Abstract
Deleting the OB folds encoding the telomeric single-stranded DNA (ssDNA)-binding activity of the human telomeric protein POT1 induces significant telomere elongation, suggesting that at least one critical aspect of the regulation of telomere length is disrupted by this POT1ΔOB mutant protein. POT1 is known to associate with two proteins through the protein interaction domain retained in POT1ΔOB—the telomeric double-stranded DNA-binding protein TRF2 and the telomere-associated protein TPP1. We report that introducing a mutation that reduces association of POT1 with TRF2, but not a mutation that reduces the association with TPP1, abrogates the ability of POT1ΔOB to promote telomere elongation. Mechanistically, expression of POT1ΔOB reduced the association of TRF2 with POT1, RAP1, and TIN2; however, of these proteins, only ectopic expression of POT1 suppressed the telomere elongation induced by POT1ΔOB. Lastly, replacing endogenous POT1 with a full-length POT1 mutant defective in the association with TRF2 induced telomere elongation. Thus, we conclude that the association of POT1 with both ssDNA and TRF2 is critical for telomere length homeostasis.
Telomeres are DNA-protein complexes that protect the ends of eukaryotic chromosomes from degradation and detection as sites of DNA damage (reviewed in reference 23). Telomeric DNA is composed of tandem arrays of repetitive double-stranded DNA (dsDNA), wherein the G-rich strand extends beyond the C-rich strand. The resultant 3′ single-stranded DNA (ssDNA) overhang can invade the dsDNA, forming a lariat structure termed the t-loop (28).
The regulation of telomere length affects mammalian biology at both the cellular and organismal levels. In normal human somatic cells, telomeres progressively shorten (32, 33) to a critical length before entering a state of permanent growth arrest termed senescence (1, 12, 21, 34, 35). Abnormally short telomeres in humans and mice are associated with various anemias, cirrhosis of the liver, and other disorders due to the premature induction of senescence, particularly in highly proliferative tissues (7, 11, 61). Conversely, de novo elongation of the telomere by the reverse transcriptase telomerase (27, 54) ensures maintenance of telomere length in the germ line and endows cultured cells with an immortal life span (8, 17, 18, 20, 30, 36, 70, 78). Moreover, telomerase activation and subsequent stabilization of telomere length occur in the vast majority of cancer cells (41, 62) and are required for cellular immortalization and the tumorigenic conversion of normal human cells (19, 30, 31, 36, 78).
The dsDNA portion of telomeres is bound directly by two proteins, TRF1 and TRF2 (9, 23). In turn, TRF1 and TRF2 are bridged by the protein TIN2 (23, 43, 76) and bind other telomere-associated proteins such as tankyrase 1 (23, 63, 64) and RAP1 (48, 56). Telomeric ssDNA is bound directly by the protein POT1 (4, 5, 23, 52). POT1 acts in a heterodimer with the protein TPP1; this heterodimer promotes POT1 binding to telomeric ssDNA at least in vitro (72, 73), protects telomeres from being detected as sites of DNA damage (3, 24, 29, 37, 73, 74), and inhibits replication protein A (RPA) localization to telomeres (3). In vitro-transcribed and -translated POT1 has also been shown to associate with recombinant glutathione S-transferase-TRF2 (75). Additionally, endogenous TRF2 has been found in some (3), but not all (51), cases to coimmunoprecipitate with exogenous epitope-tagged POT1, although different antibodies were used to detect TRF2. Endogenous POT1 has also been reported to coimmunoprecipitate with endogenous TRF2 (75). Moreover, NAAIRS substitution mutations in POT1 that reduced its ability to coimmunoprecipitate TRF2, but not to coimmunoprecipitate TPP1 or to bind telomeric ssDNA, failed to associate with telomeric chromatin, as assessed by chromatin immunoprecipitation and immunofluorescence studies (3). Taken together, these data suggest that POT1 also associates with TRF2, potentially directly, and this interaction facilitates POT1 association with telomeric chromatin (3, 37, 53, 75). All the aforementioned proteins are found in larger complexes containing other proteins involved in telomere length regulation, DNA damage response, and sister chromatid association (13, 16, 22, 49, 56, 57, 60, 67).
Inhibition of any of the proteins that directly bind telomeric DNA (TRF1, TRF2, or POT1) or many of their interacting partners (TPP1, TIN2, or RAP1) in telomerase-positive human cells results in telomere elongation (10, 38, 43, 47, 51, 56, 66, 69, 71, 74, 77). Such inhibition has also been reported to disrupt telomere protein complexes (57) and induce the mislocalization of other telomere-associated proteins (37, 39, 50, 51, 75, 77). Thus, direct and indirect effects of disrupting multiple protein interactions may underlie such alterations in telomere length.
