Abstract
The adapter protein SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) is an essential mediator of signaling from the T-cell antigen receptor (TCR). We report here that SLP-76 also mediates signaling downstream of integrins in T cells and that SLP-76-deficient T cells fail to support adhesion to integrin ligands. In response to both TCR and integrin stimulation, SLP-76 relocalizes to surface microclusters that colocalize with phosphorylated signaling proteins. Disruption of SLP-76 recruitment to the protein named LAT (linker for activation of T cells) inhibits SLP-76 clustering downstream of the TCR but not downstream of integrins. Conversely, an SLP-76 mutant unable to bind ADAP (adhesion and degranulation-promoting adapter protein) forms clusters following TCR but not integrin engagement and fails to support T-cell adhesion to integrin ligands. These findings demonstrate that SLP-76 relocalizes to integrin-initiated signaling complexes by a mechanism different from that employed during TCR signaling and that SLP-76 relocalization corresponds to SLP-76-dependent integrin function in T cells.
Coordinated signals from the extracellular environment direct T-cell functions, including cell trafficking and antigen-driven cell activation. One way that T cells make contact with their environment is through integrins expressed on the cell surface. Integrins are heterodimeric proteins that bind adhesion molecules in the extracellular matrix and on the surface of other cells (26). The predominant integrins expressed on T cells are leukocyte function-associated antigen 1 (LFA-1 [αLβ2]) and very late antigen 4 (VLA-4 [α4β1]). By associating with both extracellular adhesion molecules and the cytoskeleton, integrins bridge the cell exterior and interior. Integrin function is critical for T-cell development, trafficking through the vasculature and tissues, the formation of conjugates with antigen-presenting cells, T-cell activation, and directed cytokine secretion (18, 38, 43, 47).
Extensive studies of proximal signaling downstream of the engaged T-cell antigen receptor (TCR) have provided a model of how T cells respond to extracellular cues (44). TCR stimulation triggers the activation of the protein tyrosine kinases (PTKs) Lck and ζ-associated protein of 70 kDa (ZAP-70), resulting in the phosphorylation of the transmembrane adapter protein linker for activation of T cells (LAT). Another critical adapter protein, SH2 domain-containing leukocyte protein of 76 kDa (SLP-76), is recruited to phosphorylated LAT via its binding partner Grb2-related adapter downstream of Shc (Gads) (42). Together, SLP-76 and LAT form a multimolecular signaling complex at the cell membrane, resulting in activation of effector proteins and reorganization of the actin cytoskeleton. The dynamic relocalization of SLP-76 to TCR-initiated surface signaling complexes has been characterized using biochemical and live cell imaging approaches (7, 42, 51). In these studies, the TCR-induced recruitment of SLP-76 to microclusters coincident with the presence of PTKs and other signaling proteins was visualized in real time. As these reports show, SLP-76 microclusters arise at the periphery of the spreading T cell and then migrate inward (5) in a process shown recently to be modulated by TCR coligation with integrins (5, 35).
Integrins in hematopoietic cells are maintained in an inactive conformation and possess low affinity for their ligands (40). “Inside-out” signals originating at either the TCR or chemokine receptors upregulate integrin affinity (47). Integrins in the active conformation then bind adhesion molecule ligands and initiate “outside-in” signals, resulting in altered cell morphology and enhanced adhesive and proliferative responses (reviewed in reference 3). Work to elucidate outside-in signaling pathways in various hematopoietic lineages has uncovered a role for PTKs as well as adapter proteins downstream of engaged integrins. Src and Syk family kinases have been shown to initiate signals from stimulated integrins, phosphorylating target adapter proteins (including SLP-76), and the adhesion and degranulation-promoting adapter protein (ADAP) (2, 15, 17, 49). SLP-76 and ADAP have also been found to organize integrin signals in myeloid lineages and platelets and to contribute to integrin-dependent cell functions (20, 22, 34, 46).
While considerable progress has been made in defining how PTKs and adapters organize signaling from the TCR, much less is understood about how these molecules function downstream of integrins in T cells. Although a number of recent elegant papers have suggested that the immunoreceptor tyrosine-based activation motif (ITAM) and integrin second-messenger pathways intersect at a point very close to the membrane, how signals are then differentiated by the cell remains unclear (30, 52, 53). For this report, we made use of an inducible in vivo model to control SLP-76 expression and test the role of this adapter protein in integrin-initiated signaling in T cells. Our findings demonstrate an essential role for SLP-76 in T-cell integrin function that correlates with the relocalization of SLP-76 to signaling microclusters initiated by integrin ligation. Interestingly, the requirements for SLP-76 partner proteins and the domains of SLP-76 that they bind are distinct with respect to SLP-76 functions downstream of integrins versus the TCR, suggesting that adapter proteins mediate both overlapping and distinct signaling pathways from these receptors.
MATERIALS AND METHODS
Reagents, cDNA constructs, and stable cell lines.
SLP-76 G2 and arginine-to-lysine (RK) mutants and LAT, Gads-binding fragment (GBF), and DsRed2 murine stem cell virus-based vectors have been described previously (8, 42). Jurkat cells were electroporated (at 310 V) with 10 μg of plasmid DNA. J14, JCaM1, JCaM2, and P116 Jurkat lines have been described previously (reviewed in reference 1). JDAP cells were a gift from Ann Huang (Children's Hospital of Philadelphia) (16). Stable cell lines were generated by fluorescence-activated cell sorting for green fluorescent protein (GFP). PP1 (Calbiochem) was used at 10 μM.
Retroviral infection of T cells.
Peripheral T cells were induced to proliferate with 20 μg of phorbol myristate acetate (PMA)/ml-200 μg of ionomycin/ml-40 U of interleukin-2/ml, infected with retroviral supernatant-0.8 μg Polybrene/ml (Sigma) by spinning at 2,000 rpm for 90 min on two sequential days, cultured in T-cell media (TCM) (Iscove's modification of Dulbecco's medium-10% fetal bovine serum), and analyzed 3 to 5 days later. For naïve T cells, SLP-76−/− fetal liver cells were retrovirally transduced with GFP-SLP-76 to reconstitute lethally irradiated hosts as previously described (21), and T cells were purified after 8 weeks.
Cellular imaging.
GFP-SLP-76 microclusters were visualized as previously described (42). Delta T dishes (Bioptechs) were coated with 0.01% poly-l-lysine (PLL) (Sigma)-10 μg of OKT-3/ml (eBiosciences) or 10 μg of integrin ligand human fibronectin (FN)/ml and human or murine ICAM-1 (R&D Systems). T cells in phosphate-buffered saline (PBS) were deposited onto dishes, and images were collected at the plane of the contact site. Live-cell imaging was performed on a Perkin-Elmer five-wavelength laser UltraVIEW live-cell-imaging confocal spinning-disk scanner (Yokogawa) attached to a Nikon TE-300 inverted microscope equipped with a 1.4-numerical-aperture 100× objective and a z-axis controller (Physik Instrumente). Delta T4 dish and objective heaters (Bioptechs) maintained the sample temperature at 37°C. Samples were excited using 488-nm and 568-nm laser lines with a 488/568 red-green-blue dichroic mirror. A Hamamatsu Orca-ER camera was used to collect images. Image analysis was performed using IP Labs software (Biovision).
For fixed images, T cells were added to coated coverslips and incubated at 37°C for 8 min. Cells were then fixed in 4% paraformaldehyde, permeablized in 0.5% Triton, blocked (PBS-10% bovine serum albumin), and stained for phosphotyrosine 4G10 and pLAT-Y191 (Upstate), pLck-Y192 (Santa Cruz), and pZAP-70-Y319 (Cell Signaling Technology), washed, and mounted on to slides. Image contrast was enhanced and color channels were merged using IPLabs software.
TIRF microscopy and particle tracking.
Total internal reflection fluorescence (TIRF) images were obtained using a 60× 1.45-numerical-aperture TIRF objective fitted to an inverted microscope system (Olympus IX71) and a 488-nm laser line (Coherent), and images were acquired using a charge-coupled-device camera and recorded with HCImage software (both Hamamatsu). Images were corrected for background unevenness with a Blackman filter, and localization and tracking were performed with Matlab algorithms (Mathworks). Particle trajectories, persistence, and net displacement were computed using records of spatial and temporal coordinates.
Membrane preparations.
