Abstract
(1,3)-β-d-Glucan (BG) is a component of the Pneumocystis jiroveci cell wall. Thirty-one immunocompromised patients with pneumonia (16 with presumptive pneumocystis pneumonia [PCP] and 15 with non-PCP) were evaluated for serum BG levels. Serum from all 16 presumptive PCP patients and from 2/15 patients with non-PCP was positive for BG. Results indicate that BG is a reliable marker for diagnosing PCP.
The diagnosis of pneumocystis pneumonia (PCP) due to Pneumocystis jiroveci is usually based on the microscopic detection of cysts in respiratory specimens, such as bronchoalveolar lavage specimens made by fiber-optic bronchoscopy, which has a good sensitivity although it is sometimes very difficult to perform due to the frequent severe respiratory failure of affected patients. In addition, when an appropriate specimen is obtained, the microscopic identification of P. jiroveci is strongly related to the observer's skills and experience.
Other diagnostic procedures, such as PCR, have been reported to be both sensitive and specific, although none of these procedures turned out to be a reliable method in the analysis of biological samples—sputum and pharyngeal swabs—taken using noninvasive techniques (12).
(1,3)-β-d-Glucan (BG) is a component of the cell wall of many fungal organisms, and its presence in serum had been shown to be a reliable marker of invasive fungal infection in both clinical (7, 8, 10, 11) and autoptic (6) studies. Its clinical usefulness had been demonstrated with hematologic patients when used either alone (13) or in combination with serum galactomannan (9). BG is also a component of the P. jiroveci cell wall, but no method for detecting BG in serum has so far been validated for diagnostic purposes in PCP, despite some encouraging reports (3, 10, 14).
The aim of this study was to evaluate the role of a serum BG test in the presumptive diagnosis of PCP in immunocompromised patients unable to undergo invasive diagnostic procedures.
From May 2008 to January 2009, 31 serum samples from immunocompromised patients with pneumonia were collected and stored. All patients admitted to our clinical unit and for whom invasive diagnostic procedures were unfeasible had clear risk factors (see below) for PCP. Clinical presentations of patients were either consistent or not consistent with PCP. Each patient gave informed consent. In the same period of time, 11 control sera from healthy volunteers (the investigators involved in the study and laboratory personnel) were also collected.
A case of presumptive PCP was based on the presence of a baseline clinical condition compatible with the risk of PCP (human immunodeficiency virus [HIV] disease with fewer than 200 CD4 cells/μl, administration of high-dose steroids for more than 8 weeks, or hematological malignancies treated with immunosuppressive therapy) plus, according to the modified 1993 definition of PCP as an AIDS-defining condition (1), all the following factors: (i) a history of dyspnea on exertion or nonproductive cough of recent onset (less than 3 months); (ii) arterial PO2 of less than 70 mm Hg; (iii) chest X-ray finding of bilateral interstitial infiltrate; and (iv) no evidence of bacterial pneumonia.
PCP therapy was administered according to the clinical judgment of attending physicians.
After all data collection and before the final analysis, each case was evaluated by an independent expert, who was unaware of BG test results, and classified as presumptive PCP or non-PCP pneumonia (NPCP).
Serum BG evaluation was performed according to the manufacturer's instructions by using Fungitell (Associates of Cape Cod, Inc., Cape Cod, MA), one of the tests commercially available for the detection of BG and approved in 2003 by the U.S. Food and Drug Administration for the presumptive diagnosis of invasive fungal infection. BG values of ≥80 pg/ml were considered positive according to the manufacturer's indications.
Median BG concentrations in the subgroups of patients were calculated. Results for the subgroups were reciprocally compared by means of the Mann-Whitney U test. P values of <0.05 were considered significant.
Of 31 pneumonia patients, 16 were judged as having likely PCP and 15 as having NPCP. Eight out of 16 PCP patients had HIV infection, six had hematologic malignancies, and two were being given corticosteroids due to autoimmune disorders. Four out of six patients with hematologic malignancies were also being given corticosteroids. Thirteen had a favorable clinical course with a full response to PCP therapy and discharge from our unit, while three patients died. Two of them (one of whom was HIV infected) showed a transitory clinical response (improvement of lactate dehydrogenase, PO2, and fever) to pneumonia; however, their liver function deteriorated (they were both HCV infected), contributing to death. The other patient (HIV infected) had a severe wasting syndrome, rendering poorly evaluable the clinical response to pneumonia.