Of the aforementioned proteins, POT1 is an attractive protein in terms of directly regulating telomere length for the following reasons. First, POT1 homologues in budding and fission yeasts are known to regulate the ability of telomerase to elongate telomeric telomeres (14, 53, 55, 59, 65). Second, human POT1 is the only protein to bind telomeric ssDNA (4, 52), the substrate of telomerase (27, 54), and POT1 can modulate telomerase access to its substrate at least in vitro (40, 45, 52). Third, the POT1-TPP1 heterodimer recruits telomerase to telomeres and promotes its processivity (72, 73). As an entrée to studying how POT1 regulates telomere length in telomerase-positive cells, we note that expression of POT1ΔOB, a mutant lacking the N-terminal OB folds responsible for telomeric ssDNA binding (3, 50, 51), is one of the most potent means of inducing elongation of telomeres (2, 50, 51, 74). This mutant clearly disrupts at least one critical aspect of telomere length regulation. This prompted us to investigate the mechanism by which POT1ΔOB induces telomere elongation to gain better insight into how telomere length is controlled by telomere-binding proteins.
POT1ΔOB retains the protein interaction domain of the wild-type protein, which mediates its interaction with both TPP1 and TRF2 (3, 39, 50, 74, 75, 77), and elongates telomeres in a dominant-negative fashion (2, 50, 51, 74). This suggests that one of these protein interactions, when uncoupled from telomeric ssDNA binding, underlies the ability of POT1ΔOB to elongate telomeres. To determine which protein interaction is most important in POT1-mediated regulation of telomere length, we exploited the POT1ΔOB mutant by introducing separation-of-function mutations that disrupt association with either TPP1 or TRF2 (3). We found that introducing a mutation that decreases the interaction with TRF2, but not TPP1, abrogated the ability of POT1ΔOB to promote telomere elongation. Furthermore, expression of POT1ΔOB reduced the association of TRF2 with its binding partners POT1, RAP1, and TIN2, suggesting that, mechanistically, POT1ΔOB may act in a dominant-negative manner by disrupting other protein interactions at the telomere. Notably, only ectopic expression of POT1 was able to suppress telomere elongation induced by POT1ΔOB. Finally, replacing endogenous POT1 with either POT1ΔOB or a mutant defective in associating with TRF2 (POT1ΔTRF2) induced telomere elongation. Thus, we surmise that the association of POT1 with both telomeric ssDNA and TRF2 is critical for regulating telomere length.
MATERIALS AND METHODS
Plasmids.
pBabe-puro or pBabe-hygro carrying the FLAG-tagged RNA interference (RNAi)-resistant cDNAs F-POT1, F-POT1ΔOB, F-POT12OB, F-POT1ΔTRF2, F-POT1ΔTPP1, F-RAP1, and myc-tagged TIN2 (myc-TIN2) and pCI-neo encoding F-POT1 or myc-TIN2 were previously described (3, 56). Specifically, F-POT1 encodes an N-terminal FLAG epitope tag in frame with the cDNA representing the v1 major spliced version (4, 38) of human POT1. F-POT1ΔOB encodes an N-terminal FLAG epitope tag in frame with the fragment from amino acid 127 to 634 of v1 human POT1. F-POT12OB encodes an N-terminal FLAG epitope tag in frame with the fragment from amino acid 2 to 350 of v1 human POT1 engineered with a stop codon at amino acid 350. F-POT1ΔTPP1 and F-POT1ΔTRF2 (previously termed F-POT1ΔTRF2-2) encode an N-terminal FLAG epitope tag in frame with v1 human POT1 containing NAAIRS substitution mutations at S317SGSVS or A599YPWLE, respectively (3). F-RAP1 encodes an N-terminal FLAG epitope-tagged version of the human RAP1 protein, as previously described (56). Myc-TIN2 encodes a myc-tagged version of human TIN2, a kind gift from Judith Campisi. F-POT1ΔOBΔTPP1 and F-POT1ΔOBΔTRF2 were generated by introducing the aforementioned NAAIRS substitution mutations into F-POT1ΔOB by site-directed mutagenesis and subcloned into pBABE-puro. Yellow fluorescent protein (YFP)-POT1ΔOB was generated by subcloning untagged POT1ΔOB into pEYFP-C1 (Clontech). pSUPER-Retro-puro-POT1 short hairpin RNA (shRNA) was previously described (3, 71).
Cell lines.