Isolation of detergent-resistant membranes was carried out as described previously (42). Briefly, 1 × 107 Jurkat cells were stimulated, lysed in 1× Triton detergent, resuspended in a 40% sucrose solution, and overlaid with 30% and 5% sucrose solutions. Following overnight ultracentrifugation at 44,000 rpm, fractions were taken topwise, resuspended in loading buffer, and subjected to immunoblot analysis.
Rap1 activity assays.
Activation of Rap1 was evaluated by precipitation of Rap1-GTP from cellular lysates by the use of beads conjugated to Ral-GDS (Upstate). Precipitates were purified from unstimulated T cells and T cells treated with stromal derived factor-1α (SDF-1α), processed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted for total Rap1 (Upstate).
LFA-1 affinity assays.
Primary T cells were rested, left unstimulated, or stimulated with 1 ng of SDF-1α/ml for 30 min at 37°C, washed, and incubated with 2.5 μg of mICAM-1 (R&D)/ml prelabeled with AF-647 (Molecular Probes) for 30 min at room temperature. Cells were then washed, and ICAM-1 binding was evaluated by flow-cytometry.
Mice and tamoxifen treatment.
SLP-76 knockout, Slp-76F/F, CD4-Cre, and ADAP knockout mice have been described previously (10, 27). Slp-76F/F mice expressing Rosa-YFP R26RYFP/YFP (45) were crossed with SLP-76−/+ mice hemizygous for the CreERT2 transgene (39) to produce animals of the genotypes Slp-76F/−; CreERT2; R26RYFP and Slp-76F/+;CreERT2;R26RYFP, denoted as SLP-76 conditional knockouts (cKO) and conditional heterozygotes (cHETs). Tamoxifen (Sigma) (at 20 mg/ml in corn oil) was delivered by daily oral gavage (222 μg/g of body weight, for 5 consecutive days). Mice were studied for 4 to 14 days after the end of drug administration. Mouse handling and procedures were in strict accordance with University of Pennsylvania and IACUC protocols.
T-cell migration.
Thy1.2+ purified T cells (2 × 105) were placed in the upper chamber of transwell plates (Costar), with either TCM alone or TCM-100 ng of SDF-1α (Sigma)/ml in the bottom chamber. Following 2 to 3 h of incubation at 37°C, cells were harvested from the bottom chamber and counted using a hemacytometer; data are presented as the numbers of recovered cells × 103.
Static adhesion.
T-cell adhesion was measured as previously described (12). Costar 2BX plates were coated with 0.15 μg of mICAM-1/Fc (R&D Systems)/well. Sorted T cells were labeled with calcein-AM (Molecular Probes), precoated with 10 μg of 2c11-biotin (BD)/ml on ice, added to the ICAM-coated wells containing PBS, 100 ng of SDF-1α/ml, 1 μg of streptavidin/ml, and 100 ng/ml PMA or 5 mM MgCl2-1 mM EGTA, and incubated on ice to allow cells to settle. Plates were first read (Flx800TBE; Bio-Tek) using 485-nm-excitation and 516-nm-emission filters and then warmed to 37°C for 15 min. Wells were washed three to five times with PBS and read again. Percentages of input fluorescence following washes were calculated per well and averaged for experiments conducted using triplicate wells, and results were analyzed for statistical significance using a Student's t test.
Adhesion under flow.
T-cell adhesion under conditions of 0.5 dyn/cm2 wall shear force was measured as described previously (31). Cells (106/ml) in Hanks balanced salt solution were perfused over 1 μg of SDF-1α/ml and 5 μg of ICAM-1/ml in a parallel plate chamber, and adherent cells were counted using five viewing areas in photomicrographs after 10 min.
RESULTS
SLP-76 is dispensable for chemokine-mediated integrin activation.
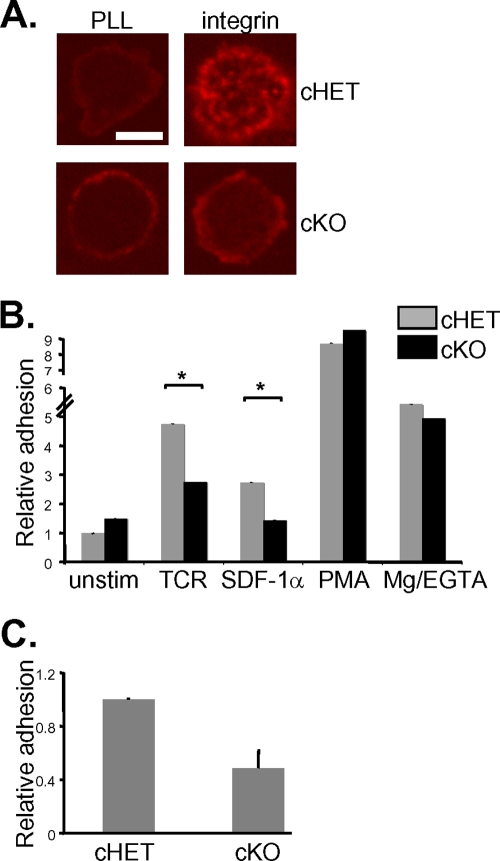
To examine the requirement for SLP-76 in integrin function, we sought to measure integrin-mediated adhesion in SLP-76-deficient T cells. Because SLP-76-dependent signaling originating at the pre-TCR is required for T-cell development in the thymus, SLP-76-deficient animals lack peripheral T cells (10, 37). Therefore, we made use of a conditional deletion approach to generate SLP-76-deficient peripheral T cells. In this system, a portion of the Lcp-2 gene encoding SLP-76 was flanked with loxP sites (27) and then inducibly deleted with a temporally controlled Cre recombinase by treatment with tamoxifen (39). A yellow fluorescent protein (YFP) reporter gene, which is expressed only following Cre activation (45), was used to identify cells from which SLP-76 has been excised (see Fig. S1 in the supplemental material). Using this approach, we generated YFP+ fl/+ SLP-76 cHET and SLP-76 fl/− cKO T cells (Fig. 1A). Characterization of SLP-76 cKO cells has demonstrated defects in proximal signaling from the TCR and T-cell effector functions (Wu et al., submitted for publication), which is a finding consistent with previous studies of Jurkat T cells and developing thymocytes (27, 50).
FIG. 1.
SLP-76 is dispensable for signaling from the chemokine receptor CXCR4. (A) Temporally controlled deletion of SLP-76 was accomplished through mice expressing a SLP-76 allele flanked with loxP restriction sites by treatment with tamoxifen. Following excision of one floxed SLP-76 allele (loxP-flanked box), cHET mice posressed one remaining copy of SLP-76 (top) and cells from cKO mice possessed no remaining SLP-76 allele (bottom). (B) SLP-76 cHET and cKO cells were allowed to migrate across a transwell barrier into medium alone (left panel) or into a source of SDF-1α (right panel), and results are presented as mean numbers of recovered cells (103) ± standard deviations determined in six independent experiments. (C) SLP-76 cHET and cKO T cells were left unstimulated (unstim) or were stimulated with 1 ng of SDF-1α/ml for 2 and 10 min, and active Rap1 was precipitated from cell lysates. Rap1 activity levels were similarly upregulated in SLP-76 cHET and cKO cells. (D) SLP-76 cHET and cKO T cells were left unstimulated or were stimulated with SDF-1α, and binding of soluble mICAM-1 was evaluated by flow cytometry as a measurement of LFA-1 affinity. cHET and cKO cells bind equivalent levels of ICAM-1 in response to SDF-1α stimulation. Max, maximum; iso, isotype.
T cells maintain surface integrins in a constitutively low-affinity conformation. To test the requirement for SLP-76 in outside-in signaling from integrins, we needed first to enable ligand binding by upregulating integrin affinity. In T cells, the two established signals whose activity results in integrin upregulation are those of the TCR and chemokine receptors (3). Given the importance of SLP-76 in proximal signaling from the TCR, TCR stimulation was not an appropriate signal to increase integrin affinity. Instead, we asked whether SDF-1α, which signals through the seven-transmembrane receptor CXCR4, could upregulate integrin affinity in the absence of SLP-76.
