Abstract
Molecules composed of β-1,4-linked N-acetylglucosamine (GlcNAc) and deacetylated glucosamine units play key roles as surface constituents of the human pathogenic fungus Cryptococcus neoformans. GlcNAc is the monomeric unit of chitin and chitooligomers, which participate in the connection of capsular polysaccharides to the cryptococcal cell wall. In the present study, we evaluated the role of GlcNAc-containing structures in the assembly of the cryptococcal capsule. The in vivo expression of chitooligomers in C. neoformans varied depending on the infected tissue, as inferred from the differential reactivity of yeast forms to the wheat germ agglutinin (WGA) in infected brain and lungs of rats. Chromatographic and dynamic light-scattering analyses demonstrated that glucuronoxylomannan (GXM), the major cryptococcal capsular component, interacts with chitin and chitooligomers. When added to C. neoformans cultures, chitooligomers formed soluble complexes with GXM and interfered in capsular assembly, as manifested by aberrant capsules with defective connections with the cell wall and no reactivity with a monoclonal antibody to GXM. Cultivation of C. neoformans in the presence of an inhibitor of glucosamine 6-phosphate synthase resulted in altered expression of cell wall chitin. These cells formed capsules that were loosely connected to the cryptococcal wall and contained fibers with decreased diameters and altered monosaccharide composition. These results contribute to our understanding of the role played by chitin and chitooligosaccharides on the cryptococcal capsular structure, broadening the functional activities attributed to GlcNAc-containing structures in this biological system.
Cryptococcus neoformans is the etiologic agent of cryptococcosis, a disease still characterized by high morbidity and mortality despite antifungal therapy (3). Pathogenic species belonging to the Cryptococcus genus also include Cryptococcus gattii, which causes disease mostly in immunocompetent individuals (24). A unique characteristic of Cryptococcus species is the presence of a polysaccharide capsule, which is essential for virulence (7-9, 19, 25, 33).
C. neoformans has a complex cell surface. The thick fungal cell wall is composed of polysaccharides (29), pigments (11), lipids (35), and proteins (36). External to the cryptococcal cell wall, capsular polysaccharides form a capsule (19). Seemingly, the assembly of the surface envelope of C. neoformans requires the interaction of cell wall components with capsular elements. Some of the cryptococcal cell wall-capsule connectors have been identified, including the structural polysaccharide α-1,3-glucan and chitooligomers (29, 30, 32).
Chitin-like molecules in fungi are polymerized by chitin synthases, which use cytoplasmic pools of UDP-GlcNAc (N-acetylglucosamine) to form β-1,4-linked oligosaccharides and large polymers. In C. neoformans, the final cellular site of chitin accumulation is the cell wall. The polysaccharide is also used for chitosan synthesis through enzymatic deacetylation (1). Eight putative cryptococcal chitin synthase genes and three regulator proteins have been identified (2). The chitin synthase Chs3 and regulator Csr2 may form a complex with chitin deacetylases for conversion of chitin to chitosan (1). Key early events in the synthesis of chitin/chitosan require the activity of glucosamine 6-phosphate synthase, which promotes the glutamine-dependent amination of fructose 6-phosphate to form glucosamine 6-phosphate, a substrate used for UDP-GlcNAc synthesis (23).
In a previous study, we demonstrated that β-1,4-linked GlcNAc oligomers, which are specifically recognized by the wheat germ agglutinin (WGA), form bridge-like connections between the cell wall and the capsule of C. neoformans (32). In fact, other reports indicate that molecules composed of GlcNAc or its deacetylated derivative play key roles in C. neoformans structural biology. For example, mutations in the genes responsible for the expression of chitin synthase 3 or of the biosynthetic regulator Csr2p caused the loss of the ability to retain the virulence-related pigment melanin in the cell wall (1, 2). These cells were also defective in the synthesis of chitosan, which has also been demonstrated to regulate the retention of cell wall melanin (1). Treatment of C. neoformans acapsular mutants with chitinase affected the incorporation of capsular components into the cell wall (32). Considering that melanin and capsular components are crucial for virulence, these results strongly suggest that GlcNAc-derived molecules are key components of the C. neoformans cell surface. The expression of GlcNAc-containing molecules is likely to be modulated during infection since chitinase expression by host cells is induced during lung cryptococcosis (37).
In this study, we used β-1,4-linked GlcNAc oligomers and an inhibitor of UDP-GlcNAc synthesis to evaluate the role played by GlcNAc-containing molecules in the surface architecture of C. neoformans. The results point to a direct relationship between the expression of GlcNAc-containing molecules and capsular assembly, indicating that chitin and chitooligomers are required for capsule organization in C. neoformans.
MATERIALS AND METHODS
Inhibitors of capsule assembly.
To study capsule assembly in C. neoformans, two different reagents related to the metabolism of GlcNAc-containing molecules were used. Chitin and chitooligomers were recently described as cryptococcal components linking the cell wall to the capsule (32). Chitin is not water soluble, but chitooligomers of two to six β-1,4-linked GlcNAc units are soluble in aqueous solvents. In our study, different chitooligomers were used for interactions with capsular components. The β-1,4-GlcNAc trimeric N, N′,N"-triacetylchitotriose (GlcNAc)3 was the prototype in capsular assembly assays. Chitin-derived oligosaccharides were obtained from Dextra Laboratories.
Glucosamine 6-phosphate, the first aminosugar used for the synthesis of UDP-GlcNAc, is synthesized by amination of fructose 6-phosphate with glutamine by glucosamine 6-phosphate synthase (23). Synthetic dipeptides containing the glutamine analog N3-(4-methoxyfumaroyl)-l-2,3-diaminopropanoic acid (FMDP) were previously described to inhibit this enzyme in Candida albicans (21, 22). One of these FMDP dipeptides, l-norvalyl-FMDP (Nva-FMDP), was shown to inhibit enzyme activity and to mediate effective antifungal effects in a mouse model of systemic candidiasis (21, 22). A protein search in the C. neoformans database (http://www.broad.mit.edu/annotation/genome/cryptococcus_neoformans.2/Blast.html) revealed the existence of two hypothetical proteins with significant homology to the C. albicans protein (loci CNAG_01164.1 and CNAG_02853.1). Therefore, we hypothesized that Nva-FMDP might also inhibit GlcNAc synthesis in C. neoformans and monitored its action by chitin staining in fluorescence-based assays.
Fungal cells.
The C. neoformans strain used in most experiments was the standard serotype A isolate H99. The serotype D isolate 24067 was used for animal infections. Yeast cells were inoculated into 100-ml Erlenmeyer flasks containing 50 ml of minimal medium composed of glucose (15 mM), MgSO4 (10 mM), KH2PO4 (29.4 mM), glycine (13 mM), and thiamine-HCl (3 μM; pH 5.5). Fungal cells were cultivated for 2 days at 30°C, with shaking. Yeast cells were obtained by centrifugation, washed in phosphate-buffered saline (PBS), and counted in a Neubauer chamber. For morphological analyses, cryptococcal yeasts (104 cells) were suspended in 50 μl of minimal medium. Using U-shaped 96-well plates, this suspension was mixed with 50 μl of the same medium containing chitotriose (GlcNAc)3 or Nva-FMDP in concentrations varying from 0 to 100 μg/ml. After 48 h of cultivation at room temperature, aliquots of the cultures were taken for microscopic analyses.
