Abstract
The use of dendritic cells (DCs) as a cellular adjuvant is a promising approach to the immunotherapy of cancer. It has previously been demonstrated that DCs pulsed ex vivo with Toxoplasma gondii antigens trigger a systemic Th1-biased specific immune response and induce protective and specific antitoxoplasma immunity. In the present study, we demonstrate that tumor antigen-pulsed DCs matured in the presence of Toxoplasma gondii components induce a potent antitumor response in a mouse model of fibrosarcoma. Bone-marrow derived DCs (BMDCs) were cultured in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4. After 5 days, tumor lysates with or without the T. gondii lysate were added to the culture for another 2 days. The cytokine production in the BMDC culture and the coculture supernatants of DCs and splenic cells was evaluated. For immunization, 7 days after tumor challenge, different groups of BALB/c mice received different kinds of DCs subcutaneously around the tumor site. Tumor growth was monitored, and 2 weeks after DC immunotherapy, the cytotoxic activity and the infiltration of CD8+ T cells were monitored in different groups. According to the findings, immunotherapy with T. gondii-matured DCs led to a significant increase in the activity of cytotoxic T cells and decreased the rate of growth of the tumor in immunized animals. Immature DCs did not cause any change in cytotoxic activity or the tumor growth rate compared to that in the healthy controls. The current study suggests that a specific antitumor immune response can be induced by DCs matured with T. gondii components and provide the basis for the use of T. gondii in DC-targeted clinical therapies.
Dendritic cells (DCs) efficiently induce T-cell activation in the secondary lymphoid organs (3, 4, 25). The Th1 arm of the immune response is very important in the battle against cancer (17). Evidence indicates that DCs play an important role in determining the type of immune response generated against antigens. Several factors can influence the development of polarized immune responses, such as the DC lineage and its activation status. Some studies have shown that distinct DC subsets are able to promote different types of responses, depending on the pathogen-derived signals and the host-derived cytokines present in the microenvironment (13, 23, 24, 37).
In microbial infections, certain molecular patterns of microbial components directly stimulate immature DCs in the periphery to differentiate into mature DCs by binding to pattern recognition receptors, such as Toll-like receptors (TLRs), which play a critical role in the innate immunity of mammals. The stimulation of TLR signaling in DCs causes an increase in the surface expression of the major histocompatibility complex (MHC) peptide for T-cell recognition, the upregulation of costimulatory molecules important for T-cell clonal expansion, and the secretion of immunomodulatory cytokines, which direct T-lymphocyte differentiation into effector cells. Remarkably, the ligation of distinct TLRs can trigger the production of different cytokines by a single DC type or result in different cytokines in distinct DC subtypes. Studying the complexity of the DC responses to TLR ligands illuminates the link between the innate and the adaptive arms of the immune system (1, 5, 36).
Toxoplasma gondii is an obligate intracellular parasite. Immunity to this organism is accomplished by the high-level production of type 1 cytokines such as gamma interferon (IFN-γ). Both interleukin-12 (IL-12) and IFN-γ are essential for resistance to this opportunistic pathogen (10, 12, 21, 31, 38). T. gondii is a potent stimulus for IL-12 production, which in turn is required to skew the immune response toward Th1 (16, 33). Innate immune cells, such as polymorphonuclear neutrophils, DCs, and macrophages, are important sources of IL-12 during T. gondii infection (7). TLR2 and myeloid differentiation factor 88 are critical for protective immunity against T. gondii infection (27). In recent years, TLR4 has also been found to be essential in the T. gondii-induced activation of DCs (2).
The present study was designed on the basis of the role of T. gondii in the induction of Th1 (cellular arm) immune responses and the importance of this arm in anticancer immunity. In the present study, the maturation state and the cytokine production capabilities of T. gondii-treated DCs, as well as their potential adjuvant effect on tumor immunotherapy of an experimental model, were evaluated.
MATERIALS AND METHODS
Animals and cell line.