We used several complementary approaches to evaluate SDF-1α signaling from CXCR4 in T cells deficient in SLP-76. We first evaluated the cellular response to SDF-1α in a functional assay. CXCR4 stimulation results in mobilization of the cytoskeleton and in T-cell migration. SLP-76 cHET and cKO T cells migrated similarly across a transwell barrier to a source of SDF-1α, suggesting that CXCR4 function is normal in the absence of SLP-76 (Fig. 1B). Next we investigated activation of Rap1, a critical mediator of chemokine signaling and cell migration (38), in the absence of SLP-76. SLP-76 cKO T cells supported robust activation of Rap1 in response to SDF-1α treatment (Fig. 1C) but did not do so downstream of the TCR (data not shown), further suggesting that SLP-76 is dispensable for SDF-1α signaling in T cells. Finally, we examined the affinity of surface integrins in SDF-1α-stimulated cHET and cKO T cells. Using binding of soluble ICAM-1 to measure LFA-1 affinity, we showed that SLP-76 cHET and cKO cells bind equivalent levels of soluble ICAM-1 in response to SDF-1α stimulation (Fig. 1D). These data strongly indicate that signaling downstream of SDF-1α and CXCR4 is normal in T cells deficient in SLP-76.
SLP-76 is required for integrin signaling and adhesion to integrin ligands downstream of both the TCR and chemokines.
Work performed in our laboratory (reviewed in reference 3) and by others (2) has shown that integrin stimulation activates PTKs and produces a cascade of phosphotyrosine-mediated signaling. Since the SLP-76 adapter function serves to coordinate PTKs with their signaling molecule targets, we wondered whether phosphotyrosine signaling from integrins would be diminished in the absence of SLP-76. To test the requirement for SLP-76 in proximal signaling from integrins, SDF-1α-treated cHET and cKO cells were deposited onto ICAM-1-coated surfaces to stimulate outside-in signaling from the integrin LFA-1. cHET T cells upregulate protein tyrosine phosphorylation in response to integrin stimulation and accumulate tyrosine-phosphorylated proteins within microclusters at the contact site with ICAM-1 (Fig. 2A). By contrast, SLP-76 cKO T cells fail to support the formation of tyrosine-rich signaling microclusters, suggesting that proximal phosphotyrosine signaling from integrins requires SLP-76.
FIG. 2.
SLP-76 is required for T-cell integrin signaling and adhesion to ICAM-1. (A) Proximal signaling from integrins is defective in SLP-76 cKO cells, as measured by immunofluorescence microscopy. LFA-1 on SLP-76 cHET and cKO T cells was stimulated by pretreatment with SDF-1α followed by deposition onto the ligand ICAM-1. Following 10 min of stimulation, cells were fixed and stained for total phosphotyrosine. Clusters of phosphotyrosine staining appear in cHET but not in cKO cells. Bar, 5 μm. (B) Adhesion of SLP-76 cHET and cKO cells to ICAM-1 was measured among cells subjected to the indicated stimuli. Adhesion levels ranged from 5 to 10% in unstimulated (unstim) cells to 80% at maximum. Data were normalized to unstimulated cHET cell results and represent means ± standard deviations of the results of 10 independent experiments. Asterisks indicate a statistical significance of P < 0.05. (C) Levels of adhesion of SLP-76 cKO and cHET cells to SDF-1α and ICAM-1 under flow conditions were compared. Data represent means ± standard deviations normalized to unstimulated cHET cell results obtained in three independent experiments.
To test the functional outcome of defective integrin outside-in signaling, we compared the ability of cHET and cKO T cells to support adhesion to integrin ligands in static and dynamic flow adhesion assays. While unstimulated T cells supported only very low levels of static adhesion to ICAM-1, treatment with SDF-1α or TCR cross-linking increased adhesion of SLP-76 cHET T cells by three- to fivefold. SLP-76-deficient cKO T cells, however, were unable to support either SDF-1α or TCR-induced adhesion to ICAM-1 (Fig. 2B). Therefore, SLP-76 is dispensable for SDF-1α-induced integrin affinity upregulation but is required for subsequent T-cell adhesive function. SLP-76 cHET and cKO T cells demonstrated comparable levels of integrin-mediated adhesion when treated with PMA or MgCl2, suggesting that cKO cells were able to adhere to integrin ligands when SLP-76-mediated outside-in signaling was bypassed. Similar results were obtained with adhesion assays performed under conditions of hydrodynamic flow. Compared to cHET cells, SLP-76-deficient T cells demonstrated a twofold reduction in adhesion to SDF-1α- and ICAM-1-coated surfaces (Fig. 2C). These results demonstrate that SLP-76 is required for outside-in integrin function in T cells.
SLP-76 relocalizes to surface microclusters in response to both TCR and integrin stimulation.
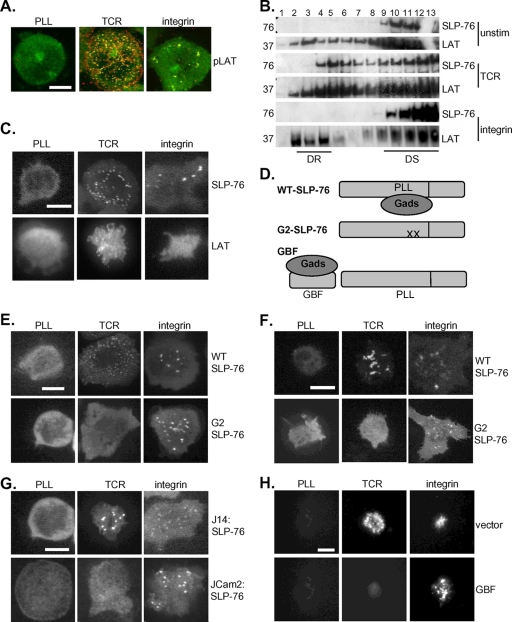
Multiple biochemical and imaging studies have demonstrated that SLP-76 relocalizes to microclusters at the cell membrane in response to TCR stimulation (7, 42). We therefore investigated whether SLP-76 might similarly relocalize in response to integrin ligation. Primary T cells and the Jurkat T-cell line were employed in complementary studies to localize SLP-76 during integrin signaling. First, we purified membrane and cytosol preparations from unstimulated, TCR-stimulated, and integrin-stimulated Jurkat T cells by ultracentrifugation. In resting cells, SLP-76 partitioned exclusively with the cytosol. By contrast, some SLP-76 partitioned with cell membranes in both TCR and integrin-stimulated cells (data not shown). To determine whether SLP-76 relocalizes to surface microclusters in response to integrin engagement, we used confocal microscopy to visualize the movement of GFP-tagged SLP-76 expressed in J14 Jurkat T cells deficient in endogenous SLP-76. Confocal micrographs revealed that SLP-76 is recruited to microclusters at the cell contact site with both integrin and TCR stimuli (Fig. 3A). To ensure that integrin-initiated SLP-76 relocalization was not a phenomenon unique to the immortalized Jurkat cell line, GFP-tagged SLP-76 was expressed in primary murine T cells by retroviral transduction. In both previously activated and naïve primary cells (Fig. 3B and C), GFP-SLP-76 relocalized to microclusters in response to engagement of the TCR and CD28 and also in response to ligation of LFA-1 with ICAM-1.
FIG. 3.

SLP-76 relocalizes to microclusters at the cell surface in response to TCR and integrin stimulation. (A) J14:GFP-SLP-76 T cells were deposited onto PLL, TCR, and integrin (ICAM-1)-stimulating surfaces, and recruitment of GFP-SLP to microclusters at the contact site is shown. Bars, 5 μm. (B and C) GFP-SLP-76 was recruited to microclusters in primary T-cell blasts (B) and naïve murine T cells (C). Image results are representative of at least five independent experiments.
Both TCR- and integrin-initiated SLP-76 microclusters are associated with PTK signaling.
Engagement of both the TCR and integrins causes rapid activation of PTKs. We next asked whether integrin-initiated SLP-76 microclusters, like those induced by the TCR, are rich in tyrosine-phosphorylated proteins. Both TCR and integrin stimulation resulted in a robust phosphotyrosine response, and phosphotyrosine-containing proteins colocalized with SLP-76 in response to both stimuli (Fig. 4A). Known mediators of TCR signaling cascades, the phosphorylated PTKs Lck (Fig. 4B) and ZAP-70 (Fig. 4C), were found in the SLP-76 microclusters following engagement of the TCR or integrins.
FIG. 4.