Fluorescence microscopy.
The staining reagents used in fluorescence microscopy included the lectin WGA, with known affinity for β-1,4-linked GlcNAc oligomers, calcofluor white, which has been extensively used to stain chitin in fungal cells due to its ability to recognize the (GlcNAc-β1,4-GlcNAc)n polymer, and the monoclonal antibody (MAb) 18B7, a mouse immunoglobulin G1 (IgG1) with high affinity for glucuronoxylomannan (GXM) of different cryptococcal serotypes (5). Yeast cells (106) were suspended in 4% paraformaldehyde cacodylate buffer (0.1 M; pH 7.2) and incubated for 30 min at room temperature. Fixed yeast cells were washed twice in PBS and incubated in 1% bovine serum albumin in PBS (PBS-BSA) for 1 h. The cells were then suspended in 100 μl of a 5 μg/ml solution of the Alexa Fluor 594 conjugate of WGA (Molecular Probes) and incubated for 30 min at 37°C. After the cells were washed in PBS, they were incubated with 25 μM calcofluor white (Invitrogen) under the same conditions. The cells were washed again and incubated for 1 h in the presence of MAb 18B7 (1 μg/ml). After the cells were washed in PBS, they were finally incubated with a fluorescein isothiocyanate-labeled goat anti-mouse IgG (Fc specific) antibody (Sigma). For a negative control we used an isotype-matched irrelevant IgG at the same concentrations used for MAb 18B7. To eliminate the possibility that the fluorescence pattern was derived from a specific sequential use of reagents, the order of the reagents was changed, and the results were the same (data not shown). Cell suspensions were mounted over glass slides as described above and analyzed under an Axioplan 2 (Zeiss, Germany) fluorescence microscope. Images were acquired using a Color View SX digital camera and processed with the software system analySIS (Soft Image System). Images were finally processed using ImageJ (provided by NIH; http://rsb.info.nih.gov/ij/).
Capture of complexes containing GXM and chitin-like structures.
The presence of complexes containing GXM and chitooligomers in supernatants was first determined by modification of conventional capture enzyme-linked immunosorbent assays (ELISAs). Ninety-six-well polystyrene plates were coated with 50 μl of a WGA solution at 1 μg/ml and incubated for 1 h at 37°C. After removal of unbound lectin molecules, the plate was blocked with PBS-BSA. Different dilutions of culture supernatants of C. neoformans after growth in concentrations of (GlcNAc)3 varying from 0 to 100 μg/ml were added to the wells, and the plates were incubated for 1 h at 37°C. The plates were then washed five times with a solution of Tris-buffered saline supplemented with 0.1% Tween 20, blocked again, and incubated with MAb 18B7 for 1 h. The plate was again washed and incubated with an alkaline phosphatase-conjugated goat anti-mouse IgG1 for 1 h. Reactions were developed after the addition of p-nitrophenyl phosphate disodium hexahydrate, followed by reading at 405 nm with a microplate reader (TP-reader; Thermo Plate). Controls included sterile culture medium or incubation of the complete reaction mixture in plates that were not initially coated with WGA.
The production of complexes containing GXM and chitooligomers in C. neoformans cultures was evaluated by affinity chromatography using an agarose conjugate of WGA (Sigma). The lectin-containing resin (1 ml) was packaged into a plastic chromatographic column (0.5-cm diameter) and extensively washed with sterile minimal medium. The column was loaded with a 10-ml sample of 48 h-culture supernatant of C. neoformans. After collection of unbound fractions and exhaustive washing with minimal medium, fractions that were retained by the lectin were eluted by passage of 10 ml of a 0.5 M GlcNAc solution through the column. Ten fractions (1 ml each) were collected and assessed for the presence of GXM by quantitative ELISA (6).
GXM binding to chitin and chitooligomers.
To analyze the affinity of GXM for β-1,4-linked GlcNAc-containing structures, 25 mg of chitin obtained from crab shells (Sigma) was exhaustively washed with minimal medium and suspended in 0.5 mg/ml GXM solution (1 ml; in minimal medium). This suspension was incubated for 1 h at 25°C under gentle shaking, followed by removal of unbound GXM by extensive washing of the insoluble particles with minimal medium. The insoluble residue was then suspended in minimal medium (1 ml) and transferred to a plastic chromatographic column (0.5-cm diameter). This material was packaged into the column with minimal medium, and chitin-binding fractions of GXM were finally eluted with a step gradient of NaCl at concentrations ranging from 0.1 to 4 M. Three samples (1 ml each) were collected for each salt concentration, and the content of GXM in these fractions was determined by quantitative ELISA (6).
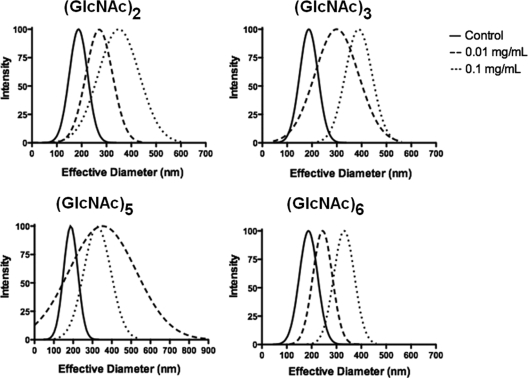
Binding of chitooligomers to GXM fractions was alternatively evaluated by the analysis of changes in the effective diameter of the polysaccharide after incubation in the presence of chitooligosaccharides. GXM extracellular fractions obtained by ultrafiltration (27) were dissolved in minimal medium to form 1 mg/ml solutions and incubated for 1 h at 25°C (control). Alternatively, the GXM solutions were supplemented with different GlcNAc-containing oligosaccharides (N,N′-diacetyl chitobiose; N,N′,N"-triacetyl chitotriose; N,N′,N″,N‴,N″″-pentaacetyl chitopentaose; N,N′,N",N‴,N″″,N″″ ′-hexaacetyl chitohexaose) to form 0.01 or 0.1 mg/ml solutions (final oligomer concentration) and then incubated as described above. Effective diameters of the GXM fractions incubated under control conditions or in the presence of the chitooligomers were determined by dynamic light scattering, following the methodology recently described by Frases and colleagues (14).
India ink counterstaining and morphological analysis.
C. neoformans suspensions grown under control conditions or in the presence of the inhibitors of capsule assembly were placed onto glass slides and mixed with similar volumes of India ink. The suspensions were covered with glass coverslips and analyzed with an Axioplan 2 (Zeiss, Germany) microscope. Images were acquired as described for fluorescence microscopy. Capsule sizes were defined as the distances between the cell wall and the outer border of the capsule. Capsule measurements were determined by using the ImageJ software. For sonication experiments, C. neoformans suspensions were placed in microcentrifuge tubes and pulsed 10 times with an Ultraclear 800 (Unique) sonifier. The capsules were then visualized as described above.
Scanning electron microscopy.