Female BALB/c mice were purchased from the Institute Pasteur of Iran. The mice were used at ages ranging from 6 to 8 weeks. All experiments with animals were performed according to the guidelines of the local ethical committee. BALB/c mouse-derived fibrosarcoma (WEHI 164) and colon carcinoma (CT26) cell lines were maintained by in vitro culture in RPMI 1640 (Sigma, Steinhem, Germany) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY), 2 mM l-glutamine (Sigma), 100 μg/ml streptomycin, and 100 U/ml penicillin.
Preparation of Toxoplasma gondii lysate.
Tachyzoites of T. gondii (strain RH) were maintained by intraperitoneal passage in female mice, as described previously (28). After 48 h, the tachyzoites were harvested from the peritoneal cavity, washed, sonicated, and centrifuged. The protein concentration of the supernatant was determined by the method of Lowry et al. (22).
Preparation of tumor lysate.
A total of 5 × 106 WEHI 164 cells were injected subcutaneously in the right flank of BALB/c mice, and tumor growth was monitored. Tumors were harvested surgically from tumor-bearing mice after they were killed, and a single cell suspension was made. Cell lysates were generated by repeated freeze-thaw cycles. After centrifugation (900 × g, 10 min), the supernatants were collected and passed through a 0.2-μm-pore-size filter. The protein concentration of the lysate was determined by the method of Lowry et al. (22).
DC preparation and culture.
Bone marrow (BM)-derived DCs (BMDCs) were generated as described by Inaba et al. (18), with slight modifications. Briefly, murine BM cells were harvested from the femurs and tibias of mice that had been killed. Contaminating erythrocytes were lysed with distilled water and 10× phosphate buffer. Cells (1 × 106 cells/ml) were placed in 24-well plates with RPMI 1640 supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, 100 U/ml of recombinant murine granulocyte-macrophage colony-stimulating factor (Bender Medsystems, Vienna, Austria), and 50 U/ml of recombinant murine IL-4 (Roche, Indianapolis, IN). On day 3, nonadherent cells were collected and fresh medium was added. On day 5, 100 μg/ml of tumor lysate was added to immature DC cultures. After 10 h, 70 μg/ml of T. gondii lysate or 1 μg/ml lipopolysaccharide (LPS; catalog no. L-6526; Sigma) were added to different wells. On day 7, mature DCs were collected and used for immunotherapy.
Flow cytometric analysis.
To determine the phenotypes of the cultured DCs, DCs from the day 5 and 7 cultures were stained with phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies (MAbs) against cell surface molecules CD40, CD80, CD86, CD11c, and MHC class II (MHC-II) (all purchased from BD PharMingen, San Diego, CA) and analyzed with a fluorescence-activated cell sorter analysis system (Becton Dickinson). In all experiments, isotype controls were included by using an appropriate MAb of the same immunoglobulin (Ig) class or subclass.
MLR assays.
To determine the antigen-presenting capacities of DCs in vitro, a one-way mixed leukocyte reaction (MLR) was performed with DCs as stimulators and primed BALB/c splenic T cells as responders (for priming, the mice were injected subcutaneously with the tumor cell lysate 2 weeks prior to collection of the splenocytes for the MLR). Cultures were established in triplicate in 96-well, round-bottom microculture plates (Nunclon) at two different ratios of stimulator cells to responder cells (20:1 and 50:1) for 96 h. The proliferation of T cells was determined by a colorimetric immunoassay for the quantification of cell proliferation on the basis of the measurement of the amount of bromodeoxyuridine (BrdU) incorporated during DNA synthesis. The BrdU enzyme-linked immunosorbent assay (ELISA) method was performed according to the manufacturer's instructions (Roche Applied Science). Briefly, stimulator and responder cells were cocultured for 96 h, and 20 μl/well BrdU of a 200-μM BrdU labeling solution was added during the final 18 h of culture. After 96 h, the plates were centrifuged, denatured with FixDenat solution, and then incubated for 90 min with 1:100-diluted mouse anti-BrdU MAbs conjugated to peroxidase (100 μl/well). After removal of the unbound antibody conjugate, 100 μl tetramethylbenzidine substrate solution was added for 20 min and the reaction was stopped by adding 1 mol/liter H2SO4 solution. The absorbance was measured at 450 nm with a reference wavelength at 690 nm by using an ELISA plate reader (Hyperion Micro Reader 4 plus).