All patients had a positive BG test result.
Two of 15 patients with NPCP had a positive BG test result. Both of them were HIV positive with a CD4 cell count of less than 200/μl. One had lobar pneumonia with Streptococcus pneumoniae bacteremia, a possible cause of BG positivity (4), while the other had bilateral pneumonia treated successfully with ciprofloxacin.
All 11 healthy volunteers had negative results for BG.
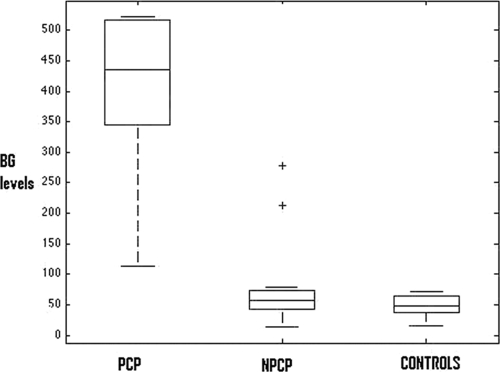
The median BG levels for the three groups are reported in Table 1. In a comparison of the BG concentrations for the two subgroups of patients by means of the Mann-Whitney U test, the difference was statistically significant (P < 0.0001). Box plots of BG values divided by subgroups are shown in Fig. 1.
TABLE 1.
Median and range of BG concentrations
| Subjects | BG level (pg/ml)
|
|
|---|---|---|
| Median | Range | |
| PCP | 423 | 113->523 |
| NPCP | 60 | 13-278 |
| Controla | 48 | 15-71 |
Healthy volunteers.
FIG. 1.
Distribution of serum BG values (pg/ml). Boxes show interquartile range; whiskers show 95% confidence intervals. Plus signs represent outliers.
PCP is a potentially life-threatening clinical event, to be taken into consideration among patients at risk with fever, progressive dyspnea, and nonproductive cough (15).
The diagnostic criterion of PCP based on a favorable response to specific therapy is largely adopted in the clinical setting. However, therapy with high-dose trimethoprim-sulfamethoxazole is often associated with significant adverse effects, including potentially severe hypersensitivity reactions, dose-related bone marrow suppression, and hepatotoxicity. Thus, such a choice should be carefully weighed in a risk/benefit analysis, considering the poor clinical conditions of many patients at risk for PCP.
In our study, we tried to reproduce a situation many clinicians have to deal with: diagnosing PCP without the aid of invasive diagnostic tests due to the poor respiratory and/or general conditions of affected patients. Therefore, we chose to assess the usefulness of the BG test at a single time point (patients' admission) in order to get potentially useful information for starting an appropriate therapy as soon as possible.
The BG assay had, retrospectively, a good concordance with clinical diagnosis of PCP, with positive results for all presumptive cases of PCP. Although we did not have definite proof of P. jiroveci infection in our cases defined as PCP, the adopted rigorous clinical criteria made the diagnosis very likely. We ruled out possible confounding factors, such as cross-reactivity of BG with antimicrobials, as has been suggested elsewhere (2), since none of the patients was being given antibiotics at BG sampling time.
In our study, we did not find any significant difference in BG levels between HIV and non-HIV patients with presumptive PCP, as showed by a recent report (5). In this light, it seems BG does not necessarily reflect the pneumocystis burden in the lungs.
We had two patients in the NPCP group with positive BG results. One of them was affected by a clinical condition (bacteremia due to S. pneumoniae) known to potentially give BG positivity, while for the other we could not find any possible interfering factor. Three patients in the PCP group (two of them HIV infected) died while in treatment with trimethoprim-sulfamethoxazole. Although we cannot completely rule out any other infection as a cause of death and therefore a BG false-positive result, we think the clinical presentation was indeed consistent with PCP and the lack of a therapeutic response was due to their respective underlying diseases (liver cirrhosis and HIV-related wasting syndrome) rather than indicating an alternative diagnosis.