C. neoformans cells obtained after growth under control conditions or in the presence of Nva-FMDP were washed three times with PBS and fixed in 2.5% glutaraldehyde for 1 h at room temperature. Samples of fungal cells were serially dehydrated in alcohol, fixed in a critical-point drier (Samdri-790; Tousimis, Rockville, MD), coated with gold-palladium (Desk-1; Denton Vacuum, Inc., Cherry Hill, NJ), and analyzed with a JEOL (Tokyo, Japan) JSM-6400 scanning electron microscope.
Influence of Nva-FMDP on diameter and monosaccharide composition of capsular fibers.
For capsule extraction, C. neoformans cells cultivated under control conditions or in the presence of Nva-FMDP (10 μg/ml; 3 × 108 cells) were suspended in dimethyl sulfoxide (DMSO; 15 ml) and incubated for 15 min with shaking at room temperature (18). Supernatants containing released capsular polysaccharides were collected by centrifugation, and the pellet was again suspended in 15 ml of DMSO for a second extraction under the same conditions. Supernatants were combined and extensively dialyzed against water for subsequent lyophilization and dry weight determination. GXM fractions obtained by DMSO extraction were analyzed by dynamic light scattering for effective diameter determination (14) or by gas chromatography-mass spectrometry analysis after per-O-trimethylsilylation of the products of acidic methanolysis (27).
In vivo analysis and WGA staining.
To establish localized pulmonary infection, male Fischer rats weighing 200 to 250 g were inoculated intratracheally with 1 × 107 C. neoformans cells, ATCC strain 24067, using an otoscope. This strain has previously been shown to elicit lung chitinase activity in the rat model (37), and its staining pattern by WGA was similar to patterns observed for other cryptococcal strains (see Fig. 9 and reference 32). To establish brain infection, rats were infected intracisternally with 1 × 103 C. neoformans cells. In most cases, rats were sacrificed at 2 weeks, and organs were removed. In some instances, pulmonary infection was allowed to progress for as long as 1 year prior to organ harvesting. At the time of sacrifice, organs were removed, fixed in formalin, embedded in paraffin, and cut into approximately 6-μm sections. All animal work was carried out with the approval of the Animal Use Committee at the Albert Einstein College of Medicine.
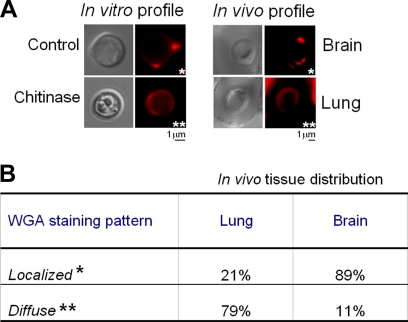
FIG. 9.
WGA reactivity of C. neoformans cells in infected lungs and brains. (A) Tissue sections were stained with fluorescent WGA and analyzed microscopically. Results obtained in vivo were compared with the profiles of lectin reactivity observed in vitro. A limited staining of specific surface sites (localized pattern; single asterisks) was observed in untreated control cells compared to the diffuse pattern (double asterisks) of lectin reactivity in chitinase-treated cells. (B) Quantitative analysis revealed that the same fluorescence pattern observed for chitinase-treated cells in vitro is abundant in infected lungs, whereas the lectin binding profile of untreated control cells is more often detected in infected brains (P < 0.0001).
After tissue sections were blocked with PBS-BSA, they were stained with a 5 μg/ml solution of the Alexa Fluor 594 conjugate of WGA. To characterize the staining patterns of C. neoformans in lung and tissue, approximately 70 yeast cells per slide were counted, and the average from 10 different tissue sections was determined. Statistical analysis was performed with the chi-square test, using the Prism for Windows software (version 4.02). The morphological features of WGA-stained C. neoformans from infected tissues were compared with those obtained in vitro, which included control or chitinase-treated cells. For chitinase treatment, PBS-washed yeast cells (106) cultivated in vitro were suspended in 100 μl of 0.01 M phosphate buffer (pH 6.0) containing chitinase (100 μg/ml; purified from Streptomyces griseus [Sigma]), followed by incubation at 37°C for 12 h. The cell suspensions were incubated overnight at 37°C and centrifuged at 4,000 rpm for cell removal. Controls included cells treated in buffer containing no enzyme.
RESULTS
Binding of GXM to chitin and chitooligomers.
Chitooligomers were recently described by our group as candidate molecules that connect the cell wall to capsular GXM (32). The ability of GXM to bind to β-1,4-linked GlcNAc-containing structures was then explored by incubation of the cryptococcal polysaccharide with chitin and elution of bound fractions with increasing concentrations of NaCl. Analysis of eluted fractions by ELISA revealed one major GXM peak, in addition to several minor peaks corresponding to binding of the cryptococcal polysaccharide to chitin (Fig. 1A). This result suggests that GXM binding to chitin may occur at different levels of affinity.
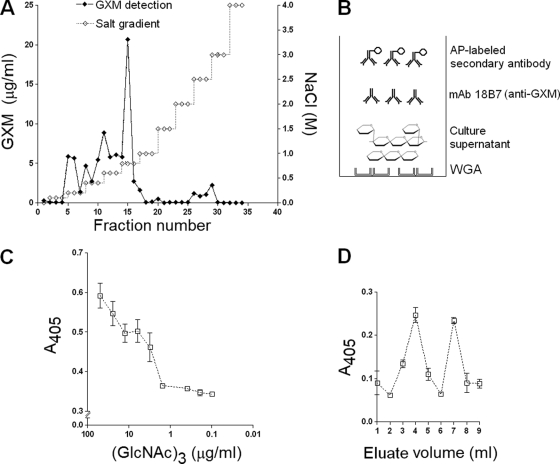
FIG. 1.
Association of GXM and chitin-like molecules. (A) Binding of GXM to chitin followed by elution with a step gradient of NaCl resulted in different GXM peaks. (B) Experimental model used to detect β-1,4-linked GlcNAc oligomers in complex with GXM in culture supernatants. WGA was used to capture chitooligomers. Lectin-coated wells of ELISA microplates were incubated with culture supernatants for further detection of carbohydrate complexes using a MAb raised to GXM. (C) Chitotriose was added to C. neoformans cultures at different concentrations, and the chitooligomer-GXM complexes were captured by WGA on ELISA microplates and detected by anti-GXM monoclonal antibody. (D) Two peaks corresponding to chitotriose-GXM complexes in C. neoformans culture supernatant could be separated by affinity binding to an agarose-WGA column. AP, alkaline phosphatase.
The ability of GXM to bind chitin under controlled conditions led us to investigate whether such interaction would normally occur in C. neoformans under the growth conditions used. A capture ELISA was then used to detect chitooligomer (chitotriose)-GXM complexes (Fig. 1B), and the results confirmed that GXM-chitotriose complexes formed during fungal growth (Fig. 1C). Nonspecific binding of the polysaccharide to the surface of the ELISA plate, which might result in false-positive results, apparently did not influence serological reactions, as inferred from the results of running the reaction in the absence of WGA (plates coated with PBS-BSA only) (data not shown).
