Biosciences) and a phycoerythrin-labeled MAb to CD8 (MAb H35.17-2; BD Biosciences) in the presence of 7-amino actinomycin D, after which they were washed with PBS containing 1% fetal calf serum and fixed in 1.6% formaldehyde. Subsequently, they were permeabilized with 0.1% saponin and stained with a fluorescein isothiocyanate-conjugated MAb to IFN-γ (MAb XMG1.2; BD Biosciences) or isotype control immunoglobulin G. In another set of experiments, C57BL/6J mice vaccinated intradermally with the indicated dose of BCG-pMV or BCG-SM for 4 weeks were challenged in the footpad with 5 × 103 of M. leprae per mouse. Thirty or 31 weeks later, the footpads and spleens were processed for further analyses. The number of M. leprae bacilli that grew in the footpads was enumerated by the method of Shepard and McRae (21), and the splenocytes were used to assess the level of IFN-γ production by the ELISA method and for intracellular staining for IFN-γ by flow cytometry (FACSCalibur flow cytometer; BD Biosciences). The animal experiments were reviewed and approved by the Animal Research Committee of Experimental Animals of the National Institute of Infectious Diseases and were conducted according to established guidelines.
Statistical analysis.
Student's t test and the Mann-Whitney-Wilcoxon test were used to determine statistical differences.
RESULTS
Production of M. leprae-derived Ag-responsive T cells in C57BL/6J mice vaccinated with BCG-SM.
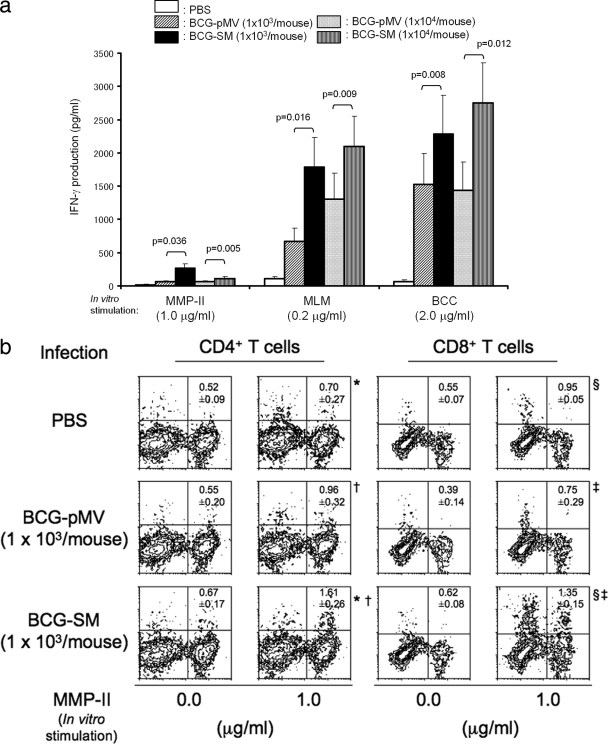
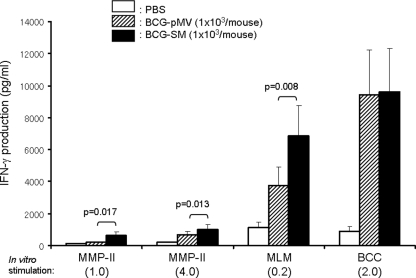
The purpose of vaccination is to produce T cells which can respond to M. leprae or M. leprae-derived Ags. C57BL/6J mice were intradermally infected with either BCG-pMV or BCG-SM; and their splenocytes were restimulated in vitro with the recombinant MMP-II, the MLM protein, or the BCC protein (Fig. 1a). While the splenocytes obtained from C57BL/6J mice inoculated with PBS minimally responded to these Ags, mice infected with either BCG-pMV or BCG-SM significantly responded to the Ags. However, splenocytes from the BCG-SM-vaccinated mice responded to all Ags more strongly and produced levels of IFN-γ higher than those from BCG-pMV-vaccinated mice. In order to define the T cells responsible for IFN-γ production, the T cells producing the intracellular cytokines were determined (Fig. 1b). Both CD4+ T cells and CD8+ T cells obtained from mice inoculated with PBS, BCG-pMV, or BCG-SM produced IFN-γ on stimulation with MMP-II. However, the two subsets of T cells from BCG-SM-infected mice responded to the stimulation more strongly than T cells from BCG-pMV-infected mice, and more than 1.0% of both CD4+ T cells and CD8+ T cells produced IFN-γ.