Detection of cytokines by ELISA.
DC and T-cell cytokine production was detected in the supernatants of a DC culture (IL-12 p70) and a coculture supernatant of DCs and splenic cells (IL-10, IFN-γ). The supernatants were collected and kept frozen at −20°C. The cytokine concentrations were measured with an ELISA kit (BenderMed System, Austria), according to the manufacture's instructions.
Immunization.
Seven days after tumor challenge, the mice were injected with 106/200 μl phosphate-buffered saline (PBS) of BMDCs matured with T. gondii lysate (T. gondii lysate-DCs) or LPS (LPS-DCs) and immature DCs (IM-DCs) subcutaneously around the tumor site. The control group received PBS. Tumor measurements were performed every 2 days with calipers, which spanned the shortest and longest surface diameters. The mice were killed when the tumor diameter reached >400 mm2.
Cytotoxicity assay.
Two weeks after immunization the splenocytes were isolated and used as effector cells. Cells of the WEHI 164 and CT26 tumor cell lines were used as target cells. Cytotoxic activity was measured with a lactate dehydrogenase (LDH) cytotoxicity detection kit (Roche Applied Science). After the effector and target cells were washed with assay medium (RPMI 1640 with 1% bovine serum albumin), the effector cells were cocultured with the target cells in a 96-well round-bottom plate for 6 h at 37°C. In some wells, target or effector cells were cultured alone to determine the amount of LDH spontaneously released by these cells. In some other wells containing target cells, 0.1 ml of 2% Triton X-100 lysing agent was added to measure the maximum amount of LDH releasable by target cells. The plates were then centrifuged, and the supernatants were transferred to another flat-bottom ELISA plate. The LDH detection mixture (0.1 ml) was added to each well and the plates were incubated for 30 min at room temperature. The absorbance at 490 nm was measured with an ELISA reader. The percentage of cell-mediated cytotoxicity was determined by the following equation: (experimental release − spontaneous target release − spontaneous effector cell release)/(maximal target release − spontaneous target release) × 100.
Immunohistochemistry.
Tumors were resected 14 days after DC immunization and were embedded in optimum cutting temperature compound (Tissue Tek; Sakura Finetechnical, Tokyo, Japan), and the embedded tumors were frozen in liquid nitrogen. Cryostat sections (5 μm) were thaw mounted on slides, air dried, and stored desiccated at −20°C. Sections were fixed for 2 min in cold acetone, hydrated in PBS, and incubated in protein-blocking solution (2% human albumin) for 15 min. Endogenous peroxidase activity was blocked with 0.3% H2O2. To detect murine CD8+ T cells, the sections were then incubated for 1 h with anti-CD8 MAb (clone 53.6.72; BD Biosciences). After the sections were washed in PBS, they were incubated with biotin-labeled anti-rat IgG and subjected to treatment with avidin-biotin-peroxidase complex by using a peroxidase kit (BD Bioscenices). The color reaction was developed in 3,3-diaminobenzidine solution, and counterstaining was performed with Mayer's hematoxylin solution. The number of CD8+ cells per 10 high-power fields (magnification, ×400) was determined by light microscopy. The CD8-positive cells in each specimen were evaluated by two independent observers, and the percentage of these cells was obtained by dividing the number of stained cells by the total number of cells counted. The results were expressed as the mean ± standard error for each group.
Statistical analysis.
The results are expressed as the means ± standard errors. Statistical analysis of all data except the survival data was performed by Student's t test. The survival data were analyzed by the Kaplan-Meier log-rank test. A P value of <0.05 was considered significant. All calculations were performed with SPSS for Windows (version 10.0) software.