Using previously described approaches (3, 58), immortal HA1 cells (population doubling [PD] 200) (17) were stably infected with the indicated combinations of retroviruses derived from pBabe-puro carrying no transgene (empty vector) or encoding F-POT1, F-POT1ΔTPP1, F-POT1ΔTRF2, F-POT1ΔOB, F-POT1ΔOBΔTPP1, F-POT1ΔOBΔTRF2, or F-RAP1 or with retroviruses derived from pBabe-hygro carrying no transgene (empty vector) or encoding F-POT1, F-POT1ΔOB, F-POT1ΔTPP1, F-POT1ΔTRF2, or myc-TIN2. As indicated, some cell lines were subsequently infected with pSUPER-Retro-puro carrying no transgene (empty vector) or POT1 shRNA (71). All cells lines were selected with puromycin (Sigma) or hygromycin B (Sigma) as previously described (2), and the first confluent population of cells following antibiotic selection was defined as PD 0.
Detection of telomeres.
Telomere lengths were assessed using 32P-labeled (CCCTAA)3 Southern hybridization of HinFI- and RsaI-digested genomic DNA as previously described (2, 25). Mean telomere lengths were determined using the Telometric program (26). Where indicated, statistical significance was determined using Student's t test.
Immunoprecipitation and immunoblotting.
Using previously described approaches (2, 3), F-POT1 and F-RAP1 proteins stably expressed in HA1 cells were detected by immunoprecipitation with anti-FLAG M2 agarose affinity resin (Sigma), followed by elution with FLAG-peptide (Sigma) and immunoblotting with an anti-FLAG M2 antibody (1:1,200, Sigma). myc-TIN2 stably expressed in HA1 cells was detected by immunoprecipitation with an anti-myc antibody (Invitrogen), followed by incubation with G-Sepharose beads (Amersham Biosciences) and immunoblotting with the aforementioned anti-myc antibody (1:3,000). Coimmunoprecipitation of endogenous TRF2 with stably expressed F-POT1, F-POT12OB, or F-POTΔOB was detected by immunoprecipitation and elution of the FLAG-tagged proteins as described above, followed by immunoblotting with an anti-TRF2 antibody (1:250; Imgenex). Coimmunoprecipitation of endogenous TRF2 with ectopic F-POT1, F-RAP1, or myc-TIN2 was detected by transient transfection of 293T cells using the FuGENE 6 reagent (Roche) with the plasmid pCI-neo carrying no transgene or encoding F-POT1 or myc-TIN2 or pBABE-puro encoding F-RAP1 in the absence or presence of transiently cotransfected YFP-POT1ΔOB. Forty-eight hours later, FLAG- and myc-tagged proteins were immunoprecipitated as described above, followed by immunoblotting with the aforementioned anti-FLAG M2, anti-myc, and anti-TRF2 antibodies. Protein expression of YFP-POT1ΔOB was validated by immunoblotting with an anti-green fluorescent protein antibody (1:1,000, Roche).
Reverse transcription-PCR (RT-PCR).
Endogenous POT1, or GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a control, was reverse-transcribed and PCR amplified as previously described (3).
RESULTS
Association with TRF2 is required for POT1ΔOB to induce telomere elongation.
POT1 binds telomeric ssDNA (4, 44, 52), the substrate of telomerase (27, 52), and can inhibit the access of telomerase to telomeric ssDNA at least in vitro (40, 45, 52). Moreover, expression of a POT1 mutant lacking OB folds, which mediate binding of telomeric ssDNA (POT1ΔOB), is one of the most potent means of inducing telomere elongation (2, 50, 51, 74). Thus, we investigated how POT1ΔOB promotes telomere elongation to understand how POT1 normally regulates telomere length. Given that POT1ΔOB acts in a dominant-negative fashion (2, 50, 51, 74) and retains the protein interaction domain through which it associates with both TPP1 and TRF2 (3, 39, 50, 74, 75, 77), we hypothesized that one of these protein interactions, when uncoupled from telomeric ssDNA binding, may mediate the telomere elongation observed in cells overexpressing POT1ΔOB.