FIG. 1.
(a) Production of T cells responsive to M. leprae-derived Ags by inoculation with recombinant BCG in mice. Five-week-old C57BL/6J mice were intradermally inoculated with the indicated dose of either BCG-pMV (the vector control BCG strain) or BCG-SM (an MMP-II-secreting BCG strain). Four weeks after the inoculation, splenocytes were restimulated in vitro with the indicated doses of various Ags for 4 days in vitro, and the level of IFN-γ production in the cell supernatant was measured by ELISA. The assays were performed in triplicate for each mouse, and the results for three mice per group are shown as the means ± standard deviations. The representative results of one of three separate experiments are shown. The titers were compared statistically by Student's t test. (b) Intracellular IFN-γ production by CD4+ and CD8+ T cells in mice intradermally inoculated with BCG by secondary stimulation. Five-week-old C57BL/6J mice were intradermally infected with 1 × 103 CFU of either BCG-pMV or BCG-SM per mouse. Four weeks after the inoculation, splenocytes (2 × 105/well) were stimulated with 1.0 μg/ml of recombinant MMP-II for 3 days in vitro. The CD4+ T cells and CD8+ T cells were gated separately and analyzed for the intracellular production of IFN-γ. The number at the top right-hand corner of each panel represents the mean percentage of IFN-γ-producing cells ± standard deviation (for three mice) among the gated T-cell population. A representative plot of one of three separate experiments is shown. The titers were compared statistically by Student's t test. *, P < 0.0001; †, P < 0.005; §, P < 0.05; ‡, P < 0.05.
Human leprosy usually manifests long after the infection with M. leprae. Therefore, we evaluated the long-term effect of vaccination with BCG-SM (Fig. 2). C57BL/6J mice were vaccinated intradermally with the recombinant BCG strain for 34 weeks, and their splenocytes were examined for a secondary response to M. leprae-derived Ags. While the response to BCC did not differ between BCG-pMV- and BCG-SM-infected mice, significantly higher levels of IFN-γ were produced in splenocytes from BCG-SM-vaccinated mice than in those from BCG-pMV-vaccinated mice on in vitro restimulation with both the recombinant MMP-II and MLM. These results indicate that the effect of the BCG-SM vaccination persisted for a long time.
FIG. 2.
Long-term effects of vaccination with recombinant BCG on the production of T cells responsive to M. leprae-derived Ags. C57BL/6J mice were intradermally infected with 1 × 103 CFU of either BCG-pMV or BCG-SM per mouse. Thirty-four weeks after the inoculation, splenocytes were stimulated with the indicated dose of various Ags for 4 days in vitro, and the amount of IFN-γ produced in the cell supernatant was measured. Assays were carried out in triplicate for each mouse, and the results for three mice per group are shown as the means ± standard deviations. The titers were compared statistically by Student's t test.
Effect of BCG-SM vaccination on multiplication of M. leprae in vivo.