RESULTS
Effect of T. gondii lysate and LPS on DC maturation.
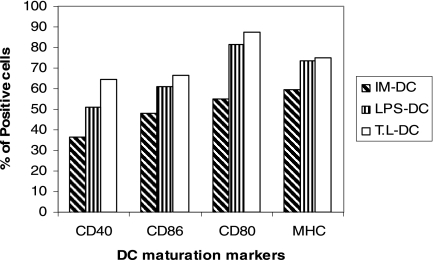
To investigate the effect of the T. gondii lysate on the phenotype and maturation of DCs, BMDCs were cultured in the presence of the T. gondii lysate. The expression of MHC-II, CD40, CD80, and CD86 was analyzed by flow cytometry. About 74% of the DCs expressed the mouse-specific DC marker CD11c. T. gondii lysate-DCs expressed high levels of maturation compared to those for IM-DCs (Fig. 1).
FIG. 1.
Phenotypic changes of BMDCs in response to LPS and T. gondii lysate (T.L). Day 5 immature and day 7 mature BMDCs were analyzed by flow cytometry for the expression of DC maturation markers. The data are presented as the percentages of positive cells for each marker and are representative of those from three separate experiments.
IL-12 production by DCs.
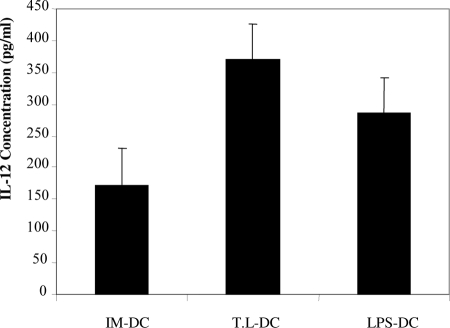
To determine the abilities of BMDCs to produce IL-12, the cytokine secreted in the supernatants of DC culture was quantified by ELISA. As shown in Fig. 2 a significant increase in the ability to produce IL-12 was detected in T. gondii lysate-DCs compared with that of LPS-DCs (P < 0.001) and IM-DCs (P < 0.001). The ability of T. gondii lysate-DCs and LPS-DCs to produce IL-12 was significantly higher than that of IM-DCs (P < 0.001).
FIG. 2.
Production of IL-12 by IM-DCs and after maturation with T. gondii lysate (T.L) and LPS. The cytokines in the supernatants of IM-DCs, T. gondii lysate-DCs, and LPS-DCs were quantified by an IL-12 (p70)-specific ELISA.
Antigen-specific proliferation and cytokine production.
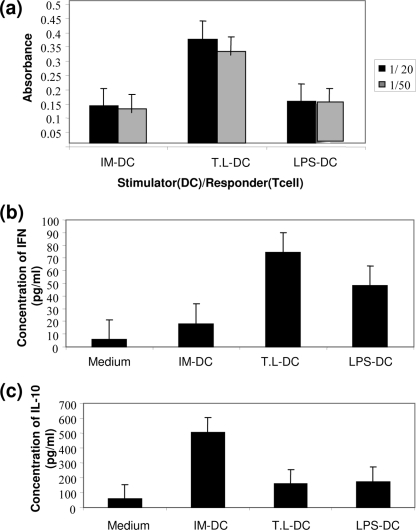
To compare the abilities of DCs of different groups to support T-cell proliferation, MLR was performed with tumor antigen-primed mouse splenocytes as the responder cells. MLR was performed by coculturing these cells with various numbers of irradiated DCs derived from each group of BALB/c mice for 5 days. In addition, the supernatants of day 2 cocultures were collected and assessed for their cytokine (IFN-γ, IL-10) contents. The cell proliferation of the responders was estimated by measurement of BrdU uptake by ELISA. Compared with both LPS-DCs (P < 0.001) and IM-DCs (P < 0.001), T. gondii lysate-DCs had a markedly increased capacity to generate MLR with BALB/c responder lymphocytes (Fig. 3a).