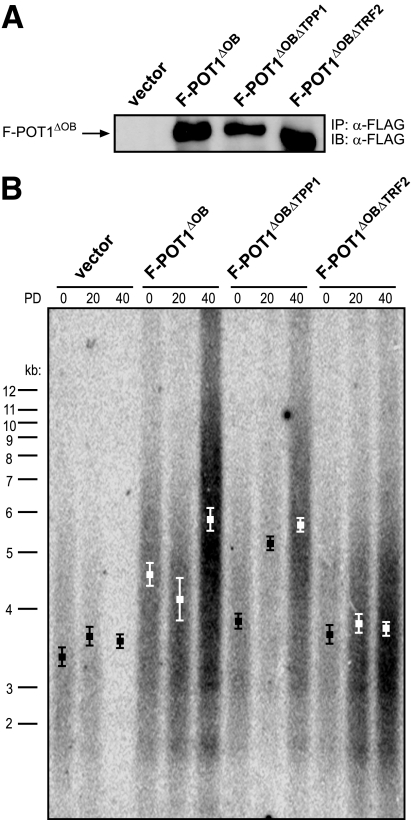
To determine if the protein interaction of POT1 with either TPP1 or TRF2 is required for the telomere elongation induced by POT1ΔOB, separation-of-function mutations previously shown to reduce the interaction of POT1 with TPP1 or TRF2 (3) were introduced into an N-terminal FLAG-tagged version of POT1ΔOB, yielding the mutants F-POT1ΔOBΔTPP1 and F-POT1ΔOBΔTRF2, respectively (see Fig. S1 in the supplemental material). F-POT1ΔOBΔTPP1 and F-POT1ΔOBΔTRF2 were verified to coimmunoprecipitate TRF2 but not TPP1, or TPP1 but not TRF2, respectively (data not shown). We note the caveat that coimmunoprecipitation does not differentiate between direct and indirect binding, and hence we cannot discount the possibility that these mutations disrupt other protein interactions that are indirectly manifested as a loss in the association with TPP1 or TRF2. Empty vector as a negative control, F-POT1ΔOB as a positive control, F-POT1ΔOBΔTPP1, or F-POT1ΔOBΔTRF2 was stably expressed in the telomerase-positive, immortal simian virus 40-transformed human embryonic kidney cell line HA1. These cells were chosen because they maintain short telomeres in a telomerase-dependent fashion over many cell divisions (17), and hence perturbations that result in telomere elongation are easily detected. Appropriate transgene expression was confirmed by immunoprecipitation and immunoblotting with an anti-FLAG antibody (Fig. 1A). Genomic DNA from the resultant cell lines was isolated at regular intervals and digested with restriction enzymes to liberate the repetitive telomeric DNA, which was detected by Southern hybridization with a 32P-labeled telomeric probe.
FIG. 1.
Association with TRF2 is required for POT1ΔOB to induce telomere elongation. (A) Detection of the indicated FLAG-tagged POT1 (F-POT1) proteins stably expressed in HA1 cells by immunoprecipitation (IP) and immunoblotting (IB) with an anti-FLAG antibody. (B) Telomere length, as assessed by Southern hybridization with a 32P-labeled telomeric probe, of HA1 cells stably expressing the indicated transgenes at PDs 0, 20, and 40. Boxes indicate mean telomere length ± standard deviation calculated from three independently derived sets of cell lines.
Negative control vector cells maintained a mean telomere length of ∼3.6 kb over the observed period of 40 PDs, whereas the telomeres of positive control cells expressing F-POT1ΔOB were elongated to ∼6.0 kb over the same number of PDs (Fig. 1B). We note a slight decrease in telomere length in cells expressing F-POT1ΔOB at PD 20, which was lost by PD 40, perhaps reflecting a selective pressure in the polyclonal population over time. This transient decrease in telomere length was not observed in all cases (e.g., compare Fig. 1B and 4B). Telomeres of cells expressing F-POT1ΔOBΔTPP1 elongated to a length similar to that observed in positive control F-POT1ΔOB-expressing cells, suggesting that the interaction with TPP1 does not underlie the ability of F-POT1ΔOB to induce telomere elongation. Conversely, the telomeres of cells expressing F-POT1ΔOBΔTRF2 maintained a mean telomere length of ∼3.9 kb, just slightly longer than vector control cells (Fig. 1B). This supports the notion that association with TRF2 is critical to the telomere phenotype induced by POT1ΔOB. These results were reproducible, as similar telomere dynamics were observed in two additional independently derived sets of cell lines (Fig. 1B). In summary, the association of F-POT1ΔOB with TRF2, but not with TPP1, promotes telomere elongation.