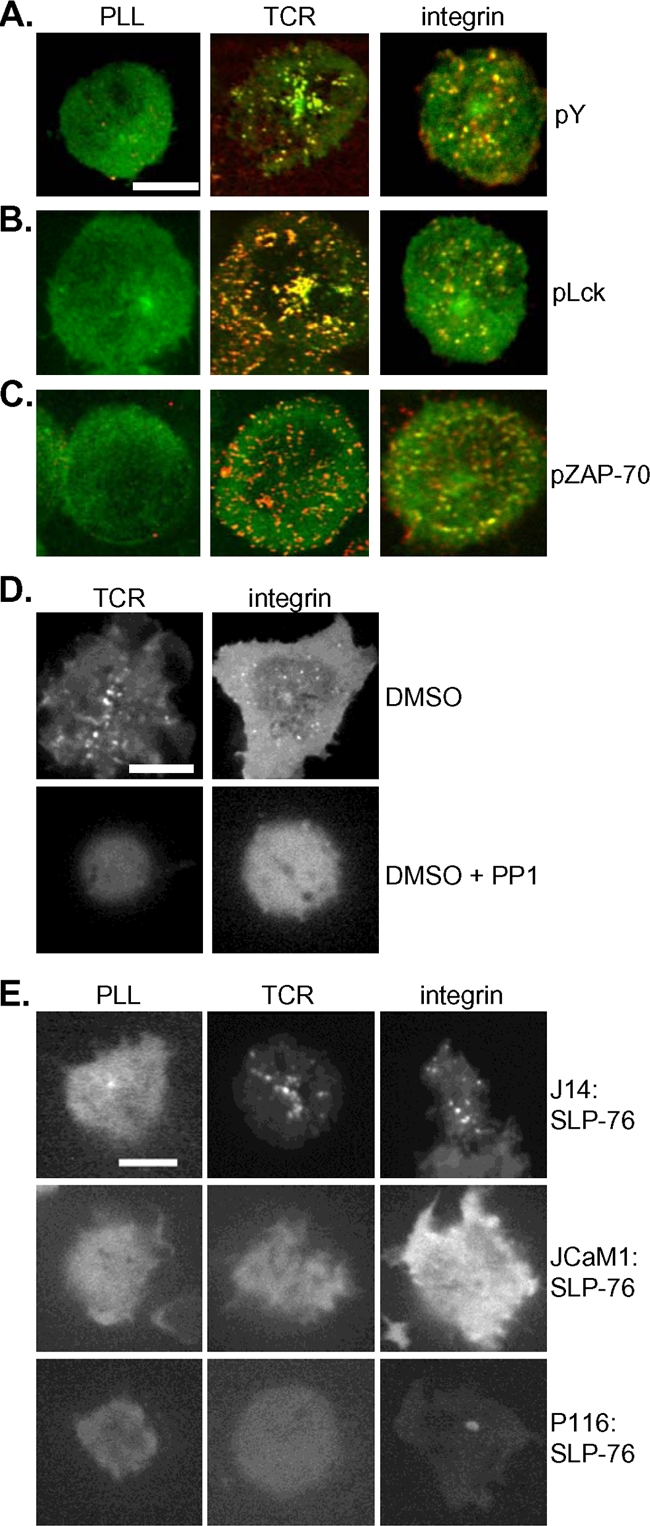
PTKs direct SLP-76 relocalization to integrin-initiated microclusters. (A) J14 cells expressing GFP-SLP-76 (green) were deposited onto unstimulatory or TCR or integrin (FN) stimulatory surfaces, fixed, and stained for total phosphorylated tyrosine (pY) (red); merged images are shown. Bars, 5 μm. (B) GFP-SLP-76 (green) colocalized with phospho-Lck (pLck) (red). (C) GFP-SLP-76 (green) colocalized with phospho-ZAP-70 (pZAP-70) (red). (D) J14:GFP-SLP-76 cells were preincubated with either dimethyl sulfoxide (DMSO) carrier alone or DMSO plus PP1 and were stimulated by TCR or integrin (FN). (E) JCaM1 and P116 Jurkat T cells transfected with GFP-SLP-76 were deposited onto TCR and integrin (FN) stimulatory surfaces; the resulting GFP-SLP-76 clusters are shown. All image results are representative of at least three independent experiments.
Because GFP-SLP-76 microclusters are coincident with the presence of phosphotyrosine, we wondered whether PTKs direct recruitment of SLP-76 to microclusters downstream of engaged integrins. Pharmacological PP1 inhibition of Src-family kinase (SFK) activity blocked formation of SLP-76 microclusters in cells stimulated by either TCR or integrins (Fig. 4D). To specifically test the requirement for the PTKs Lck and ZAP-70 in integrin-induced SLP-76 relocalization, GFP-SLP-76 was expressed in the Jurkat cell lines JCaM1 and P116, which are deficient in Lck and ZAP-70, respectively (1). In both cell lines, SLP-76 failed to relocalize to microclusters in response to integrin ligation (Fig. 4E), suggesting that phosphotyrosine signaling by Lck and ZAP-70 directs SLP-76 relocalization to microclusters downstream of both TCR and integrin engagement.
The dynamics of SLP-76 microclusters differ in response to TCR and integrin ligation.
Having shown that integrin-initiated SLP-76 microclusters correspond to sites of intracellular signaling, we next sought to compare and contrast their features to those of TCR-initiated microclusters. TIRF microscopy detected SLP-76 microclusters induced by both TCR and integrins, suggesting that SLP-76 localizes within the same range of distance, approximately 100 nm, from the cell surface in response to either stimulus.
Prior studies have provided an understanding of the dynamic regulation of TCR-initiated SLP-76 microclusters: they first arise at the periphery of the T-cell contact site, rapidly move centrally from their point of origin to accumulate near the microtubule-organizing center, and ultimately disappear at later time points (5). Our data confirm these findings but reveal different patterns of SLP-76 dynamic relocalization downstream of integrins. While SLP-76 clusters initiated at integrins also arise at the cell periphery, they do not centralize as they do in cells stimulated by the TCR (Fig. 5A; see also movie S2 in the supplemental material). Integrin-initiated microclusters exhibit decreased displacement and persist longer than those stimulated by the TCR (Fig. 5). Yet integrin-initiated clusters are not fixed and move at velocities comparable to those seen with SLP-76 microclusters downstream of the TCR (data not shown). These data suggest that, while common signaling events initiate SLP-76 relocalization downstream of the TCR and integrins, the temporal and spatial regulation of SLP-76 signaling clusters downstream of these receptors is different.
FIG. 5.
TCR and integrin-initiated SLP-76 microclusters exhibit different dynamic properties. (A) J14:GFP-SLP-76 cells were deposited onto TCR and integrin (FN) stimulatory surfaces and imaged over time by TIRF microscopy, and snapshots from indicated time points (indicated in seconds) are shown. Bars, 5 μm. (B) GFP-SLP-76 clusters were tracked at all time points, and trajectories are shown overlaid on the sample cell. (C) (Left panel) The displacement of GFP-SLP-76 clusters was calculated, and the results are shown with respect to elapsed time, with TCR results represented in black and integrin (FN) results in gray. (Right panel) Numbers of GFP-SLP-76 clusters in representative cells were counted at multiple time points, and percentages representing the maximum numbers of clusters counted at any time point are shown. Data represent means ± standard deviations of the results of 10 TCR and 4 FN experiments.
LAT directs SLP-76 relocalization downstream of the TCR but not downstream of integrins.
An emerging body of research has shown that both SLP-76 relocalization and centralization downstream of the engaged TCR are driven by the inducible association of SLP-76 with tyrosine-phosphorylated LAT. To test the requirement for LAT in SLP-76 clustering downstream of the TCR and integrins, we took multiple approaches. We first used immunofluorescence microscopy to investigate whether SLP-76 colocalized with phosphorylated LAT. Only SLP-76 clusters initiated by TCR ligation colocalized with phospho-LAT; in integrin-stimulated cells, staining for phosphorylated LAT was minimal, with little to no colocalization with GFP-SLP-76 (Fig. 6A). LAT has been shown to partition into microdomains of the plasma membrane resistant to solubilization by detergent, whereas SLP-76 constitutively resides in the cell cytosol. To test the movement of SLP-76 into LAT-containing membrane microdomains, Jurkat T cells were stimulated through use of the TCR or integrins, and detergent-resistant cell fractions were prepared by density ultracentrifugation. Whereas upon TCR stimulation some SLP-76 partitioned with LAT, SLP-76 failed to relocalize to LAT within detergent-resistant membranes in response to integrin engagement (Fig. 6B). Next, we transfected GFP-LAT into the LAT-deficient Jurkat T-cell line JCaM2 and found that, unlike SLP-76, which relocalizes downstream of either TCR or integrin engagement, GFP-LAT formed microclusters only in response to TCR ligation (Fig. 6C). These results indicate that while SLP-76 microclusters formed in response to both TCR and integrin stimulation colocalize with phosphorylated PTKs Lck and ZAP-70, SLP-76 clusters formed in response to integrin engagement do not contain the LAT adapter protein.