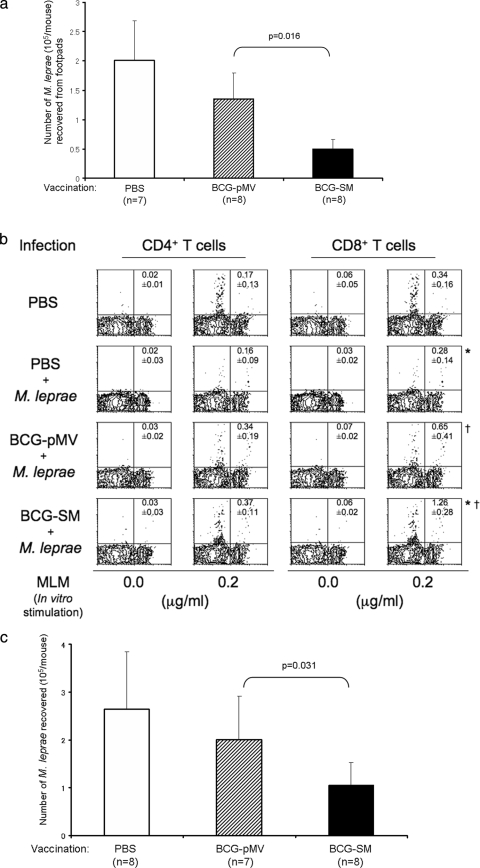
C57BL/6J mice that had been vaccinated 4 weeks earlier with either BCG-pMV or BCG-SM (1 × 104/mouse) intradermally were challenged with 5 × 103 M. leprae bacilli in the footpad. Thirty or 31 weeks later, the footpads were removed and the M. leprae bacilli recovered were enumerated (Fig. 3a). A total of 2 × 105 M. leprae were recovered from the mice inoculated with PBS and challenged with M. leprae, and BCG-pMV partially inhibited the multiplication of M. leprae. However, only 5 × 104 M. leprae bacilli were recovered from the BCG-SM-vaccinated mice, showing that BCG-SM is more effective than BCG-pMV at inhibiting the growth of M. leprae. In order to clarify the T-cell population responsible for the inhibition of M. leprae growth, CD4+ T cells and CD8+ T cells from BCG-vaccinated and M. leprae-challenged mice were restimulated with MMP-II (data not shown) or MLM (Fig. 3b) in vitro. There was no significant difference in the percentage of IFN-γ-producing CD4+ T cells among uninfected M. leprae-challenged, BCG-pMV-vaccinated M. leprae-challenged, and BCG-SM-vaccinated M. leprae-challenged mice. However, significantly higher numbers of CD8+ T cells from BCG-SM-vaccinated M. leprae-challenged mice than T cells from the other groups of mice produced intracellular IFN-γ in response to MMP-II. We then examined the effect of a lower dose of recombinant BCG on the multiplication of M. leprae in the footpads of mice. Again, a 40-fold increase in the number of M. leprae bacilli was observed in M. leprae-challenged (5 × 103/mouse) nonvaccinated mice. Also, vaccination with BCG-SM was more effective in inhibiting the growth of M. leprae than vaccination with BCG-pMV (Fig. 3c).
FIG. 3.
(a) Effect of vaccination with recombinant BCG on M. leprae multiplication. Five-week-old C57BL/6J mice were intradermally inoculated with 1 × 104 CFU of BCG-SM or BCG-pMV per mouse 4 weeks prior to challenge in the footpad with 5 × 103 of M. leprae. Thirty to 31 weeks later, the number of M. leprae bacilli recovered from the footpad was enumerated by the method of Shepard (22). The indicated number of the mice per group was used, and the numbers of bacilli recovered were compared statistically by the Mann-Whitney-Wilcoxon test. (b) Intracellular production of IFN-γ by CD4+ T cells and CD8+ T cells in mice vaccinated with BCG and challenged with M. leprae. C57BL/6J mice were vaccinated with 1 × 104 CFU of either BCG-SM or BCG-pMV per mouse for 4 weeks and challenged with 5 × 103 bacilli of M. leprae for 30 to 31 weeks. Splenocytes (2 × 105/well) were obtained from these mice and were restimulated with 0.2 μg/ml of MLM for 3 days in vitro. The CD4+ T cells and CD8+ T cells were gated separately and were analyzed for the intracellular production of IFN-γ. The number in the top right-hand corner of each panel represents the mean percentage of IFN-γ-producing cells ± standard deviation (for three mice) among the gated T-cell population. The titers were compared statistically by Student's t test. *, P < 0.01; †, P < 0.05. (c) Effect of vaccination with a low dose of BCG on the multiplication of M. leprae. Again, as described for panel a, C57BL/6J mice were inoculated but they were inoculated with a lower dose of recombinant BCG (1 × 103 CFU per mouse), and the effect on the multiplication of M. leprae was observed. The numbers of bacilli recovered were compared statistically by the Mann-Whitney-Wilcoxon test.