If we had relied on BG test results only, we would have treated two patients in excess, although the concomitant infections and the clinical presentations would have not suggested such a choice. Therefore, in our opinion, the clinical criterion should always be combined with BG test results in therapeutic management.
In conclusion, we think our study showed the BG assay was clinically reliable for patients with presumptive PCP and therefore may be suggested as an adjunctive test for PCP diagnosis for those patients unable to undergo invasive procedures.
Footnotes
Published ahead of print on 19 August 2009.
REFERENCES
- 1.Anonymous. 1993. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. 1992. MMWR Recomm. Rep. 41:1-19. [PubMed] [Google Scholar]
- 2.Marty, F. M., S. Koo, J. Bryar, and L. R. Baden. 2007. (1→3)-β-D-Glucan assay positivity in patients with Pneumocystis (carinii) jiroveci pneumonia. Ann. Intern. Med. 147:70-72. [DOI] [PubMed] [Google Scholar]
- 3.Marty, F. M., C. M. Lowry, S. J. Lempitski, D. W. Kubiak, M. A. Finkelman, and L. R. Baden. 2006. Reactivity of (1→3)-β-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob. Agents Chemother. 50:3450-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mennink-Kersten, M., D. Ruegebrink, and P. E. Verweij. 2008. Pseudomonas aeruginosa as a cause of 1,3-β-d-glucan assay reactivity. Clin. Infect. Dis. 46:1930-1931. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura, H., M. Tateyama, D. Tasato, S. Haranaga, S. Yara, F. Higa, Y. Ohtsuki, and J. Fujita. 2009. Clinical utility of serum β-D-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern. Med. 48:195-202. [DOI] [PubMed] [Google Scholar]
- 6.Obayashi, T., K. Negishi, T. Suzuki, and N. Funata. 2008R. Appraisal of the serum (1→3)-β-D-glucan assay for the diagnosis of invasive fungal infections—-a study based on autopsy cases from 6 years. Clin. Infect. Dis. 46:1864-1870. [DOI] [PubMed] [Google Scholar]
- 7.Odabasi, Z., G. Mattiuzi, E. Estey, H. Kantarjian, F. Sacki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. (1→3)-β-D-Glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 8.Ostrosky-Zeichner, L., B. D. Alexander, D. H. Kett, J. Vazquez, P. G. Pappas, F. Saeki, P. A. Ketchum, J. Wingard, R. Schiff, H. Tamura, M. A. Finkelman, and J. H. Rex. 2005. Multicenter clinical evaluation of the (1→3)-β-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 9.Pazos, C., J. Ponton, and A. Del Palacio. 2005. Contribution of (1→3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic patients: a comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 43:299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persat, F., S. Ranque, F. Derouin, A. Michel-Nguyen, S. Picot, and A. Sulahian. 2008. Contribution of the (1→3)-β-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 46:1009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickering, J. W., H. W. Sant, C. A. P. Bowles, W. L. Roberts, and G. L. Woods. 2005. Evaluation of a (1→3)-β-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohner, P., V. Jacomo, R. Studer, J. Schrenzel, and J.-D. Graf. 2008. Detection of Pneumocystis jirovecii by two staining methods and two quantitative PCR assays. Infection. doi: 10.1007/s15010-008-8027-x. [DOI] [PubMed]
- 13.Senn, L., J. O. Robinson, S. Schmidt, M. Knaup, N. Asahi, S. Satomura, S. Matsuura, B. Duvoisin, J. Bille, T. Calandra, and O. Marchetti. 2008. 1,3-β-d-Glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 46:878-885. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu, Y., N. Sunaga, K. Dobashi, M. Fueki, N. Fueki, S. Makino, and M. Mori. 2009. Serum markers in interstitial pneumonia with and without Pneumocystis jirovecii colonization: a prospective study. BMC Infect. Dis. doi: 10.1186/1471-2334-9-47. [DOI] [PMC free article] [PubMed]
- 15.Thomas, C. F., and A. H. Limper. 2004. Pneumocystis pneumonia. N. Engl. J. Med. 350:2487-2498. [DOI] [PubMed] [Google Scholar]