FIG. 6.
SLP-76 relocalization downstream of integrins is independent of LAT. (A) J14 cells expressing GFP-SLP-76 (green) were deposited onto unstimulatory (unstim) and TCR and integrin (FN) stimulatory surfaces, fixed, and stained for phospho-LAT Y-191 (red); color-merged images are shown. Bars, 5 μm. (B) J14:GFP-SLP-76 cells were stimulated by TCR and integrins (FN), and density ultracentrifugation was used to separate detergent-resistant (DR) (lanes 2 to 5) from detergent-soluble (DS) (lanes 9 to 12) protein fractions, which were then subjected to immunoblot analysis. (C) LAT−/− JCaM2 Jurkat cells were transfected with GFP-LAT and stimulated by TCR and integrins (FN). (D) The G2 SLP-76 mutant was unable to support Gads binding or Gads-mediated recruitment to LAT, while the GBF competed with SLP-76 for Gads binding. (E and F) J14 (E) and primary (F) T-cell blasts expressing GFP-G2 and WT SLP-76 were stimulated by TCR and integrin (FN and ICAM-1, respectively). (G) JCaM2 T cells supported the formation of integrin (FN)-initiated but not TCR-initiated GFP-SLP-76 microclusters. (H) Primary T-cell blasts transduced with both GFP-WT SLP-76 and either vector alone or GBF peptide were stimulated by TCR or integrins (ICAM-1). All image results are representative of at least five independent experiments.
Because integrin stimulation fails to induce LAT clustering, we speculated that SLP-76 relocalization to integrin-initiated signaling complexes may be independent of LAT. To test the requirement for LAT association during SLP-76 relocalization, we studied an SLP-76 mutant, G2-SLP-76. As a result of two amino acid substitutions, G2-SLP-76 cannot bind Gads or be recruited to LAT (25, 42) (Fig. 6D). G2-SLP-76 relocalizes like wild-type (WT) SLP-76 to microclusters initiated by integrin engagement but fails to relocalize to clusters downstream of the TCR (Fig. 6E). It was possible that G2-SLP-76 relocalizes to integrin-initiated clusters only in the absence of WT SLP-76. To confirm that G2-SLP-76 can compete with the WT for recruitment to microclusters, we expressed G2-SLP-76 in primary T cells containing endogenous SLP-76. In WT primary cells, as in J14 cells, G2-SLP-76 formed clusters following engagement of integrins but not engagement of the TCR (Fig. 6F).
To specifically test the requirement for LAT during integrin-initiated SLP-76 relocalization, we visualized GFP-SLP-76 localization in LAT−/− JCaM2 cells. Whereas SLP-76 relocalized to microclusters in response to both TCR and integrin engagement in LAT-sufficient cells, SLP-76 was recruited to microclusters in response to integrin stimulation but not TCR stimulation in LAT-deficient cells (Fig. 6G). These results confirm that LAT is dispensable for integrin-induced but not TCR-induced SLP-76 relocalization. We then wondered whether this differential requirement for LAT could be exploited to selectively disrupt SLP-76 recruitment to TCR-initiated but not integrin-initiated microclusters. Toward this goal, a GBF of SLP-76, which has been shown to compete with SLP-76 for Gads-mediated relocalization downstream of the TCR (19, 42), was expressed in primary T cells. Coexpression of the GBF peptide did not impair recruitment of GFP-SLP-76 to microclusters downstream of integrin engagement but selectively inhibited SLP-76 relocalization to TCR-initiated microclusters (Fig. 6H). In sum, these findings indicate that although critical for TCR-induced SLP-76 clusters, LAT is not responsible for SLP-76 relocalization in response to integrin engagement.
SLP-76 association with ADAP is required for relocalization downstream of integrins but not for relocalization downstream of the TCR.
The selective requirement for LAT for SLP-76 microclusters downstream of the TCR but not downstream of integrins demonstrated that these relocalization events use different molecular mechanisms. To investigate how SLP-76 relocalizes to signaling complexes independently of LAT, we focused on the adapter protein ADAP. A SLP-76 binding partner shown previously to upregulate integrins (6, 14, 36), ADAP is phosphorylated following integrin stimulation in T-cell blasts (17). To test the requirement for ADAP in integrin-initiated SLP-76 relocalization, we expressed GFP-SLP-76 in the ADAP-deficient Jurkat cell line JDAP (16). In contrast to ADAP-sufficient cells, in which SLP-76 relocalizes downstream of both TCR and integrins, SLP-76 was recruited to microclusters in response to TCR stimulation but not in response to integrin stimulation in JDAP cells (Fig. 7A). To confirm the requirement for ADAP in SLP-76 relocalization in primary cells, ADAP-deficient primary T-cell blasts were retrovirally transduced with GFP-SLP-76. As in JDAP cells, SLP-76 failed to relocalize to microclusters in response to integrin stimulation of ADAP-deficient primary T cells, despite normal relocalization downstream of the TCR (Fig. 7B).
FIG. 7.
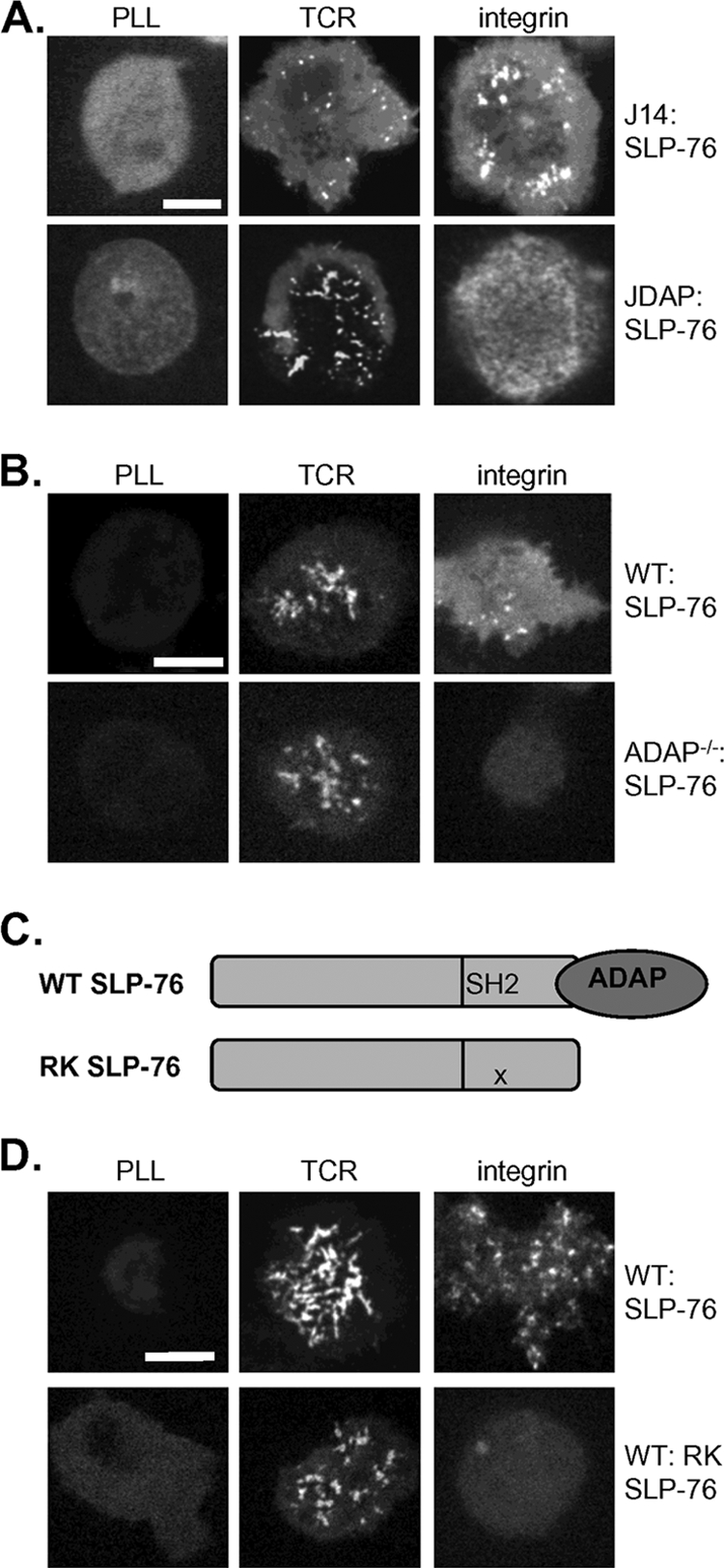
SLP-76 relocalization downstream of integrins requires ADAP. (A and B) JDAP Jurkat T cells (A) and ADAP−/− primary T-cell blasts (B) transduced with GFP-SLP-76 were stimulated by TCR and integrin (FN and ICAM-1, respectively). Bars, 5 μm. (C) The RK mutation in the SH2 domain of SLP-76 disrupts SLP-76 association with ADAP. (D) Primary T-cell blasts were transduced with WT or RK-SLP-76 and stimulated by TCR or integrins (ICAM-1). All image results are representative of at least five independent experiments.