Although the efficacy of detection of complexes containing GXM and chitooligomers in culture fluids was directly influenced by the concentration of the exogenously added oligosaccharide in the medium, we observed positive ELISA reactions even when C. neoformans grew without supplementation of chitooligosaccharides. This observation suggests that a natural association of chitooligomers with GXM may occur during cryptococcal growth. To isolate these complexes, regular culture supernatants of C. neoformans were passed through an agarose-WGA column. Bound samples were eluted with 0.5 M GlcNAc, and the presence of GXM in these fractions was monitored in ELISAs with a MAb to GXM (MAb 18B7) (6). The chromatographic profile revealed two peaks of GXM (Fig. 1D), suggesting that WGA captured GlcNAc-containing molecules from culture supernatants in association with GXM. Absorbance values related to GXM detection were relatively low. The results shown in Fig. 1A may suggest that the association of GXM with chitin at pH 5.5 involves noncovalent bonds, possibly including some electrostatic and hydrophobic interactions. Therefore, the relatively low absorbance values probably reflect the fact that GXM-chitooligomer interactions in culture fluids are likely to be dynamic and transient, and consequently no quantitative information should be inferred from these results.
Growth in the presence of chitotriose led to modified cryptococcal capsules.
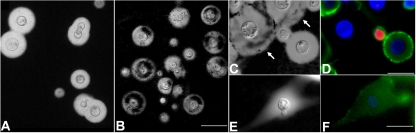
The association of GXM with chitooligomers forming soluble hybrid complexes led us to hypothesize that high concentrations of the β-1,4-linked GlcNAc oligosaccharides could affect capsule assembly by interfering with the incorporation of capsular components by growing cells. Analysis of India ink-counterstained cells revealed that, after growth in minimal medium supplemented with (GlcNAc)3 (6.25 μg/ml), the capsule of several cells of C. neoformans became permeable to India ink. The border of the capsule was apparently well preserved, but the interface of the cell wall with the capsule was impregnated with India ink, indicating the abnormal increase of capsular permeability (Fig. 2A and B). To explore the capsular structure that stained with India ink of cells grown in the presence of chitooligomers, cryptococci were stained with MAb 18B7, WGA, and calcofluor for detection, respectively, of GXM, β-1,4-linked chitooligomers, and chitin. Surprisingly, fluorescence microscopy revealed that yeast cells with India ink-permeable capsules stained neither with WGA nor with MAb 18B7 (Fig. 2C and D). In contrast, cryptococcal cells with a regular profile of India ink counterstaining exhibited normal reactivity with MAb 18B7. When C. neoformans was cultivated at higher (25 μg/ml) concentrations of (GlcNAc)3, a highly distorted capsular morphology, with longer capsular projections, was observed (Fig. 2E and F).
FIG. 2.
Morphological and antibody-reacting features of cryptococcal capsules in yeast cells grown in the presence of (GlcNAc)3. India ink counterstaining was used to analyze capsule morphology under control conditions (A) or after growth in the presence of the chitooligomer (6.5 μg/ml) (B). Scale bar, 10 μm. Yeast cells with defective capsules that became permeable to India ink (C; arrows) were not recognized by MAb 18B7 (D). Scale bar, 10 μm. Growth of C. neoformans in the presence of a higher (GlcNAc)3 concentration (25 μg/ml) results in the formation of capsules with aberrant morphology that are not permeable to India ink penetration (E). These structures are still recognized by MAb 18B7 (F). Scale bar, 10 μm. Cellular structures stained in blue and green show, respectively, recognition sites of calcofluor and MAb 18B7.
Capsule assembly is not required for expression of β-1,4-linked GlcNAc oligomers.
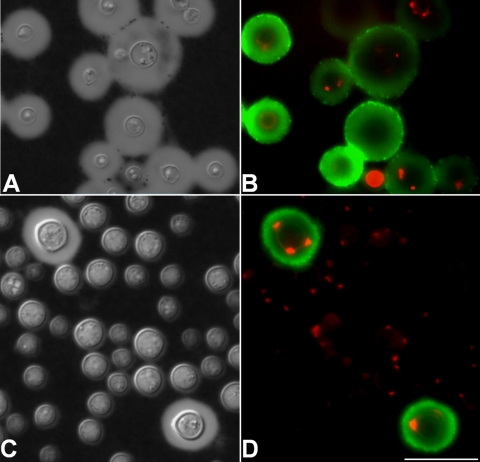
The partial sensitivity of the GXM-chitin linkage to increasing NaCl concentrations (Fig. 1A) is in line with data from previous studies showing that growth of C. neoformans in high-salt concentrations resulted in decreased expression of capsular polysaccharides (10, 34). We therefore evaluated whether cultivation of C. neoformans in minimal medium supplemented with 1 M NaCl would affect expression of surface chitooligomers. Control cells cultivated in minimal medium manifested relatively large capsules and patterns of antibody and lectin binding that were in agreement with previous observations by our group (32) (Fig. 3A and B). After cultivation in medium of high osmolarity, most of the yeast cells had no visible capsules and were not recognized by the MAb to GXM (Fig. 3C and D). The minor fraction of the fungal population that remained fully encapsulated was regularly recognized by the antibody. Growth in high-salt concentrations, however, did not affect the expression of surface β-1,4-linked oligomers, indicating that, in agreement with chromatographic analysis (Fig. 1A), high NaCl concentrations affect GXM-GlcNAc linkages without interfering with synthesis and surface exposure of chitooligomers in cryptococci.
FIG. 3.
Analysis of the C. neoformans capsule after growth in minimal medium (A and B) or in the same medium supplemented with 1 M NaCl (C and D). (A and B) India ink counterstaining and fluorescence analysis showing the regular profiles of capsule expression and staining of GXM and chitooligomers by MAb 18B7 (green fluorescence) and WGA (red fluorescence), respectively. (C) India ink counterstaining showing that the vast majority of the yeast cells have no visible capsule. (D) Only the yeast cells with normal capsules are recognized by the antibody to GXM. Most cells show the typical profile of staining of β-1,4-linked GlcNAc oligomers by WGA. Scale bar, 10 μm.
Interaction with chitooligomers increased diameters of GXM fibers.
The ability of GXM to bind structures related to chitin and previous studies showing that chitooligomers participate in GXM interactions with the cell wall (12, 32) led us to evaluate whether binding to β-1,4-linked GlcNAc-containing molecules could affect the diameter of GXM fibers. For this purpose we used the method described by Frases and coworkers (14), in which the dimensions of GXM fibers were analyzed by dynamic light scattering. Analysis of the distribution of polysaccharide molecules as a function of their effective diameter demonstrated that incubation in the presence of the β-1,4-linked GlcNAc oligomers caused a shift in the distribution curves to regions of enhanced diameters (Fig. 4). The increase in the GXM effective diameter was proportional to the chitooligomer concentration. The only exception was observed when the chitopentamer was used, when the increase in the effective diameter was apparently not influenced by oligomer concentration. Calculation of the molar ratios of chitooligomer and polysaccharide solutions using the values of GXM molecular mass proposed by McFadden and colleagues (20) showed that the oligomer-GXM ratios in our model were in the range of 20 to 1. When these values increased up to 200, no correlation between oligosaccharide concentration and polysaccharide diameter was observed (data not shown).
FIG. 4.
Interaction of GXM with chitooligomers of differential lengths affects the effective diameter of the fungal polysaccharide. A shift of the distribution curves to areas of higher diameter was observed after incubation of GXM in the presence of all β-1,4-linked GlcNAc oligosaccharides in comparison to control systems in the absence of chitooligomers.
