Abstract
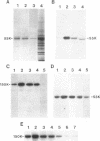
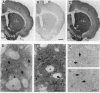
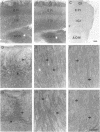
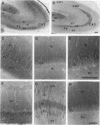
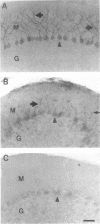
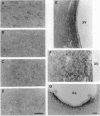
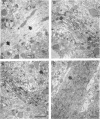
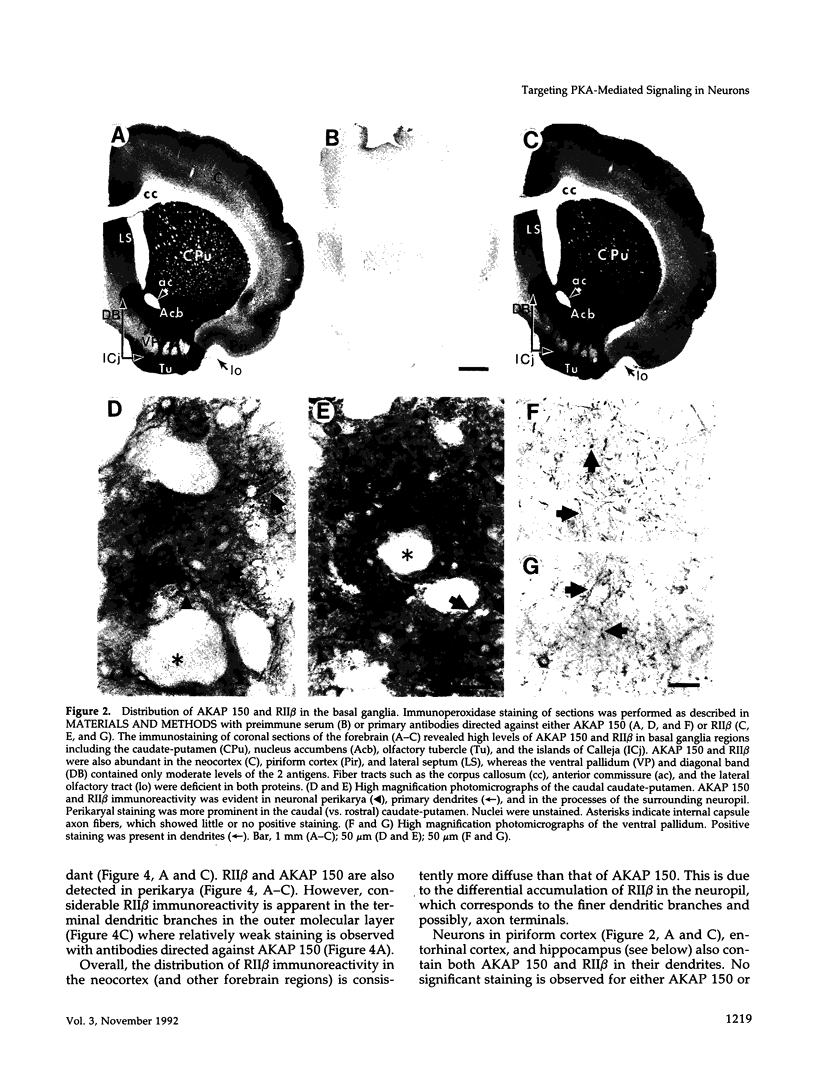
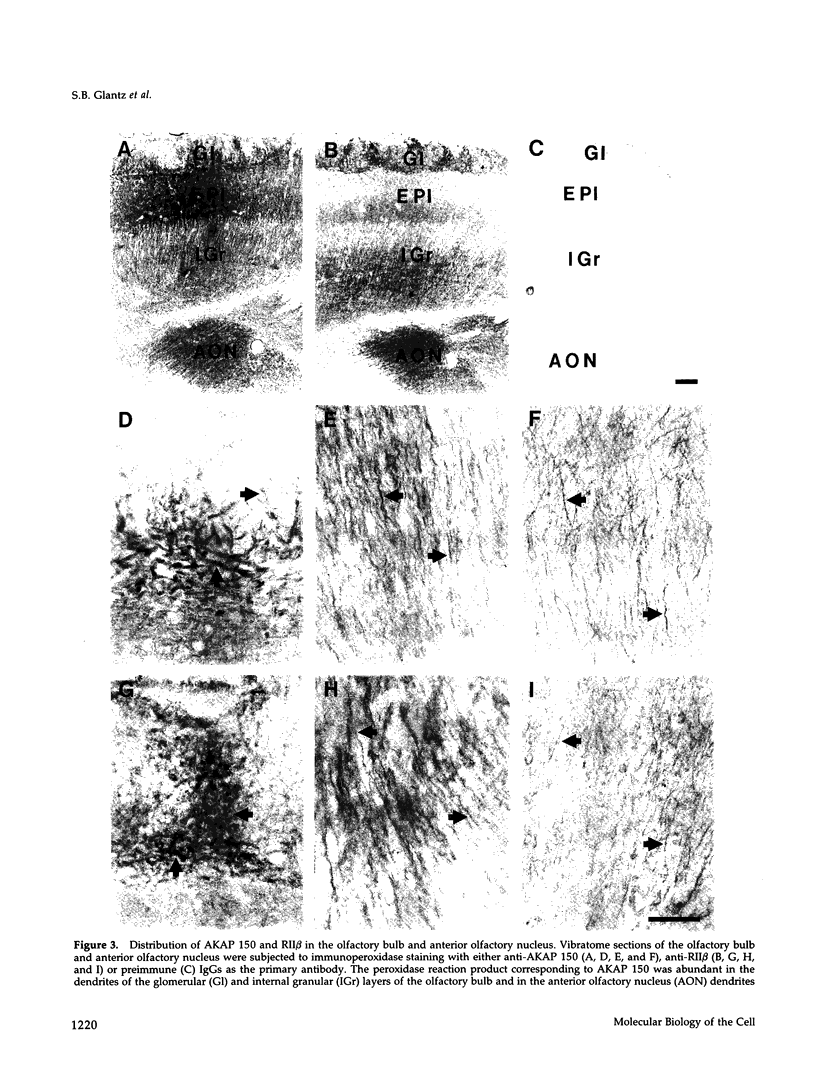
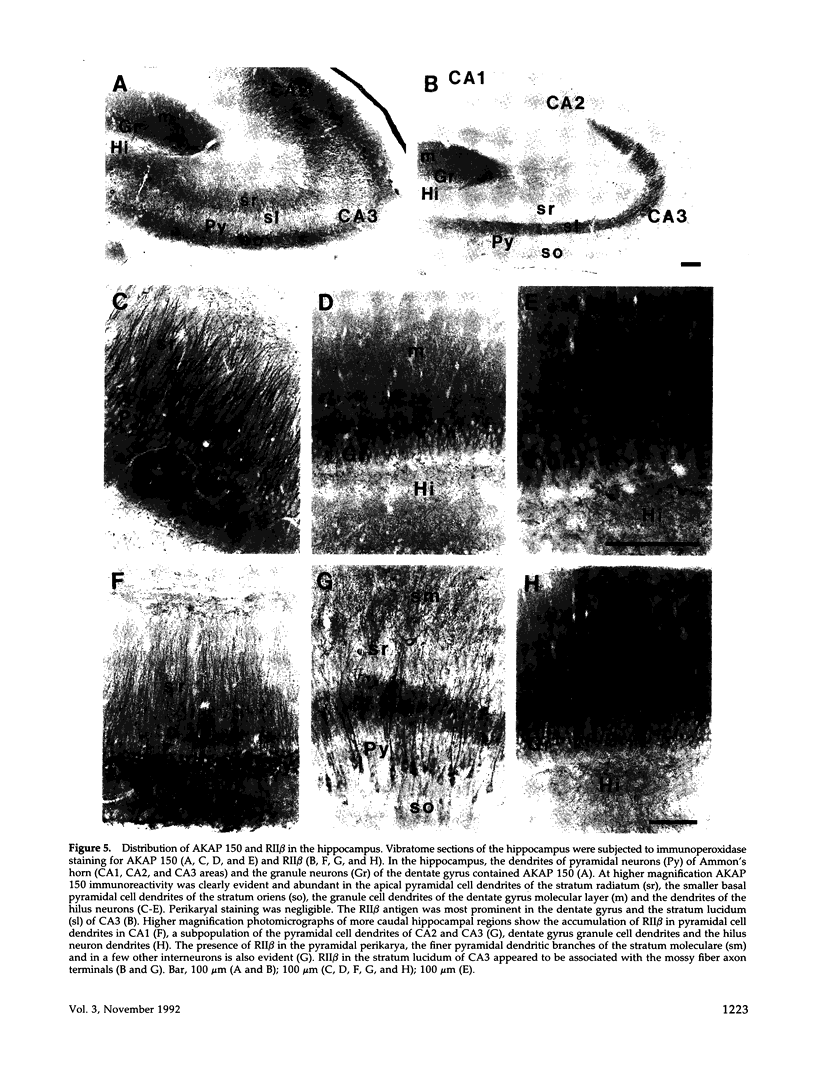
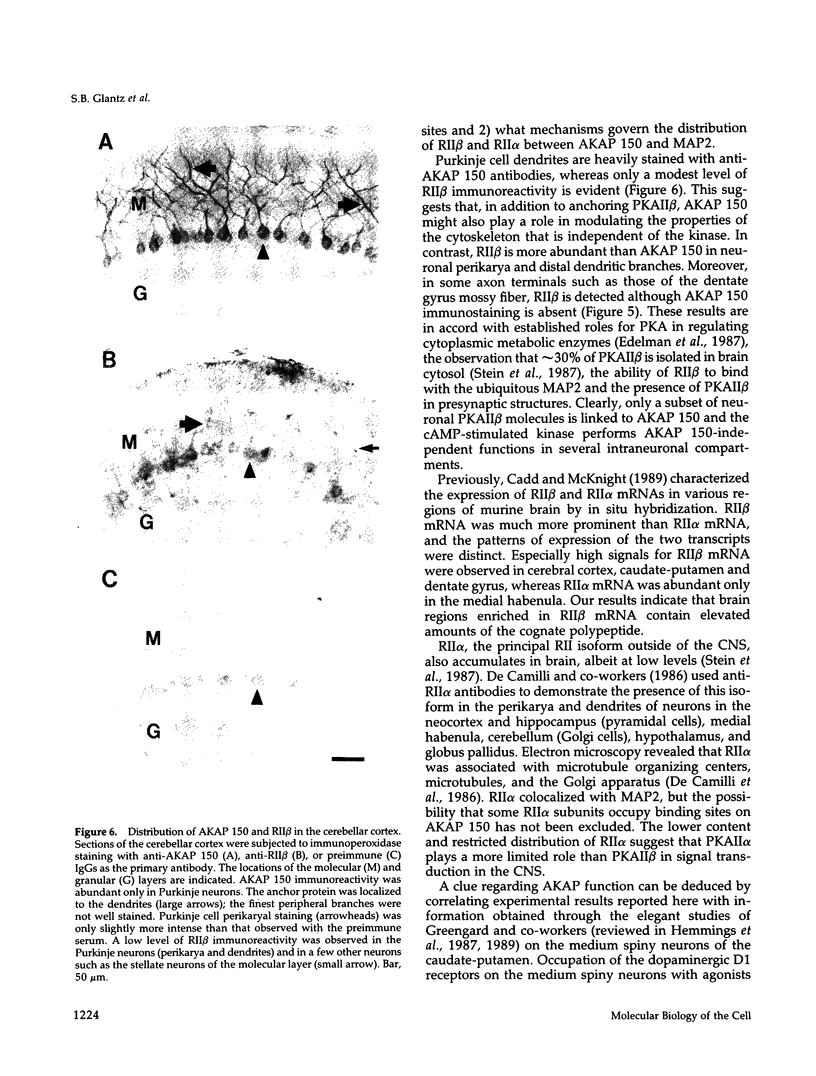
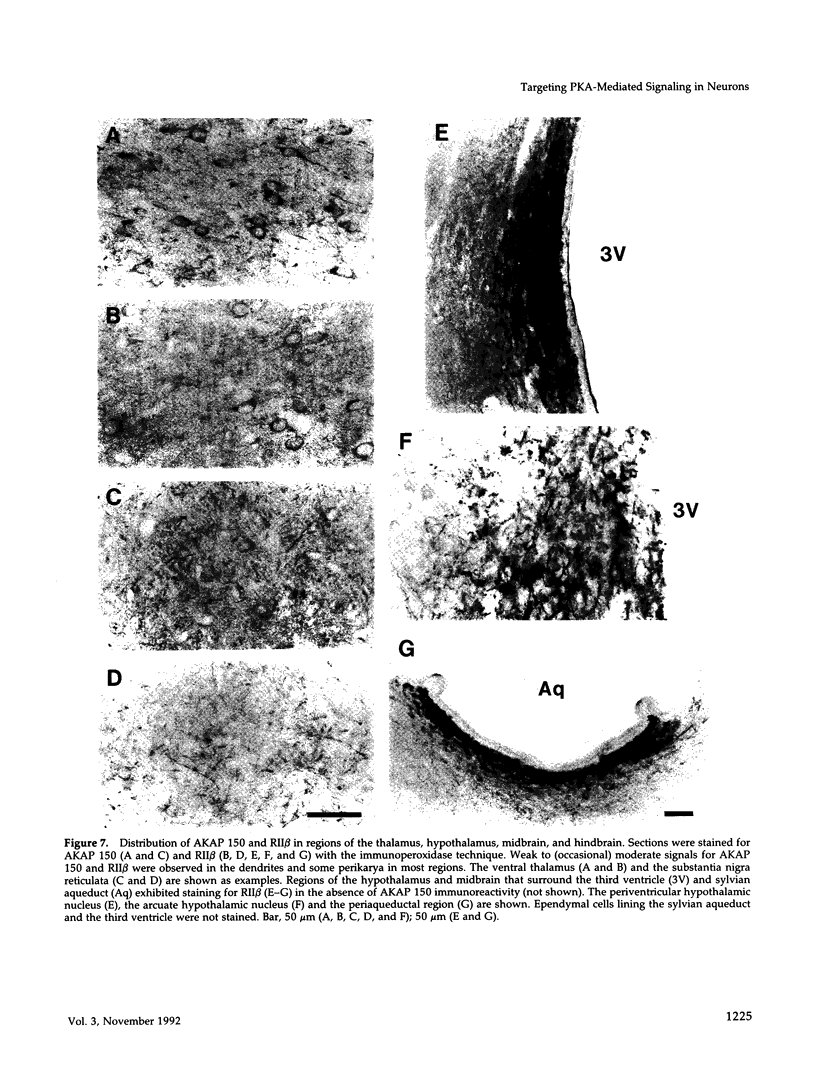
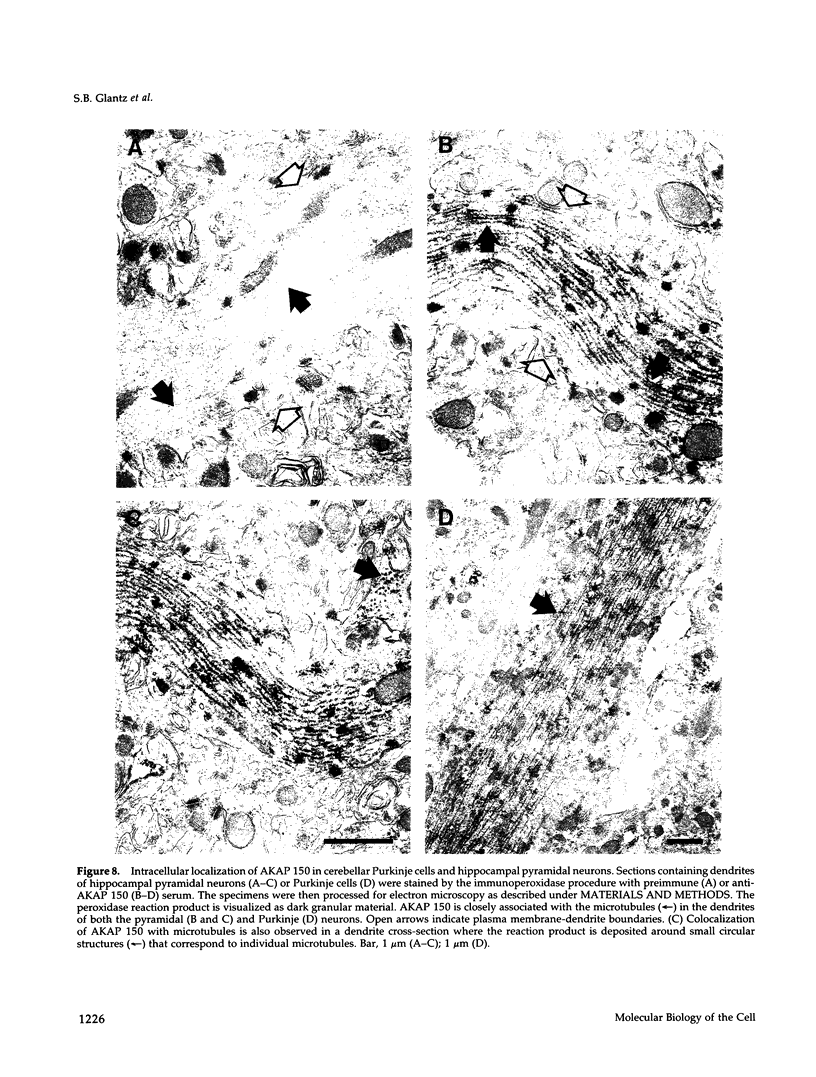
In mammalian brain, physiological signals carried by cyclic AMP (cAMP) seem to be targeted to effector sites via the tethering of cAMP-dependent protein kinase II beta (PKAII beta) to intracellular structures. Recently characterized A kinase anchor proteins (AKAPs) are probable mediators of the sequestration of PKAII beta because they contain a high-affinity binding site for the regulatory subunit (RII beta) of the kinase and a distinct intracellular targeting domain. To establish a cellular basis for this targeting mechanism, we have employed immunocytochemistry to 1) identify the types of neurons that are enriched in AKAPs, 2) determine the primary intracellular location of the anchor protein, and 3) demonstrate that an AKAP and RII beta are coenriched and colocalized in neurons