FIG. 3.
Proliferative response and cytokine production of T cells elicited by BMDCs. T cells were stimulated with autologous IM-DCs and DCs matured in the presence of T. gondii lysate (T.L) and LPS. After stimulation, T-cell proliferation (a) and the production of IFN-γ (b) and IL-10 (c) were evaluated. T-cell proliferation was measured by the BrdU-based ELISA method, and the cytokine concentrations were determined by specific ELISAs. The results are the means of three independent experiments.
As shown in Fig. 3b, a significant increase in the level of IFN-γ production was detected in T. gondii lysate-DCs compared to the levels in LPS-DCs (P < 0.001) and IM-DCs (P < 0.001), whereas the level of production of IL-10 was lower (P < 0.5 and P < 0.001, respectively) (Fig. 3c). LPS-DCs also induced significantly higher levels of T-cell proliferation (P < 0.001) and IFN-γ production (P < 0.001) and lower levels of IL-10 production than IM-DCs (P < 0.001).
CTL activity induced by intratumor injection of T. gondii lysate-DCs and LPS-DCs.
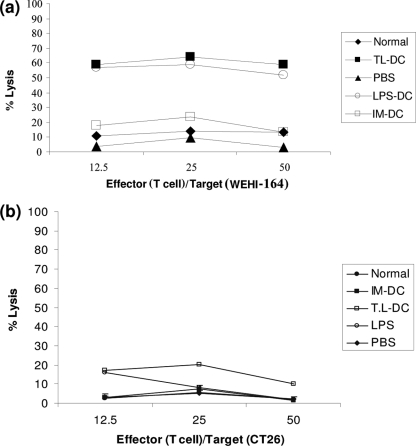
To confirm the induction of systemic and specific antitumor immunity, we examined the cytotoxic activities of T lymphocytes derived from the spleens of mice treated with DCs. Splenocytes were cultured with WEHI 164 or CT26 cells for 6 to 8 h, and the LDH release assay was performed. As shown in Fig. 4, the intratumor injection of T. gondii lysate-DCs induced significant cytotoxic T lymphocyte (CTL) activity against WEHI 164 cells compared with that induced by the other groups of DCs. Treatment with T. gondii lysate-DCs induced significantly higher levels of CTL activity (P < 0.001). A low level of CTL activity against CT26 cells was observed in all groups. These results suggest that the CTL activity induced by treatment with T. gondii lysate-DCs and LPS-DCs appeared to be specific for WEHI 164 tumor cells.
FIG. 4.
CTL responses in mice treated with DCs. Specific cytotoxicity against WEHI 164 cells as the target cells (a) and nonspecific cytotoxicity against an unrelated tumor cell line (CT26 cells) (b) were evaluated in the different groups. The mice treated with T. gondii lysate-DCs (T.L-DC) and LPS-DCs showed the highest specific CTL response when the response was compared to the responses of the other groups. In contrast, there were no significant differences in the nonspecific CTL responses between selected groups.
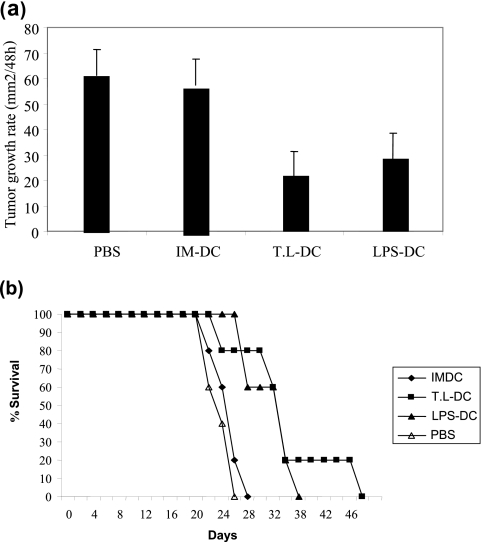
Inhibition of tumor growth in vivo.