DISCUSSION
In 1991, the World Health Assembly proposed the elimination of leprosy as a public health problem by the year 2000, since the multidrug therapy was drastically effective in reducing the number of registered leprosy cases. However, at present, more than 200,000 newly manifested leprosy cases are still reported annually (25). Therefore, in order to eliminate the disease, an effective and safe vaccine is needed. The vaccine should also be widely available at a low cost. An assessment of the cost-effectiveness of BCG vaccination on childhood tuberculosis was conducted and was found to be a highly cost-effective intervention (23). However, human immunodeficiency virus (HIV)-infected infants who were vaccinated with BCG at birth were at high risk of developing disseminated BCG disease (5, 6). Therefore, care should be taken to prevent the vaccination of HIV-exposed infants with BCG. For the prevention of leprosy, a number of field trials as well as animal experiments have been conducted to test the efficacies of heat-killed Mycobacterium leprae, Mycobacterium sp. strain w, the combination of M. leprae and Mycobacterium sp. strain w, and recombinant M. bovis BCG as candidate vaccines in regions of endemicity (1-4, 7, 15, 20, 24). Although M. bovis BCG offered a certain level of protection against leprosy, its effect needs to be bolstered (19, 26). To improve BCG, its immunostimulatory activity needs to be enhanced. It is generally believed that in the host defense against mycobacteria, including M. leprae, both CD4+ T cells and CD8+ T cells play a central role. In the initial stage of a mycobacterial infection, the cells that mainly participate are the IFN-γ-producing CD4+ T cells. The IFN-γ produced from CD4+ T cells may activate macrophages infected with the mycobacteria, and the activated macrophages may induce the intracellular killing of the mycobacteria. In contrast, in the chronic stage, cytotoxic T lymphocytes differentiated from the activated type 1 CD8+ T cells mainly act to inhibit the growth of the intracellular mycobacteria (11, 16). Thus, the activation of both CD4+ T cells and CD8+ T cells is essential for inhibiting the multiplication of mycobacteria.
We previously screened for M. leprae antigens with immunostimulatory properties and observed that a membrane protein, namely, MMP-II, stimulated human monocyte-derived DCs to produce the active form of interleukin-12 and tumor necrosis factor alpha (8). DCs pulsed with MMP-II stimulated both CD4+ and CD8+ T cells to produce IFN-γ. Therefore, we produced a recombinant BCG strain that secretes M. leprae-derived MMP-II (strain BCG-SM). The DCs infected with BCG-SM activated both human naïve CD4+ T cells and naïve CD8+ T cells more efficiently than the vector control BCG (9). T cells of both subsets which can respond to MLM as well as recombinant MMP-II were more efficiently produced from unprimed mice by inoculation with BCG-SM (Fig. 1). At 3 weeks postinoculation, no BCG could be recovered from the spleen. Moreover, it was found that BCG-SM effectively inhibited the multiplication of M. leprae in the footpads of C57BL/6J mice, possibly due to the efficient production of T cells responsive to M. leprae-derived Ags. It may be difficult to determine the T-cell subset responsible for the inhibition; however, CD8+ T cells from mice vaccinated with BCG-SM and challenged with M. leprae for 30 weeks still had the ability to produce IFN-γ after stimulation with M. leprae-derived Ag. Furthermore, M. leprae-responsive CD4+ and CD8+ T cells persisted for 34 weeks after infection with BCG-SM. Therefore, it is possible that CD8+ T cells at least partially contribute to inhibiting the growth of M. leprae in vivo.