These data suggest that ADAP is required for integrin-induced SLP-76 microclusters. To specifically test whether the association between SLP-76 and ADAP mediates SLP-76 relocalization to integrin-initiated signaling clusters, we studied the movement of a GFP-tagged SLP-76 mutant with an RK substitution in its SH2 domain, previously shown to disrupt ADAP binding (32) (Fig. 7C). RK-SLP-76 expressed in primary T cells was recruited to microclusters in response to engagement of the TCR but not in response to integrin engagement, suggesting that the direct association with ADAP recruits SLP-76 to microclusters in response to integrin ligation (Fig. 7D).
SLP-76 relocalization to integrin-initiated microclusters correlates with the ability to support integrin-mediated cell adhesion.
The results of SLP-76 relocalization studies strongly support different mechanisms for SLP-76 recruitment to signaling complexes following TCR and integrin engagement. To test whether these differences are relevant for SLP-76-mediated T-cell adhesion, we reconstituted SLP-76 cKO primary T cells with WT SLP-76, RK-SLP-76, or G2-SLP-76 by transgenic and retroviral transduction. T cells expressing WT SLP-76 support adhesion to ICAM-1 following either TCR or SDF-1α stimulation (Fig. 8A). Next, naïve cKO T cells expressing the RK SLP-76 mutant from a CD2 transgene were tested for their ability to support T-cell adhesion to integrin ligands (33). RK SLP-76 failed to support T-cell adhesion to ICAM-1 in response to either TCR or CXCR4 stimulus (Fig. 8A), suggesting that the association between SLP-76 and ADAP is critical not only for TCR-induced integrin upregulation but also for integrin signals resulting in cell adhesion.
FIG. 8.
SLP-76 relocalization correlates with support of integrin function. (A) Adhesion to ICAM-1 among naïve CD4-Cre SLP-76 cHET, cKO, cKO:RK SLP-76, or ADAP−/− T cells was measured in response to the indicated stimuli. Adhesion levels ranged from 5 to 10% in unstimulated (unstim) cells to 80% at maximum. Data represent means ± standard deviations normalized to unstimulated cHET cell results from four experiments. Asterisks indicate a statistical significance of P < 0.05. (B) Adhesion to ICAM-1 was measured among Ubc-CreT2 SLP-76 cKO T-cell blasts retrovirally reconstituted with vector only or with WT, G2, or SLP-76. Data represent means ± standard deviations normalized to unstimulated WT cell results from three experiments. ns, not significant.
Lastly, we analyzed the G2-SLP-76 mutant, which relocalizes to microclusters downstream of integrins but not downstream of the TCR. G2-SLP-76 expressed retrovirally in cKO cells supported T-cell adhesion to ICAM-1 after stimulation with SDF-1α but not after engagement of the TCR (Fig. 8B). Similar results were obtained using naïve SLP-76 cKO T cells transgenically reconstituted with a Gads-binding SLP-76 mutant (33) (data not shown). These findings demonstrate that relocalization of SLP-76 to integrin-initiated microclusters correlates with its ability to support adhesion to integrin ligands. G2-SLP-76 relocalizes to outside-in signaling complexes and promotes T-cell adhesion, whereas RK SLP-76 is unable to relocalize to integrin-initiated microclusters and fails to support adhesion. SLP-76 relocalization downstream of engaged integrins therefore promotes SLP-76-dependent activation of integrin-mediated adhesion by the use of a mechanism distinct from SLP-76-mediated signaling from the TCR.
DISCUSSION
In this report we demonstrate a requirement for the adapter protein SLP-76 for integrin-mediated T-cell adhesion. SLP-76 relocalization to signaling complexes initiated by integrin ligation was visualized by confocal and TIRF microscopy. Integrin-initiated signals that recruit SLP-76 to microclusters are dependent on the activity of PTKs, including Lck and ZAP-70, but are independent of that of the adapter protein LAT. The association between SLP-76 and LAT, although critical for SLP-76-mediated signaling downstream of the TCR, is dispensable for integrin-initiated SLP-76 relocalization as well as integrin function. By contrast, the association between SLP-76 and ADAP is critical for both SLP-76 clustering and function downstream of integrins. Together, these findings support an essential role for SLP-76 in integrin function in primary T cells and provide a model whereby signals originating at integrins and the TCR may be distinguished by the differential use of adapter proteins.
Our results indicate that SLP-76 is required for TCR-induced T-cell adhesion to integrin ligands. This finding was expected, as extensive research has shown SLP-76 to be an essential mediator of signaling originating at the TCR. Defective T-cell adhesion to integrin ligands could result solely from impaired inside-out integrin activation from the TCR or additionally from absent SLP-76-mediated integrin outside-in signals. To specifically address the requirement for SLP-76 in integrin-initiated signaling, we demonstrate that SLP-76 cKO T cells mount defective adhesion to ICAM-1 after integrin upregulation by the chemokine SDF-1α. This result implicates SLP-76 in outside-in signaling initiated at integrins that results in cell adhesion. The requirement for ADAP and the ADAP-binding domain of SLP-76 in TCR-induced T-cell adhesion corroborates earlier reports suggesting that SLP-76 directs integrin upregulation through a multimolecular complex with the Src-kinase-associated phosphoprotein of 55 kDa (SKAP-55) and Rap-1-interacting molecule (RIAM) (23, 29). Reciprocally, integrin-proximal signaling complexes containing Rap1 and RIAM may recruit SLP-76 via phosphorylated ADAP, as we have shown that ADAP is also required for SDF-1α-induced adhesion to integrin ligands. A number of additional SLP-76 binding partners, including the interleukin-2-activating tyrosine kinase (Itk) and the Rho guanine exchange factor Vav, have been implicated in adhesion to integrin ligands, suggesting that SLP-76 may direct T-cell adhesion by organizing a complex with these proteins (12, 13, 24, 48).
SLP-76 regulates the assembly of multimolecular signaling complexes in a spatiotemporal manner. The results of our live-cell imaging studies demonstrate, for the first time, that SLP-76 relocalizes to signaling complexes downstream of engaged integrins alone. SLP-76 microclusters downstream of both the TCR and integrins can be detected by TIRF microscopy, indicating that SLP-76 localizes to the same membrane-proximal zone in response to stimulation of both receptors. Preliminary analysis revealed that SLP-76 clusters initiated at integrins and the TCR are similar in size and fluorescence intensity (data not shown). Yet we were intrigued to find that, despite similar results with respect to enrichment for activated PTKs and phosphotyrosine, SLP-76 microclusters formed in response to integrin ligation exhibit dynamic behavior that differs from that of TCR-initiated clusters. Unlike TCR-induced SLP-76 clusters, which migrate to the cell center along linear trajectories, integrin-initiated SLP-76 clusters typically persist at the sites where they arise. Recent reports have described SLP-76 centralization as a component of TCR signal downregulation and as dependent on phosphotyrosine-mediated interactions between active signaling complexes and the ubiquitin E3 ligase Cbl (4, 5). Regulation of integrin-initiated and TCR-initiated SLP-76 signaling complexes therefore appears different, perhaps due to different constituent phosphoproteins. Differential regulation is consistent with the increase we observed in the duration of integrin-initiated SLP-76 microclusters. These results correlate with the recent finding that ligation of β1 integrins also increases the persistence of SLP-76 clusters formed following TCR stimulation (35). The longer lifetime of SLP-76 clusters formed downstream of integrins may drive increased temporally sustained cellular responses to integrin engagement, including adhesion and proliferation.