To examine the antitumor effects of DCs, 1× 106 LPS-DCs, T. gondii lysate-DCs, or IM-DCs were injected around the established WEHI 164 cell tumors 7 days after tumor challenge. As shown in Fig. 5a, the T. gondii lysate-DCs had the most suppressive effect on tumor growth. The T. gondii lysate-DCs and LPS-DCs suppressed the growth of tumors more significantly than the IM-DCs (P = 0.007 and P = 0.043, respectively).
FIG. 5.
Protective effect of T. gondii lysate-DC (T.L-DC) and LPS-DC in a subcutaneous challenged WEHI 164 tumor model. Mean tumor growth rate per 48 h (a) and the percentage of animals that survived (b) are shown for each group. Each group consisted of five mice.
The 26-day rates of survival after tumor inoculation for mice treated with T. gondii lysate-DCs, LPS-DCs, IM-DCs, and the control group were 100, 100, 20, and 0%, respectively (Fig. 5b). The log-rank test revealed that treatment with T. gondii lysate-DCs and LPS-DCs significantly prolonged survival compared to the length of survival achieved with IM-DCs and PBS (control treatment) (P < 0.05).
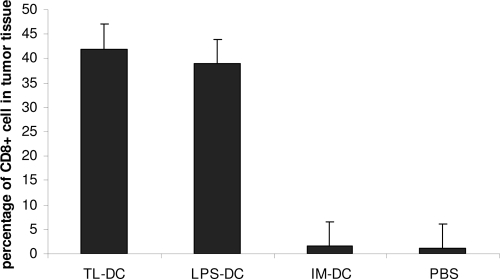
Tumor infiltration of CD8+ T cells.
The CD8+ T-cell infiltration into tumor cells in different groups of mice immunized with DCs was determined by an immunohistochemical method. The infiltration of a larger number of CD8+ T cells was shown in mice receiving T. gondii lysate-DCs and LPS-DCs than in mice in the control groups. As shown in Fig. 6, the percent CD8+ cell infiltration was determined for all groups. The level of infiltration of CD8+ T cells in the T. gondii lysate-DC group was 41% and that in the LPS-DC group was about 39%, whereas it was about 1% in the IM-DC and PBS groups.
FIG. 6.
Percentage of CD8+ cells in tumor tissue in different groups treated with various types of DCs.
DISCUSSION
Previous studies have revealed that DCs exhibit different activation responses to different classes of pathogens in vitro and that there appears to be a correlation between the activation status of DCs and the type of T-helper cell response that they are able to induce (26). However, DCs are used extensively for antigen-specific immunotherapy of cancer because they are the only antigen-presenting cells capable of inducing the initial immune response (15).
T. gondii is an intracellular protozoan that induces a strong Th1 response during acute infection; the development of this response and the production of IFN-γ are required for host survival during both the acute and the chronic stages of Toxoplasma infection (11). In this study, we analyzed the effects of T. gondii cellular components on DC maturation, cytokine production, and T-cell activation in vitro and their efficacies as maturation factors for DCs for use as immunotherapy for experimental model of tumors in vivo.
DCs control T-cell responses by the production of cytokines. DC-derived IL-12 is the major Th1-driving factor (20, 34). In the present study, we show that T. gondii-matured DCs produce large amounts of IL-12 and small amounts of IL-10. Splenic DCs in mice infected with T. gondii became highly activated, and infection with T. gondii resulted in dramatically increased levels of expression of activation markers and elevated levels of IL-12 production by DCs. In comparison with LPS, used as the standard maturation factor for DCs (14), T. gondii significantly enhanced the production of IL-12. The IFN-γ secreted by T cells can exert direct antiproliferative and antimetabolic effects on a wide variety of tumor cells, while IL-10 inhibits the stimulatory capacity of DCs and impairs DC-mediated Th1 cell differentiation, promoting the Th2 phenotype and T-cell anergy (8, 9). In this study, T cells cocultured with T. gondii-matured DCs produced large amounts of IFN-γ. T. gondii lysate-DCs consistently caused a significant decrease in the level of IL-10 production of T cells compared to that induced by IM-DCs. Therefore, T. gondii can stimulate DCs to shift the immune response toward the type 1 response. According to the findings of previous studies, live but not killed tachyzoites of T. gondii upregulate CD40, CD80, CD86, and MHC-II molecules on human DCs and activated DCs induce the production of large amounts of IFN-γ by T cells (35).