that utilize the adenylate cyclase-cyclic AMP-dependent protein kinase (PKA) signaling pathway. Antibodies directed against rat brain AKAP 150 were used to elucidate the regional, cellular and intracellular distribution of a prototypic anchor protein in the CNS. AKAP 150 is abundant in Purkinje cells and in neurons of the olfactory bulb, basal ganglia, cerebral cortex, and other forebrain regions. In contrast, little AKAP 150 is detected in neurons of the thalamus, hypothalamus, midbrain, and hindbrain. A high proportion of total AKAP 150 is concentrated in primary branches of dendrites, where it is associated with microtubules. We also discovered that the patterns of accumulation and localization of RII beta (and PKAII beta) in brain are similar to those of AKAP 150. The results suggest that bifunctional AKAP 150 tethers PKAII beta to the dendritic cytoskeleton, thereby creating a discrete target site for the reception and propagation of signals carried by cAMP.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. P., Reddington M. The cellular localization of adenosine receptors in rat neostriatum. Neuroscience. 1989;28(3):645–651. doi: 10.1016/0306-4522(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984 Jun 20;226(2):203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Bregman D. B., Bhattacharyya N., Rubin C. S. High affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II-B. Cloning, characterization, and expression of cDNAs for rat brain P150. J Biol Chem. 1989 Mar 15;264(8):4648–4656. [PubMed] [Google Scholar]
- Bregman D. B., Hirsch A. H., Rubin C. S. Molecular characterization of bovine brain P75, a high affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II beta. J Biol Chem. 1991 Apr 15;266(11):7207–7213. [PubMed] [Google Scholar]
- Cadd G., McKnight G. S. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989 Jul;3(1):71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Collins S., Caron M. G., Lefkowitz R. J. From ligand binding to gene expression: new insights into the regulation of G-protein-coupled receptors. Trends Biochem Sci. 1992 Jan;17(1):37–39. doi: 10.1016/0968-0004(92)90425-9. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Miller P. E., Navone F., Theurkauf W. E., Vallee R. B. Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience. 1984 Apr;11(4):817–846. [PubMed] [Google Scholar]
- De Camilli P., Moretti M., Donini S. D., Walter U., Lohmann S. M. Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: evidence for its intracellular accumulation on microtubules, microtubule-organizing centers, and in the area of the Golgi complex. J Cell Biol. 1986 Jul;103(1):189–203. doi: 10.1083/jcb.103.