An inhibitor of glucosamine 6-phosphate synthesis affects cell wall and capsule morphology in C. neoformans.
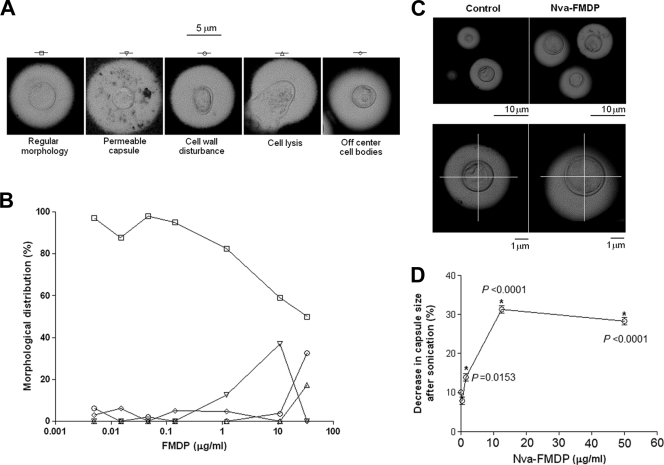
Nva-FMDP is a dipeptide inhibitor that interferes with early steps of UDP-GlcNAc synthesis, resulting in defective chitin biosynthesis (21). The fact that growth of C. neoformans in the presence of chitooligomers affected capsular morphology led us to evaluate the effects of Nva-FMDP on C. neoformans morphology. Yeast growth was barely affected by the inhibitor (data not shown). In contrast, an overview of the cellular morphology after fungal cultivation in the presence of Nva-FMDP revealed several abnormal features (Fig. 5). Four major morphological alterations were observed for cells growing in the presence of inhibitor: increased capsule permeability, changed cell wall architecture, increased cell lysis, and dislocation of cell bodies from the central position in relation to the capsule. Quantification of these morphological cell types showed that as the number of C. neoformans cells with regular morphology decreased, the number of the other types fluctuated (off-center cell body position, cells with increased capsule permeability, apparent cell lysis, and cell wall with irregular border) (Fig. 5B). In all cases, the morphological changes induced by the inhibitor suggested that cell wall and capsular components were affected. The disturbances in the cell wall morphology (Fig. 5A) and, especially, the dislocation of the cell body in relation to the capsule center (Fig. 5C) suggested that connection of the capsule to the cell wall in C. neoformans had been affected after fungal growth in the presence of the inhibitor. In fact, capsules of cells grown with Nva-FMDP were more easily detached from the cell surface by sonication (Fig. 5D).
FIG. 5.
Inhibition of glucosamine 6-phosphate synthesis by Nva-FMDP affects the morphology and cell wall-capsule connections in C. neoformans. (A) Major morphological features of control cells (regular morphology) or of yeast cells cultivated in the presence of Nva-FMDP. (B) Distribution of the morphological alterations shown in panel A after growth of C. neoformans with Nva-FMDP. (C) Off-center cell bodies suggesting a possible loose connection between the cell wall and the capsule as induced by the inhibitor. (D) Growth in the presence of increasing concentrations of Nva-FMDP rendered yeast cells more susceptible to capsule release by sonication. Asterisks denote inhibitor concentrations at which capsule release by sonication was significantly more efficient than under control conditions (no inhibitor).
Lack of cell wall chitin is correlated with reduced capsular dimensions in C. neoformans.
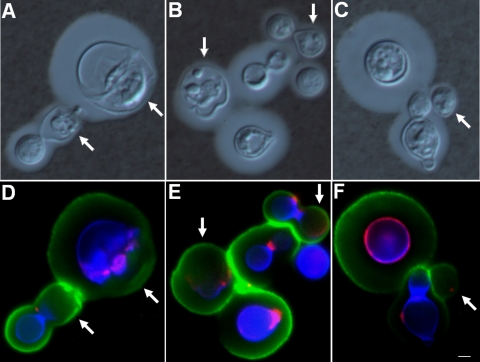
The cellular activity of Nva-FMDP in the Cryptococcus model was monitored by staining fungal cells with calcofluor white, based on the assumption that lack of calcofluor binding to cell walls implies a deficient chitin biosynthesis. Therefore, the effects of Nva-FMDP on the surface architecture of C. neoformans were evaluated by fluorescence microscopy in combination with India ink counterstaining.
Approximately 40% of the cells grown with 25 μg/ml of the inhibitor had altered chitin expression, as concluded from a comparison between the numbers of calcofluor-stained yeast cells grown under control conditions with the number of stained cells from inhibitor-containing cultures. Several cells from cultures supplemented with Nva-FMDP were regularly stained with calcofluor and WGA; these cells showed normal capsules that reacted with MAb 18B7 (Fig. 6). However, other cells showed no reactivity with calcofluor. In addition, some cells were stained for chitin in specific regions of the cell wall and lacked calcofluor reactivity at other surface sites. In all cases, negative calcofluor staining was correlated with the observation of capsular structures with noticeably reduced dimensions, as determined by India ink counterstaining in combination with tests of capsular reactivity with MAb 18B7. Negative calcofluor staining did not result in lack of MAb 18B7 reactivity, indicating that GXM was also associated with the cell wall of C. neoformans even in cells manifesting reduced capsules.
FIG. 6.
Nva-FMDP interferes with chitin synthesis in C. neoformans cells. In this assay, calcofluor staining (blue) shows a regular chitin expression; lack of calcofluor reactivity indicates defective chitin biosynthesis. Staining in red and green indicates, respectively, detection of chitooligomers and GXM. Panels A to C show the morphological features of India ink-counterstained C. neoformans after growth with Nva-FMDP. Panels D to F show the corresponding fluorescence images. Regions of the cell surface with defective chitin biosynthesis were associated with reduced capsule size (arrows). Panels A and D, B and E, and C and F represent three different experiments prepared under the same conditions and producing similar results. Scale bar, 1 μm.
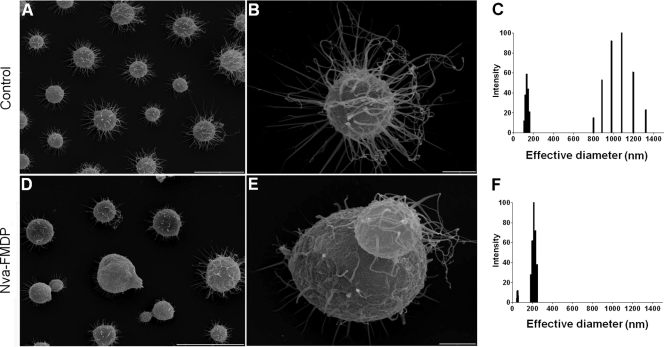
The fluorescence profile and related changes in capsule expression observed after growth of C. neoformans in the presence of Nva-FMDP led us to evaluate the morphological aspects of the capsule by scanning electron microscopy (SEM). The capsular fibrils of yeast cells exposed to the inhibitor were noticeably shorter than those in control cells (Fig. 7A, B, D, and E). In fact, extraction of wall-associated capsular material with DMSO followed by light-scattering analysis confirmed that the capsular fibers of cells grown in the presence of the inhibitor (30 μg/ml) were shorter than those extracted from control systems (Fig. 7C and F). Similar results were observed when C. neoformans cells were cultivated with 10 μg/ml Nva-FMDP (data not shown). These results strongly suggested that GlcNAc-containing molecules are required for extension of capsular fibers in C. neoformans, as initially inferred from the fact that chitooligomers can regulate GXM effective diameter (Fig. 4).