SLP-76 localization is governed by its protein binding partners. SLP-76 relocalizes downstream of the TCR via its association with LAT via Gads. Yet SLP-76 recruitment to microclusters in response to integrin engagement was independent of LAT, supporting the idea of a unique mechanism for SLP-76 recruitment to integrin signaling complexes. Our imaging studies of Jurkat and primary T cells suggest that the adapter protein ADAP is responsible for recruiting SLP-76 to integrin-initiated signaling complexes. In addition to its role in TCR proximal signaling, ADAP has been implicated in integrin outside-in signaling, as LFA-1 stimulation promotes ADAP association with cytoskeletal structures that facilitate T-cell adhesion (46). A signaling function of the actin cytoskeleton in response to integrin engagement was also suggested by research showing that integrins modulate the internalization of TCR-induced SLP-76 microclusters (35). Our finding that inhibition of actin polymerization with latrunculin B blocks formation of new integrin-initiated SLP-76 microclusters (data not shown) supports this possibility. Recent findings establishing unique requirements for different SFK members in signaling from neutrophil adhesion receptors (52) may also implicate ADAP in integrin signaling, given that Fyn, and not the predominant T-cell SFK Lck, is responsible for ADAP phosphorylation (11, 17, 28, 41).
The visualization of TCR-induced microclusters of signaling proteins has generated considerable interest and excitement. The formation of TCR microclusters downstream of TCR ligation has been demonstrated to be a very early event, corresponding to the rapid and localized initiation of PTK and calcium signaling as well as rearrangement of the actin cytoskeleton (9). Imaging-based descriptions of the immune synapse have documented clusters not only of the TCR but also of LFA-1 and ZAP-70 that undergo dynamic and regulated movement (51). Likewise, TCR-initiated SLP-76 microclusters have been demonstrated to colocalize with ZAP-70, LAT, and actin-reorganizing signaling proteins in a dynamic fashion (7). While inhibitors of cellular signaling have been shown to block both the formation and stability of protein microclusters, it remains unclear what role microclusters play in the activation of cell signaling. To our knowledge, this is the first report of signaling clusters formed downstream of stimulated adhesion receptors alone. Interestingly, while many common signaling elements were detected in clusters downstream of both the TCR and integrins, there were critical differences in protein composition. The adapter LAT is not present in integrin-initiated microclusters, and it is not required for their appearance. These differences, which correspond to functional requirements for integrin signaling resulting in T-cell adhesion, provide additional support for a model of intracellular signaling carried out by microclusters of protein kinases and adapter molecules.
A number of recent reports have demonstrated overlapping genetic requirements for signaling downstream of integrins and ITAM-bearing receptors such as the TCR, suggesting a proximal convergence of TCR and integrin signaling in T cells. ITAM domains as well as Src and Syk family PTKs have been shown to be essential for integrin as well as ITAM signaling and resulting cell functions in myeloid cells (30, 52, 53). These findings emphasize the shared components of surface signaling in hematopoietic cells and further pose the question of how cells distinguish signals originating at different surface receptors. Our finding that SLP-76 mediates integrin signaling and function in T cells by the use of a mechanism distinct from its role in signaling downstream of the TCR provides an attractive model for how this might be accomplished. Adapter proteins, through their diversity of protein-protein associations, may be able to selectively assemble signaling complexes in response to distinct cell stimulations. Understanding how the cell promotes these differences offers avenues toward the sophisticated control of immune cell functions.
Supplementary Material
Acknowledgments
We thank Taku Kambayashi and Laurie Lenox for critical reading of the manuscript, Yoji Shimizu and Christopher DeNucci and members of the Burkhardt laboratory for technical advice, and Justina Stadanlick for editorial assistance.
J.S.M. is a John Merrill Scholar of the American Society of Nephrology. This work was supported by the American Heart Association (R.G.B.) and the NIH (G.A.K., D.A.H., and T.B.).
Footnotes
Published ahead of print on 10 August 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abraham, R. T., and A. Weiss. 2004. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 4:301-308. [DOI] [PubMed] [Google Scholar]
- 2.Arias-Salgado, E. G., S. Lizano, S. Sarkar, J. S. Brugge, M. H. Ginsberg, and S. J. Shattil. 2003. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA 100:13298-13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, R. G., and G. A. Koretzky. 2008. Regulation of T cell integrin function by adapter proteins. Immunol. Res. 42:132-144. [DOI] [PubMed] [Google Scholar]
- 4.Balagopalan, L., V. A. Barr, C. L. Sommers, M. Barda-Saad, A. Goyal, M. S. Isakowitz, and L. E. Samelson. 2007. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol. Cell. Biol. 27:8622-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr, V. A., L. Balagopalan, M. Barda-Saad, R. Polishchuk, H. Boukari, S. C. Bunnell, K. M. Bernot, Y. Toda, R. Nossal, and L. E. Samelson. 2006. T-cell antigen receptor-induced signaling complexes: internalization via a cholesterol-dependent endocytic pathway. Traffic 7:1143-1162. [DOI] [PubMed] [Google Scholar]
- 6.Boerth, N. J., B. A. Judd, and G. A. Koretzky. 2000. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J. Biol. Chem. 275:5143-5152. [DOI] [PubMed] [Google Scholar]
- 7.Bunnell, S. C., D. I. Hong, J. R. Kardon, T. Yamazaki, C. J. McGlade, V. A. Barr, and L. E. Samelson. 2002. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158:1263-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunnell, S. C., A. L. Singer, D. I. Hong, B. H. Jacque, M. S. Jordan, M. C. Seminario, V. A. Barr, G. A. Koretzky, and L. E. Samelson. 2006. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol. Cell. Biol. 26:7155-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campi, G., R. Varma, and M. L. Dustin. 2005. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202:1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements, J. L., B. Yang, S. E. Ross-Barta, S. L. Eliason, R. F. Hrstka, R. A. Williamson, and G. A. Koretzky. 1998. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science 281:416-419. [DOI] [PubMed] [Google Scholar]
- 11.da Silva, A. J., O. Janssen, and C. E. Rudd. 1993. T cell receptor zeta/CD3-p59fyn(T)-associated p120/130 binds to the SH2 domain of p59fyn(T). J. Exp. Med. 178:2107-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein, L. D., Y. Shimizu, and P. L. Schwartzberg. 2005. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J. Immunol. 175:5923-5930. [DOI] [PubMed] [Google Scholar]
- 13.García-Bernal, D., N. Wright, E. Sotillo-Mallo, C. Nombela-Arrieta, J. V. Stein, X. R. Bustelo, and J. Teixido. 2005. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1. Mol. Biol. Cell 16:3223-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths, E. K., C. Krawczyk, Y. Y. Kong, M. Raab, S. J. Hyduk, D. Bouchard, V. S. Chan, I. Kozieradzki, A. J. Oliveira-Dos-Santos, A. Wakeham, P. S. Ohashi, M. I. Cybulsky, C. E. Rudd, and J. M. Penninger. 2001. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293:2260-2263. [DOI] [PubMed] [Google Scholar]
- 15.Hsia, D. A., S.-T. Lim, J. A. Bernard-Trifilo, S. K. Mitra, S. Tanaka, J. den Hertog, D. N. Streblow, D. Ilic, M. H. Ginsberg, and D. D. Schlaepfer. 2005. Integrin α4β1 promotes focal adhesion kinase-independent cell motility via α4 cytoplasmic domain-specific activation of c-Src. Mol. Cell. Biol. 25:9700-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, Y., D. D. Norton, P. Precht, J. L. Martindale, J. K. Burkhardt, and R. L. Wange. 2005. Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 280:23576-23583. [DOI] [PubMed] [Google Scholar]