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis A., Bouhelal R., Sebben M., Cory R., Bockaert J. A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol Pharmacol. 1988 Dec;34(6):880–887. [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Sarkar D., Fleischer N., Rubin C. S. Identification of two subclasses of type II cAMP-dependent protein kinases. Neural-specific and non-neural protein kinases. J Biol Chem. 1980 Sep 10;255(17):8179–8184. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Duncan G. E., Fornaretto M. G., Dearry A., Gingrich J. A., Breese G. R., Caron M. G. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand V. I., Bershadsky A. D. Microtubule dynamics: mechanism, regulation, and function. Annu Rev Cell Biol. 1991;7:93–116. doi: 10.1146/annurev.cb.07.110191.000521. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Hirsch A. H., Glantz S. B., Li Y., You Y., Rubin C. S. Cloning and expression of an intron-less gene for AKAP 75, an anchor protein for the regulatory subunit of cAMP-dependent protein kinase II beta. J Biol Chem. 1992 Feb 5;267(4):2131–2134. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jahnsen T., Hedin L., Lohmann S. M., Walter U., Richards J. S. The neural type II regulatory subunit of cAMP-dependent protein kinase is present and regulated by hormones in the rat ovary. J Biol Chem. 1986 May 25;261(15):6637–6639. [PubMed] [Google Scholar]