Earlier efforts to produce a vaccine against leprosy have not been particularly successful. Some reports indicated that a mixture of refined components of M. leprae was protective, while others emphasized DNA-based vaccines (13, 14). To date, BCG-based vaccines seem to be more promising in terms of their applicability in the field due to the safety and history of global usage of BCG. Taken together, the present study indicates that a recombinant BCG strain that secretes MMP-II could be a useful candidate as a vaccine against leprosy.
Acknowledgments
We acknowledge Y. Shimohakamada and M. Gidoh for assistance with the animal experiments. We also thank Y. Harada and H. Amanai for their technical support and the Japanese Red Cross Society for kindly providing PBMCs from healthy donors.
This work was supported in part by a Grant-in-Aid for Research on Emerging and Re-Emerging Infectious Diseases from the Ministry of Health, Labour, and Welfare of Japan.
Footnotes
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Cho, S. N., and P. J. Brennan. 1999. New biological tools for leprosy surveillance. Int. J. Lepr. Other Mycobact. Dis. 67:59-62. [PubMed] [Google Scholar]
- 2.Convit, J., C. Sampson, M. Zúñiga, P. G. Smith, J. Plata, J. Silva, J. Molina, M. E. Pinardi, B. R. Bloom, and A. Salgado. 1992. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet 339:446-450. [DOI] [PubMed] [Google Scholar]
- 3.Gelber, R. H., V. Mehra, B. Bloom, L. P. Murray, P. Siu, M. Tsang, and P. J. Brennan. 1994. Vaccination with pure Mycobacterium leprae proteins inhibits M. leprae multiplication in mouse footpads. Infect. Immun. 62:4250-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gormus, B. J., G. B. Baskin, K. Xu, M. S. Ratterree, L. N. Martin, P. A. Mack, R. P. Bohm, Jr., W. M. Meyers, and G. P. Walsh. 2000. Antileprosy protective vaccination of sooty mangabey monkeys with BCG or BCG plus heat-killed Mycobacterium leprae: immunologic observations. Int. J. Lepr. Other Mycobact. Dis. 68:434-443. [PubMed] [Google Scholar]
- 5.Hesseling, A. C., M. F. Cotton, C. Fordham von Reyn, S. M. Graham, R. P. Gie, and G. D. Hussey. 2008. Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants. Int. J. Tuberc. Lung Dis. 12:1376-1379. [PubMed] [Google Scholar]
- 6.Hesseling, A. C., B. J. Marais, R. P. Gie, H. S. Schaaf, P. E. Fine, P. Godfrey-Faussett, and N. Beyers. 2007. The risk of disseminated bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine 25:14-18. [DOI] [PubMed] [Google Scholar]
- 7.Karonga Prevention Trial Group. 1996. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 8.Maeda, Y., T. Mukai, J. Spencer, and M. Makino. 2005. Identification of an immunomodulating agent from Mycobacterium leprae. Infect. Immun. 73:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makino, M., Y. Maeda, and K. Inagaki. 2006. Immunostimulatory activity of recombinant Mycobacterium bovis BCG that secretes major membrane protein II of Mycobacterium leprae. Infect. Immun. 74:6264-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott-Lancaster, R. D., T. Ito, K. Kohsaka, C. C. Guelpa-Lauras, and J. H. Grosset. 1987. Multiplication of Mycobacterium leprae in the nude mouse, and some applications of nude mice to experimental leprosy. Int. J. Lepr. Other Mycobact. Dis. 55:889-895. [PubMed] [Google Scholar]
- 11.Modlin, R. L. 1994. Th1-Th2 paradigm: insights from leprosy. J. Investig. Dermatol. 102:828-832. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura, M. 1994. Elimination of contaminants in a homogenate of nude-mouse footpad experimentally infected with Mycobacterium leprae. Jpn. J. Lepr. 64:47-50. [DOI] [PubMed] [Google Scholar]