Mature DCs are specialized in the activation of T cells because of the high level of expression of MHC and costimulatory molecules (3). In the present study, T. gondii increased the levels of expression of MHC-I, MHC-II, CD80, CD86, and CD40 molecules by DCs. MLR showed that the proliferative response of T cells to T. gondii lysate-DCs was significantly higher than that to LPS and IM-DCs.
Activation of DCs is crucial for the priming of CTLs, which have a critical role in tumor immunity (29, 32). In the cytotoxicity assay, we showed that a DC vaccine generated in the presence of T. gondii induced a strong specific CTL response against our murine fibrosarcoma tumor model. The immunohistochemical study also showed that the level of CD8+ T-cell infiltration of the tumor in T. gondii lysate-DC-treated animals was significantly higher than that in animals treated with LPS-DCs, IM-DCs, and PBS (controls). Our data indicate that vaccinations with T. gondii-stimulated DCs are even more protective and inhibit tumor growth more than LPS-stimulated DCs.
In this study we did not characterize the exact component of Toxoplasma which caused a significant effect on DCs. However according to the findings from different investigations, the initiation of immune responses by DCs is promoted by various exogenous or endogenous stimuli, including LPS, tumor necrosis factor alpha, and heat shock protein (6, 19, 30). It was demonstrated that T. gondii heat shock protein 70 is capable of inducing the phenotypic and functional differentiation and maturation of murine BMDCs, and this effect is dependent on TLR4 (2).
In summary, in this study, we demonstrated that the administration of T. gondii-matured DCs as a therapeutic vaccine is able to induce a pronounced CTL-mediated antitumor immune response which leads to retarded tumor growth and the prolonged survival of the tumor-bearing mice. This approach is applicable to the generation of DC-based antitumor vaccines which could be evaluated in clinical trials as a treatment for appropriate human cancers.
Acknowledgments
We are grateful to Ahmad Jalili of the Medical University of Vienna for helpful comments.
This study was supported by a grant 132/10601 from the Tehran University of Medical Sciences.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5-11. (Erratum, 15:238.) [DOI] [PubMed] [Google Scholar]
- 2.Aosai, F., M. S. Rodriguez Pena, H. S. Mun, H. Fang, T. Mitsunaga, K. Norose, H. K. Kang, Y.-S. Bae, and A. Yano. 2006. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4. Cell Stress Chaperones 11:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Barton, G. M., and R. Medzhitov. 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380-383. [DOI] [PubMed] [Google Scholar]
- 6.Basu, S., R. J. Binder, R. Suto, K. M. Anderson, and P. K. Srivastava. 2000. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 12:1539-1546. [DOI] [PubMed] [Google Scholar]
- 7.Bliss, S. K., A. J. Marshall, Y. Zhang, and E. Y. Denkers. 1999. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J. Immunol. 162:7369-7375. [PubMed] [Google Scholar]
- 8.Caux, C., C. Massacrier, B. Vanbervliet, C. Barthelemy, Y. J. Liu, and J. Banchereau. 1994. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int. Immunol. 6:1177-1185. [DOI] [PubMed] [Google Scholar]
- 9.Corinti, S., C. Albanesi, A. la Sala, S. Pastore, and G. Girolomoni. 2001. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 166:4312-4318. [DOI] [PubMed] [Google Scholar]
- 10.Denkers, E. Y. 2003. From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol. Med. Microbiol. 39:193-203. [DOI] [PubMed] [Google Scholar]
- 11.Denkers, E. Y. 1999. T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect. 1:699-708. [DOI] [PubMed] [Google Scholar]