FIG. 7.
Capsule fibrils are affected by growth of C. neoformans in the presence of Nva-FMDP. SEM of control cells at low (A) and high (B) magnifications show the regular aspects of capsule fibers. These structures were extracted from yeast cells with DMSO, and their effective diameters were determined by light scattering (C). SEM of fungal cells grown with Nva-FMDP (D and E) showed a reduction in the size of capsular fibers, which was confirmed by light-scattering analysis of DMSO extracts of capsule material (F). Scale bars, 10 μm (A and D) and 1 μm (B and E).
Capsule composition is influenced by the synthesis of GlcNAc-containing molecules.
The altered dimensions of capsule fibers observed after cultivation of C. neoformans in medium supplemented with Nva-FMDP suggested that capsular structures were modified under these conditions. The monosaccharide composition of capsular extracts of control cells and of Nva-FMDP-treated cryptococci was analyzed by gas chromatography-mass spectrometry (Fig. 8). Mannose, xylose, and glucuronic acid were the main sugar constituents in control capsular extracts, which also showed trace amounts of glucose and galactose. Growth in the presence of Nva-FMDP, however, changed the molar carbohydrate content, with a reduced relative amount of glucuronic acid and increased glucose. Therefore, in addition to the morphological alterations caused by inhibition of GlcNAc synthesis in yeast cells, the carbohydrate content of C. neoformans capsule was also affected by Nva-FMDP.
FIG. 8.
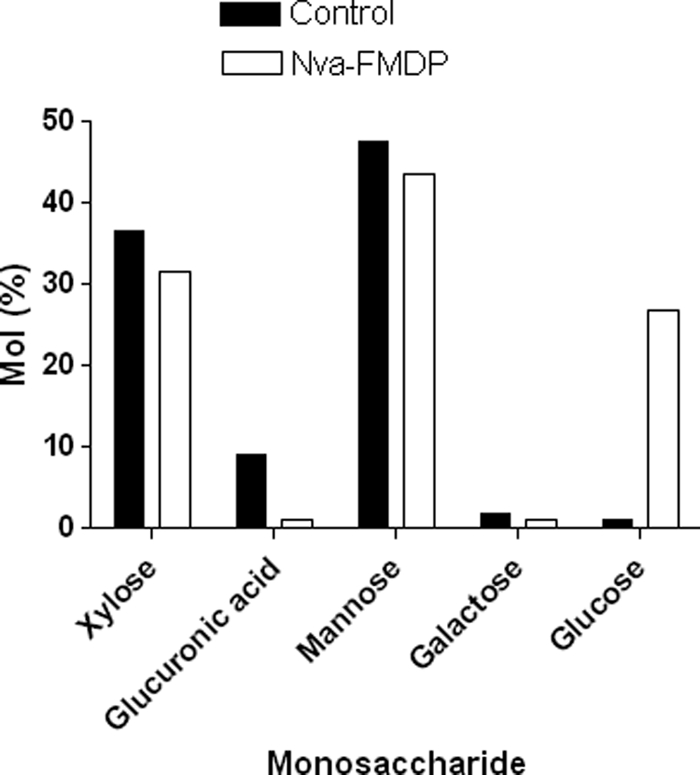
Monosaccharide composition of capsular extracts of C. neoformans after growth under control conditions or in the presence of Nva-FMDP. Capsular extracts from C. neoformans cultivated in the presence of the inhibitor showed eightfold less glucuronic acid and a 10-fold increase in the content of glucose.
Expression of chitooligomers in vivo.
Intratracheal inoculation of C. neoformans induces chitinase activity in the lung and bronchoalveolar lavage fluid of infected rats (37). Since chitin and chitooligomers participate in the organization of C. neoformans cell envelope (1, 2, 32), we investigated the profile of WGA staining of yeast cells from experimental cryptococcosis in rats. Sections of lungs and brains from infected animals were stained with fluorescent WGA and analyzed microscopically. Two patterns of lectin staining were observed (Fig. 9A). The first staining profile corresponded to reactivity of the lectin with restricted areas of the cell surface, which was similar to the pattern observed in control C. neoformans cells (32). The second staining profile corresponded to a diffuse pattern of WGA binding. Diffuse lectin staining is usually observed after treatment of yeast cells with chitinase, which converts the localized profile of WGA binding to C. neoformans to a diffuse pattern of cell wall labeling (32). This effect is attributed to the enhanced generation of cell wall chitooligosaccharides after partial enzymatic hydrolysis of chitin (12, 32). Considering that WGA interacts with β-1,4-linked GlcNAc oligomers but not with chitin in its polymeric form, chitinase treatment generates an increased number of cellular sites for lectin binding (32). Therefore, we classified the two visible profiles of C. neoformans lectin staining described above as localized and diffuse patterns of WGA binding to the cell surface.
In infected lungs of rats, the diffuse pattern observed for chitinase-treated cells predominated in comparison with the number of fungal cells presenting the regular, localized profile of WGA staining (P < 0.0001) (Fig. 9B). As expected, due to the much reduced concentration of organ chitinase (15, 28, 37), the opposite pattern was observed in the infected rat brain.
DISCUSSION
Hexosamine metabolism in fungi is crucial for the biosynthesis of several macromolecules, including chitin, chitosan, and glycoproteins (23). In C. neoformans, synthesis of chitin and its deacetylated derivative, chitosan, is directly linked with cell wall assembly and fungal growth (1, 2). At least 14 different genes are associated with the synthesis of these polymers in cryptococci, including eight putative chitin synthases, three putative regulator proteins, and three deacetylases (1, 2). The relatively high gene redundancy observed for chitin synthases may be responsible for the low susceptibility of C. neoformans to nikkomycin, an inhibitor of chitin synthesis (17).
Anchoring of the capsule or capsule precursors to the cell wall is crucial for the pathogenicity of C. neoformans since capsular components are required for virulence. The interactions between the components of the cell wall and the capsule responsible for attaching the capsule to the cell wall remain poorly understood, but various reports suggest that the surface connections are complex and could involve multiple cell wall components (29, 30, 32). α-1,3-Glucan was the first cell wall component associated to capsule anchoring. Cryptococcal cells with disrupted α-glucan synthase genes had normal biosynthesis of capsular material but lacked a surface capsule (30). Mutant cells lacking α-1,3-glucan also had modified cell walls (30), strengthening the notion that the lack of cell wall glucans could impair buildup of the cell wall and cell wall-capsule connections.
In a recent report, we demonstrated the existence of chitooligomers that form singular round or hook-like connections between the cell wall and the capsule of C. neoformans (32). Similar observations with Trichosporon asahii were described by our group (12). These results suggested that, in addition to its expected role in the synthesis of cell wall components, GlcNAc metabolism in fungi could be linked to GXM assembly at the cell surface. In this context, the role played by chitooligomers and chitin in the process of capsule assembly was further investigated.