- Ludvig N., Ribak C. E., Scott J. D., Rubin C. S. Immunocytochemical localization of the neural-specific regulatory subunit of the type II cyclic AMP-dependent protein kinase to postsynaptic structures in the rat brain. Brain Res. 1990 Jun 18;520(1-2):90–102. doi: 10.1016/0006-8993(90)91694-c. [DOI] [PubMed] [Google Scholar]
- Luo Z., Shafit-Zagardo B., Erlichman J. Identification of the MAP2- and P75-binding domain in the regulatory subunit (RII beta) of type II cAMP-dependent protein kinase. Cloning and expression of the cDNA for bovine brain RII beta. J Biol Chem. 1990 Dec 15;265(35):21804–21810. [PubMed] [Google Scholar]
- McLean J. H., Shipley M. T., Nickell W. T., Aston-Jones G., Reyher C. K. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol. 1989 Jul 15;285(3):339–349. doi: 10.1002/cne.902850305. [DOI] [PubMed] [Google Scholar]
- Obar R. A., Dingus J., Bayley H., Vallee R. B. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron. 1989 Nov;3(5):639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Rainbow T. C., Parsons B., Wolfe B. B. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino H. M., Dammerman M., Shafit-Zagardo B., Erlichman J. Localization and characterization of the binding site for the regulatory subunit of type II cAMP-dependent protein kinase on MAP2. Neuron. 1989 Nov;3(5):631–638. doi: 10.1016/0896-6273(89)90273-0. [DOI] [PubMed] [Google Scholar]
- Sarkar D., Erlichman J., Rubin C. S. Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J Biol Chem. 1984 Aug 10;259(15):9840–9846. [PubMed] [Google Scholar]
- Schubart U. K., Fields K. L. Identification of a calcium-regulated insulinoma cell phosphoprotein as an islet cell keratin. J Cell Biol. 1984 Mar;98(3):1001–1009. doi: 10.1083/jcb.98.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D. Cyclic nucleotide-dependent protein kinases. Pharmacol Ther. 1991;50(1):123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- Stein J. C., Farooq M., Norton W. T., Rubin C. S. Differential expression of isoforms of the regulatory subunit of type II cAMP-dependent protein kinase in rat neurons, astrocytes, and oligodendrocytes. J Biol Chem. 1987 Mar 5;262(7):3002–3006. [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982 Mar 25;257(6):3284–3290. [PubMed] [Google Scholar]
- Trinczek B., Schwoch G. Immunoelectron microscopical localization of the catalytic subunit of cAMP-dependent protein kinases in brain microtubules and neurofilaments. FEBS Lett. 1990 Dec 17;277(1-2):167–170. doi: 10.1016/0014-5793(90)80835-7. [DOI] [PubMed] [Google Scholar]