- 12.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Smedt, T., E. Butz, J. Smith, R. Maldonado-Lopez, B. Pajak, M. Moser, and C. Maliszewski. 2001. CD8alpha− and CD8alpha+ subclasses of dendritic cells undergo phenotypic and functional maturation in vitro and in vivo. J. Leukoc. Biol. 69:951-958. [PubMed] [Google Scholar]
- 14.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong, L., and E. G. Engleman. 2000. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 18:245-273. [DOI] [PubMed] [Google Scholar]
- 16.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 17.Ikeda, H., K. Chamoto, T. Tsuji, Y. Suzuki, D. Wakita, T. Takeshima, and T. Nishimura. 2004. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 95:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688-5694. [DOI] [PubMed] [Google Scholar]
- 20.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263-266. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman, L. A., and C. A. Hunter. 2002. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int. Rev. Immunol. 21:373-403. [DOI] [PubMed] [Google Scholar]
- 22.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 23.MacDonald, A. S., A. D. Straw, B. Bauman, and E. J. Pearce. 2001. CD8-dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 167:1982-1988. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado-Lopez, R., C. Maliszewski, J. Urbain, and M. Moser. 2001. Cytokines regulate the capacity of CD8alpha+ and CD8alpha− dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 167:4345-4350. [DOI] [PubMed] [Google Scholar]
- 25.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 26.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 27.Mun, H.-S., F. Aosai, K. Norose, M. Chen, L.-X. Piao, O. Takeuchi, S. Akira, H. Ishikura, and A. Yano. 2003. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Immunol. 15:1081-1087. [DOI] [PubMed] [Google Scholar]
- 28.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1976. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp. Parasitol. 39:365-376. [DOI] [PubMed] [Google Scholar]
- 29.Salio, M., N. Dulphy, J. Renneson, M. Herbert, A. McMichael, A. Marchant, and V. Cerundolo. 2003. Efficient priming of antigen-specific cytotoxic T lymphocytes by human cord blood dendritic cells. Int. Immunol. 15:1265-1273. [DOI] [PubMed] [Google Scholar]
- 30.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scharton-Kersten, T., E. Y. Denkers, R. Gazzinelli, and A. Sher. 1995. Role of IL12 in induction of cell-mediated immunity to Toxoplasma gondii. Res. Immunol. 146:539-545. (Erratum, 147:121, 1996.) [DOI] [PubMed] [Google Scholar]
- 32.Schuurhuis, D. H., S. Laban, R. E. Toes, P. Ricciardi-Castagnoli, M. J. Kleijmeer, E. I. van der Voort, D. Rea, R. Offringa, H. J. Geuze, C. J. Melief, and F. Ossendorp. 2000. Immature dendritic cells acquire CD8+ cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J. Exp. Med. 192:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sher, A., and C. Reis e Sousa. 1998. Ignition of the type 1 response to intracellular infection by dendritic cell-derived interleukin-12. Eur. Cytokine Network 9:65-68. [PubMed] [Google Scholar]
- 34.Straw, A. D., A. S. MacDonald, E. Y. Denkers, and E. J. Pearce. 2003. CD154 plays a central role in regulating dendritic cell activation during infections that induce Th1 or Th2 responses. J. Immunol. 170:727-734. [DOI] [PubMed] [Google Scholar]
- 35.Subauste, C. S., and M. Wessendarp. 2000. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-gamma. J. Immunol. 165:1498-1505. [DOI] [PubMed] [Google Scholar]
- 36.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 37.Vieira, P. L., E. C. de Jong, E. A. Wierenga, M. L. Kapsenberg, and P. Kaliski. 2000. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 164:4507-4512. [DOI] [PubMed] [Google Scholar]
- 38.Yap, G. S., and A. Sher. 1999. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology 201:240-247. [DOI] [PubMed] [Google Scholar]