In our model, growth of C. neoformans under conditions that hampered capsule expression (high salt concentration) resulted in normal expression of chitin oligomers (Fig. 3), suggesting that these structures are constitutively synthesized and assembled at the cell surface, even in cells lacking visible capsules. Using chromatographic and ELISA-based approaches in combination with dynamic light scattering (Fig. 1 and 4), we demonstrated that GXM can interact with chitin and chitooligomers. The reactivity of the captured complexes with MAb 18B7 depended on the concentration of the oligomers in the culture, and, interestingly, positive reactions were observed even when chitotriose was not added to the medium (data not shown). In fact, complexes containing GXM and β-1,4-linked GlcNAc-containing structures were isolated from culture supernatants (Fig. 1D), suggesting that such carbohydrate-carbohydrate interactions are common events in the cryptococcal physiology.
The ability of GXM to bind to insoluble chitin and the detection of complexes containing GXM and chitooligomers led us to hypothesize that soluble β-1,4-linked GlcNAc-containing structures could modify the molecular architecture of C. neoformans capsular components. Consistent with this hypothesis, yeast cells grown in the presence of chitotriose showed an altered capsular morphology that was most prominent at the cell wall-capsule interface (Fig. 2). Of particular interest we note that the permeabilization to India ink penetration observed after growth of C. neoformans in the presence of chitooligomers resulted in the loss of reactivity of capsular material with MAb 18B7, suggesting that the epitopes recognized by the antibody are somehow modified in these defective capsules. In fact, β-1,4-linked GlcNAc oligomers altered the effective diameter of GXM fibrils (Fig. 4), which suggests that aggregated structures containing larger GXM fibers and chitooligomers could form compact capsular regions that increase the permeability to India ink penetration. In addition, polysaccharide aggregation mediated by chitooligomers would likely promote hindrance of O-acetyl epitopes, which are required for MAb reactivity (16). Chitooligomers could also modify GXM structure and immunogenicity through the ionic association of glucuronic acid residues with GlcNAc units. The amino group on chitin has a pKa of 6.1 (26), implying that chitooligomers would likely be charged at pHs between 5 and 6, which was the range used for fungal growth and also in most assays in this study.
To pursue the role of GlcNAc-containing molecules in capsule architecture, we explored the effect of interference with hexosamine metabolism in C. neoformans using an inhibitor of the synthesis of glucosamine 6-phosphate, a key step in the UDP-GlcNAc biosynthetic process (23). Nva-FMDP, the inhibitor used in this study, shows a high antifungal efficacy against C. albicans (21), but its anticryptococcal activity was limited to alterations in cellular morphology. Budding in the presence of the inhibitor was not affected (data not shown), which warranted its use to control the expression of GlcNAc-containing molecules in viable cryptococcal populations. Different analyses showed that the interference with the metabolism of GlcNAc resulted in loosened capsule-cell wall connections (Fig. 5) and suggested a direct relationship between a lack of chitin synthesis and reduced capsule size. Conversely, addition of chitooligomers to the culture medium at relatively high concentrations resulted in the formation of aberrant capsules with increased dimensions (Fig. 2E and F). Staining of fungal cells with MAb 18B7 and observation by fluorescence microscopy and SEM (Fig. 6 and 7) revealed that the major capsular component, GXM, was still associated to the cell wall in Nva-FMDP-treated cells. These analyses, in association with light-scattering determinations, revealed that capsular fibers showed reduced effective diameters and, apparently, were less abundant in the cell surface. The reduced effective diameters of GXM fibrils in cells that had altered hexosamine metabolism are in agreement with the observation that chitooligomers may regulate the size of GXM fibers (Fig. 4). Since inhibition of GlcNAc synthesis resulted in reduced capsule sizes but did not abolish capsule anchoring to the cell wall, we speculate that part of the capsular material in C. neoformans cells could be associated to α-1,3-glucans and related molecules at the cell wall (29, 30). The fact that cell wall components other than chitin-like structures are required for capsule anchoring was supported by the fact that Saccharomyces cerevisiae yeast cells, which also express WGA-binding structures, were unable to incorporate exogenous GXM (data not shown), indicating that chitin-like structures and chitooligomers are involved in the external organization of capsular components rather than in their attachment to the cell wall.
The changes in capsule morphology and physical properties induced by Nva-FMDP raised the question as to how the capsular composition would be affected after growth in the presence of the inhibitor. Under this condition the relative concentration of glucuronic acid in cell extracts was reduced eightfold (Fig. 8). We have shown previously that the negative charges of glucuronic acid are required for ion-mediated GXM aggregation and capsule enlargement (27). The reduced concentration of this acidic sugar could, therefore, account for the formation of smaller capsules caused by the inhibitor. Conversely, polysaccharide fractions from Nva-FMDP-treated cells showed an increased content of glucose. The presence of glucose as a minor component of cryptococcal capsular extracts has been demonstrated before (13), but presently the amount of glucose was close to that of xylose, a major GXM constituent.
In the lungs of mice, but not in whole brain, acidic mammalian chitinase is the sole detectable endogenous chitinase (4). In rat tissues a comparable acidic chitinase was observed. The acidic chitinase in rodents is distinct from human chitotriosidase, and the protein could be identified in alveolar macrophages of silicotic rats (15), suggesting that in the rat lung this enzyme could be generated by macrophages. Since chitinase activity is induced in infected lungs by cryptococcal infections (37) and not in infected brain tissue (28), it was not surprising that the profiles of expression of chitooligomers in lung and brain from rats infected with C. neoformans were different. In this study, we demonstrate that most of the C. neoformans yeast cells in infected lungs of rats resemble the pattern of chitinase-treated cells in vitro while only 21% of the fungal cells infecting the brain showed the same profile of WGA staining (Fig. 9). These results suggest that the expression of chitin-derived molecules in fungi may be modulated by enzymatic activities derived from the host, which vary according to the infected anatomic site. Such variability could be related to the previously described organ-dependent variation of capsule thickness in C. neoformans during animal infection (31).
In summary, this study establishes a link between synthesis and surface distribution of chitin-like structures and the architecture of the capsule in C. neoformans. As suggested in prior reports (1, 2), understanding the different steps required for synthesis of chitin and chitin-like molecules could reveal the existence of promising antifungal targets. The fact that chitin-like molecules are both modulated in vivo and associated with capsule assembly reinforces this idea. In addition, cryptococcal virulence could be related to the synthesis of chitooligomers, which makes chitin metabolism and related pathways attractive areas to be explored within the field of fungal pathogenicity.
Acknowledgments
M.L.R., L.R.T., and L.N. are supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de São Paulo (Brazil), and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (Brazil). A.C. is supported by NIH grants AI033142, AI033774, AI052733, and HL059842. M.L.R., L.N., and L.R.T. are CNPq research fellows. R.J.B.C. is supported by the Training Program in Cellular and Molecular Biology and Genetics, T32 GM007491. Carbohydrate analyses were performed at the Complex Carbohydrate Research Center, University of Georgia (Atlanta, GA), which is supported in part by the Department of Energy-funded Center for Plant and Microbial Complex Carbohydrates (grant DE-FG-9-93ER-20097).