- 13.Ngamying, M., P. Sawanpanyalert, R. Butraporn, J. Nikasri, S. N. Cho, L. Levy, and P. J. Brennan. 2003. Effect of vaccination with refined components of the organism on infection of mice with Mycobacterium leprae. Infect. Immun. 71:1596-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomaguchi, H., T. Mukai, F. Takeshita, M. Matsuoka, Y. Maeda, T. M. Aye, N. Jahan, Y. Yogi, M. Endo, Y. Sato, and M. Makino. 2002. Effect of hsp65 DNA vaccination carrying immunostimulatory DNA sequences (CpG motifs) against Mycobacterium leprae multiplication in mice. Int. J. Lepr. Other Mycobact. Dis. 70:182-190. [PubMed] [Google Scholar]
- 15.Ohara, N., M. Matsuoka, H. Nomaguchi, M. Naito, and T. Yamada. 2001. Protective responses against experimental Mycobacterium leprae infection in mice induced by recombinant bacillus Calmette-Guérin over-producing three putative protective antigen candidates. Vaccine 19:1906-1910. [DOI] [PubMed] [Google Scholar]
- 16.Ottenhoff, T. H., and T. Mutis. 1990. Specific killing of cytotoxic T cells and antigen-presenting cells by CD4+ cytotoxic T cell clones. A novel potentially immunoregulatory T-T cell interaction in man. J. Exp. Med. 171:2011-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pönnighaus, J. M., P. E. Fine, J. A. Sterne, R. J. Wilson, E. Msosa, P. J. Gruer, P. A. Jenkins, S. B. Lucas, N. G. Liomba, and L. Bliss. 1992. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 339:636-639. [DOI] [PubMed] [Google Scholar]
- 18.Salazar, L., H. Fsihi, E. de Rossi, G. Riccardi, C. Rios, S. T. Cole, and H. E. Takiff. 1996. Organization of the origins of replication of the chromosomes of Mycobacterium smegmatis, Mycobacterium leprae and Mycobacterium tuberculosis and isolation of a functional origin from M. smegmatis. Mol. Microbiol. 20:283-293. [DOI] [PubMed] [Google Scholar]
- 19.Setia, M. S., C. Steinmaus, C. S. Ho, and G. W. Rutherford. 2006. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect. Dis. 6:162-170. [DOI] [PubMed] [Google Scholar]
- 20.Sharma, P., R. Mukherjee, G. P. Talwar, K. G. Sarathchandra, R. Walia, S. K. Parida, R. M. Pandey, R. Rani, H. Kar, A. Mukherjee, K. Katoch, S. K. Benara, T. Singh, and P. Singh. 2005. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8-10 years. Lepr. Rev. 76:127-143. [PubMed] [Google Scholar]
- 21.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. Other Mycobact. Dis. 36:78-82. [PubMed] [Google Scholar]
- 22.Shepard, C. C. 1983. Animal vaccination studies with Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 51:519-523. [PubMed] [Google Scholar]
- 23.Trunz, B. B., P. Fine, and C. Dye. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173-1180. [DOI] [PubMed] [Google Scholar]
- 24.Walia, R., K. G. Sarathchandra, R. M. Pandey, S. K. Parida, S. A. Zaheer, H. K. Kar, A. Mukherjee, R. Mukherjee, and G. P. Talwar. 1993. Field trials on the use of Mycobacterium w vaccine in conjunction with multidrug therapy in leprosy patients for immunotherapeutic and immunoprophylactic purposes. Lepr. Rev. 64:302-311. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2008. Global leprosy situation, beginning of 2008. Wkly. Epidemiol. Rec. 33:293-300. [PubMed] [Google Scholar]
- 26.Zodpey, S. P. 2007. Protective effect of bacillus Calmette Guerin (BCG) vaccine in the prevention of leprosy: a meta-analysis. Indian J. Dermatol. Venereol. Leprol. 73:86-93. [DOI] [PubMed] [Google Scholar]