- 17.Hunter, A. J., N. Ottoson, N. Boerth, G. A. Koretzky, and Y. Shimizu. 2000. Cutting edge: a novel function for the SLAP-130/FYB adapter protein in beta 1 integrin signaling and T lymphocyte migration. J. Immunol. 164:1143-1147. [DOI] [PubMed] [Google Scholar]
- 18.Huse, M., E. J. Quann, and M. M. Davis. 2008. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat. Immunol. 9:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan, M. S., J. S. Maltzman, S. Kliche, J. Shabason, J. E. Smith, A. Obstfeld, B. Schraven, and G. A. Koretzky. 2007. In vivo disruption of T cell development by expression of a dominant-negative polypeptide designed to abolish the SLP-76/Gads interaction. Eur. J. Immunol. 37:2961-2972. [DOI] [PubMed] [Google Scholar]
- 20.Judd, B. A., P. S. Myung, L. Leng, A. Obergfell, W. S. Pear, S. J. Shattil, and G. A. Koretzky. 2000. Hematopoietic reconstitution of SLP-76 corrects hemostasis and platelet signaling through alpha IIb beta 3 and collagen receptors. Proc. Natl. Acad. Sci. USA 97:12056-12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judd, B. A., P. S. Myung, A. Obergfell, E. E. Myers, A. M. Cheng, S. P. Watson, W. S. Pear, D. Allman, S. J. Shattil, and G. A. Koretzky. 2002. Differential requirement for LAT and SLP-76 in GPVI versus T cell receptor signaling. J. Exp. Med. 195:705-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasirer-Friede, A., B. Moran, J. Nagrampa-Orje, K. Swanson, Z. M. Ruggeri, B. Schraven, B. G. Neel, G. Koretzky, and S. J. Shattil. 2007. ADAP is required for normal αIIbβ3 activation by VWF/GP Ib-IX-V and other agonists. Blood 109:1018-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kliche, S., D. Breitling, M. Togni, R. Pusch, K. Heuer, X. Wang, C. Freund, A. Kasirer-Friede, G. Menasche, G. A. Koretzky, and B. Schraven. 2006. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol. Cell. Biol. 26:7130-7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krawczyk, C., A. Oliveira-dos-Santos, T. Sasaki, E. Griffiths, P. S. Ohashi, S. Snapper, F. Alt, and J. M. Penninger. 2002. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity 16:331-343. [DOI] [PubMed] [Google Scholar]
- 25.Liu, S. K., N. Fang, G. A. Koretzky, and C. J. McGlade. 1999. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9:67-75. [DOI] [PubMed] [Google Scholar]
- 26.Luo, B. H., C. V. Carman, and T. A. Springer. 2007. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25:619-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maltzman, J. S., L. Kovoor, J. L. Clements, and G. A. Koretzky. 2005. Conditional deletion reveals a cell-autonomous requirement of SLP-76 for thymocyte selection. J. Exp. Med. 202:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marie-Cardine, A., L. R. Hendricks-Taylor, N. J. Boerth, H. Zhao, B. Schraven, and G. A. Koretzky. 1998. Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J. Biol. Chem. 273:25789-25795. [DOI] [PubMed] [Google Scholar]
- 29.Ménasché, G., S. Kliche, E. J. Chen, T. E. Stradal, B. Schraven, and G. Koretzky. 2007. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol. Cell. Biol. 27:4070-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mócsai, A., C. L. Abram, Z. Jakus, Y. Hu, L. L. Lanier, and C. A. Lowell. 2006. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 7:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin, N. A., P. W. Oakes, Y. M. Hyun, D. Lee, Y. E. Chin, M. R. King, T. A. Springer, M. Shimaoka, J. X. Tang, J. S. Reichner, and M. Kim. 2008. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J. Exp. Med. 205:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musci, M. A., L. R. Hendricks-Taylor, D. G. Motto, M. Paskind, J. Kamens, C. W. Turck, and G. A. Koretzky. 1997. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 272:11674-11677. [DOI] [PubMed] [Google Scholar]
- 33.Myung, P. S., G. S. Derimanov, M. S. Jordan, J. A. Punt, Q. H. Liu, B. A. Judd, E. E. Meyers, C. D. Sigmund, B. D. Freedman, and G. A. Koretzky. 2001. Differential requirement for SLP-76 domains in T cell development and function. Immunity 15:1011-1026. [DOI] [PubMed] [Google Scholar]
- 34.Newbrough, S. A., A. Mocsai, R. A. Clemens, J. N. Wu, M. A. Silverman, A. L. Singer, C. A. Lowell, and G. A. Koretzky. 2003. SLP-76 regulates Fcgamma receptor and integrin signaling in neutrophils. Immunity 19:761-769. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen, K., N. R. Sylvain, and S. C. Bunnell. 2008. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity 28:810-821. [DOI] [PubMed] [Google Scholar]
- 36.Peterson, E. J., M. L. Woods, S. A. Dmowski, G. Derimanov, M. S. Jordan, J. N. Wu, P. S. Myung, Q. H. Liu, J. T. Pribila, B. D. Freedman, Y. Shimizu, and G. A. Koretzky. 2001. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 293:2263-2265. [DOI] [PubMed] [Google Scholar]
- 37.Pivniouk, V., E. Tsitsikov, P. Swinton, G. Rathbun, F. W. Alt, and R. S. Geha. 1998. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell 94:229-238. [DOI] [PubMed] [Google Scholar]
- 38.Rose, D. M., R. Alon, and M. H. Ginsberg. 2007. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 218:126-134. [DOI] [PubMed] [Google Scholar]
- 39.Ruzankina, Y., C. Pinzon-Guzman, A. Asare, T. Ong, L. Pontano, G. Cotsarelis, V. P. Zediak, M. Velez, A. Bhandoola, and E. J. Brown. 2007. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1:113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salas, A., M. Shimaoka, A. N. Kogan, C. Harwood, U. H. von Andrian, and T. A. Springer. 2004. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity 20:393-406. [DOI] [PubMed] [Google Scholar]
- 41.Schraven, B., A. Marie-Cardine, and G. Koretzky. 1997. Molecular analysis of the fyn-complex: cloning of SKAP55 and SLAP-130, two novel adaptor proteins which associate with fyn and may participate in the regulation of T cell receptor-mediated signaling. Immunol. Lett. 57:165-169. [DOI] [PubMed] [Google Scholar]
- 42.Singer, A. L., S. C. Bunnell, A. E. Obstfeld, M. S. Jordan, J. N. Wu, P. S. Myung, L. E. Samelson, and G. A. Koretzky. 2004. Roles of the proline-rich domain in SLP-76 subcellular localization and T cell function. J. Biol. Chem. 279:15481-15490. [DOI] [PubMed] [Google Scholar]
- 43.Smith, A., P. Stanley, K. Jones, L. Svensson, A. McDowall, and N. Hogg. 2007. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol. Rev. 218:135-146. [DOI] [PubMed] [Google Scholar]
- 44.Smith-Garvin, J. E., G. A. Koretzky, and M. S. Jordan. 2009. T cell activation. Annu. Rev. Immunol. 27:591-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srinivas, S., T. Watanabe, C. S. Lin, C. M. William, Y. Tanabe, T. M. Jessell, and F. Costantini. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki, J., S. Yamasaki, J. Wu, G. A. Koretzky, and T. Saito. 2007. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood 109:168-175. [DOI] [PubMed] [Google Scholar]
- 47.Takagi, J., B. M. Petre, T. Walz, and T. A. Springer. 2002. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110:599-611. [DOI] [PubMed] [Google Scholar]
- 48.Takesono, A., R. Horai, M. Mandai, D. Dombroski, and P. L. Schwartzberg. 2004. Requirement for Tec kinases in chemokine-induced migration and activation of Cdc42 and Rac. Curr. Biol. 14:917-922. [DOI] [PubMed] [Google Scholar]
- 49.Woodside, D. G., A. Obergfell, L. Leng, J. L. Wilsbacher, C. K. Miranti, J. S. Brugge, S. J. Shattil, and M. H. Ginsberg. 2001. Activation of Syk protein tyrosine kinase through interaction with integrin beta cytoplasmic domains. Curr. Biol. 11:1799-1804. [DOI] [PubMed] [Google Scholar]
- 50.Yablonski, D., M. R. Kuhne, T. Kadlecek, and A. Weiss. 1998. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science 281:413-416. [DOI] [PubMed] [Google Scholar]
- 51.Yokosuka, T., K. Sakata-Sogawa, W. Kobayashi, M. Hiroshima, A. Hashimoto-Tane, M. Tokunaga, M. L. Dustin, and T. Saito. 2005. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 6:1253-1262. [DOI] [PubMed] [Google Scholar]
- 52.Zarbock, A., C. L. Abram, M. Hundt, A. Altman, C. A. Lowell, and K. Ley. 2008. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J. Exp. Med. 205:2339-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zarbock, A., C. A. Lowell, and K. Ley. 2007. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity 26:773-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.