We thank Camila Franca and Emma Robertson for help with antimicrobial tests and members of the Albert Einstein College of Medicine analytical imaging facility for help with SEM.
Footnotes
Published ahead of print on 17 July 2009.
REFERENCES
- 1.Baker, L. G., C. A. Specht, M. J. Donlin, and J. K. Lodge. 2007. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 6:855-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, I. R., C. A. Specht, M. J. Donlin, K. J. Gerik, S. M. Levitz, and J. K. Lodge. 2005. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 4:1902-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bicanic, T., and T. S. Harrison. 2004. Cryptococcal meningitis. Br. Med. Bull. 72:99-118. [DOI] [PubMed] [Google Scholar]
- 4.Boot, R. G., E. F. Blommaart, E. Swart, K. Ghauharali-van der Vlugt, N. Bijl, C. Moe, A. Place, and J. M. Aerts. 2001. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 276:6770-6778. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 154:27-35. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C., and K. J. Kwon-Chung. 1998. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 66:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y. C., L. A. Penoyer, and K. J. Kwon-Chung. 1996. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 64:1977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dykstra, M. A., L. Friedman, and J. W. Murphy. 1977. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect. Immun. 16:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenman, H. C., J. D. Nosanchuk, J. B. Webber, R. J. Emerson, T. A. Camesano, and A. Casadevall. 2005. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 44:3683-3693. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca, F. L., S. Frases, A. Casadevall, O. Fischman-Gompertz, L. Nimrichter, and M. L. Rodrigues. 2009. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet. Biol. 46:496-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frases, S., L. Nimrichter, N. B. Viana, A. Nakouzi, and A. Casadevall. 2008. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot. Cell 7:319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frases, S., B. Pontes, L. Nimrichter, N. B. Viana, M. L. Rodrigues, and A. Casadevall. 2009. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc. Natl. Acad. Sci. USA 106:1228-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guoping, C., P. Fan, S. Jingxi, L. Xiaoping, J. Shiqin, and L. Yuri. 1997. Purification and characterization of a silica-induced bronchoalveolar lavage protein with fibroblast growth-promoting activity. J. Cell Biochem. 67:257-264. [DOI] [PubMed] [Google Scholar]
- 16.Kozel, T. R., S. M. Levitz, F. Dromer, M. A. Gates, P. Thorkildson, and G. Janbon. 2003. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infect. Immun. 71:2868-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, R. K., and M. G. Rinaldi. 1999. In vitro antifungal activity of nikkomycin Z in combination with fluconazole or itraconazole. Antimicrob. Agents Chemother. 43:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxson, M. E., E. Cook, A. Casadevall, and O. Zaragoza. 2007. The volume and hydration of the Cryptococcus neoformans polysaccharide capsule. Fungal Genet. Biol. 44:180-186. [DOI] [PubMed] [Google Scholar]
- 19.McFadden, D., O. Zaragoza, and A. Casadevall. 2006. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol. 14:497-505. [DOI] [PubMed] [Google Scholar]
- 20.McFadden, D. C., M. De Jesus, and A. Casadevall. 2006. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J. Biol. Chem. 281:1868-1875. [DOI] [PubMed] [Google Scholar]
- 21.Milewski, S., R. Andruszkiewicz, L. Kasprzak, J. Mazerski, F. Mignini, and E. Borowski. 1991. Mechanism of action of anticandidal dipeptides containing inhibitors of glucosamine-6-phosphate synthase. Antimicrob. Agents Chemother. 35:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milewski, S., H. Chmara, R. Andruszkiewicz, E. Borowski, M. Zaremba, and J. Borowski. 1988. Antifungal peptides with novel specific inhibitors of glucosamine 6-phosphate synthase. Drugs Exp. Clin. Res. 14:461-465. [PubMed] [Google Scholar]
- 23.Milewski, S., I. Gabriel, and J. Olchowy. 2006. Enzymes of UDP-GlcNAc biosynthesis in yeast. Yeast 23:1-14. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell, D. H., T. C. Sorrell, A. M. Allworth, C. H. Heath, A. R. McGregor, K. Papanaoum, M. J. Richards, and T. Gottlieb. 1995. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin. Infect. Dis. 20:611-616. [DOI] [PubMed] [Google Scholar]
- 25.Monari, C., F. Bistoni, and A. Vecchiarelli. 2006. Glucuronoxylomannan exhibits potent immunosuppressive properties. FEMS Yeast Res. 6:537-542. [DOI] [PubMed] [Google Scholar]
- 26.Murray, S. B., and A. C. Neville. 1998. The role of pH, temperature and nucleation in the formation of cholesteric liquid crystal spherulites from chitin and chitosan. Int. J. Biol. Macromol. 22:137-144. [DOI] [PubMed] [Google Scholar]
- 27.Nimrichter, L., S. Frases, L. P. Cinelli, N. B. Viana, A. Nakouzi, L. R. Travassos, A. Casadevall, and M. L. Rodrigues. 2007. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot. Cell 6:1400-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overdijk, B., G. J. Van Steijn, and F. C. Odds. 1999. Distribution of chitinase in guinea pig tissues and increases in levels of this enzyme after systemic infection with Aspergillus fumigatus. Microbiology 145:259-269. [DOI] [PubMed] [Google Scholar]
- 29.Reese, A. J., and T. L. Doering. 2003. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 50:1401-1409. [DOI] [PubMed] [Google Scholar]
- 30.Reese, A. J., A. Yoneda, J. A. Breger, A. Beauvais, H. Liu, C. L. Griffith, I. Bose, M. J. Kim, C. Skau, S. Yang, J. A. Sefko, M. Osumi, J. P. Latge, E. Mylonakis, and T. L. Doering. 2007. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 63:1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivera, J., M. Feldmesser, M. Cammer, and A. Casadevall. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 66:5027-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues, M. L., M. Alvarez, F. L. Fonseca, and A. Casadevall. 2008. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot. Cell 7:602-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues, M. L., F. L. Fonseca, S. Frases, A. Casadevall, and L. Nimrichter. 3 April 2009. The still obscure attributes of cryptococcal glucuronoxylomannan. Med. Mycol. doi: 10.1080/13693780902788621. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 34.Rodrigues, M. L., S. Rozental, J. N. Couceiro, J. Angluster, C. S. Alviano, and L. R. Travassos. 1997. Identification of N-acetylneuraminic acid and its 9-O-acetylated derivative on the cell surface of Cryptococcus neoformans: influence on fungal phagocytosis. Infect. Immun. 65:4937-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigues, M. L., L. R. Travassos, K. R. Miranda, A. J. Franzen, S. Rozental, W. de Souza, C. S. Alviano, and E. Barreto-Bergter. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 68:7049-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siafakas, A. R., T. C. Sorrell, L. C. Wright, C. Wilson, M. Larsen, R. Boadle, P. R. Williamson, and J. T. Djordjevic. 2007. Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J. Biol. Chem. 282:37508-37514. [DOI] [PubMed] [Google Scholar]
- 37.Vicencio, A. G., S. Narain, Z. Du, W. Y. Zeng, J. Ritch, A. Casadevall, and D. L. Goldman. 2008. Pulmonary cryptococcosis induces chitinase in the rat. Respir. Res. 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]