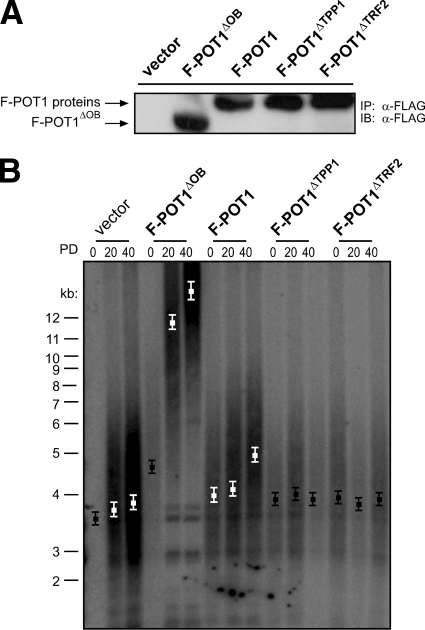
FIG. 4.
POT1ΔOB, but not POT1ΔTRF2, acts in a dominant-negative fashion to elongate telomeres. (A) Detection of the indicated FLAG-tagged POT1 (F-POT1) proteins stably expressed in HA1 cells by immunoprecipitation (IP) and immunoblotting (IB) with an anti-FLAG antibody. (B) Telomere length, as assessed by Southern hybridization with a 32P-labeled telomeric probe, of HA1 cells stably expressing the indicated proteins at PDs 0, 20, and 40. Boxes indicate mean telomere length ± standard deviation calculated from three independently derived sets of cell lines.
POT1ΔOB disrupts TRF2 protein interactions.
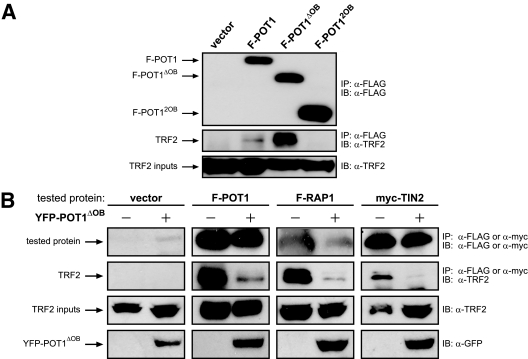
Since the association of POT1ΔOB with TRF2 was required to promote telomere elongation (Fig. 1B) and since TRF2 interacts with most proteins through its homodimerization domain (15, 25a, 43, 46, 48, 68, 76), we reasoned that ectopic POT1ΔOB might act to elongate telomeres by displacing other proteins from endogenous TRF2. To test this hypothesis, we first determined whether F-POT1ΔOB associated with TRF2. Specifically, stably expressed empty vector or a truncation mutant of F-POT1 that encodes only the telomeric ssDNA-binding activity (F-POT12OB) (3, 52) as negative controls, full-length FLAG-tagged POT1 (F-POT1) as a positive control, or F-POT1ΔOB was immunoprecipitated by virtue of the FLAG epitope and immunoblotted with an anti-TRF2 antibody to detect coimmunoprecipitated endogenous TRF2. As expected (3, 75), TRF2 coimmunoprecipitated with F-POT1 in positive control cells but not with empty vector or F-POT12OB in negative control cells (Fig. 2A). F-POT1ΔOB coimmunoprecipitated with TRF2, indicating that F-POT1ΔOB readily associated with endogenous TRF2. Of note, the interaction of endogenous TRF2 and F-POT1ΔOB was more robust than that between endogenous TRF2 and full-length F-POT1 (Fig. 2A).
FIG. 2.
POT1ΔOB disrupts TRF2 protein interactions. (A) Immunoprecipitation (IP) of stably expressed FLAG-tagged POT1 (F-POT1) proteins followed by immunoblotting (IB) to detect whether endogenous TRF2 coimmunoprecipitated with indicated F-POT1 proteins in 293T cells. (B) Immunoprecipitation of the indicated transiently expressed FLAG- or myc-tagged proteins followed by immunoblotting to detect whether endogenous TRF2 coimmunoprecipitated with empty vector, F-POT1, F-RAP1, or myc-TIN2 in the absence (−) or presence (+) of transiently coexpressed YFP-tagged POT1ΔOB (YFP-POT1ΔOB) in 293T cells.
Given that POT1ΔOB associates more strongly than full-length POT1 with endogenous TRF2 (Fig. 2A), we next explored whether POT1ΔOB reduced the binding of telomeric proteins to TRF2. TRF2 binds the proteins POT1 (75), RAP1 (47, 48, 56), and TIN2 (42, 76). All three of these proteins are known to negatively regulate telomere length (38, 43, 47, 50, 56, 71, 74, 77), which is consistent with POT1ΔOB potentially disrupting their function. Thus, F-POT1, FLAG-tagged RAP1 (F-RAP1), myc-tagged TIN2 (myc-TIN2), or an empty vector as a negative control was transiently expressed in the absence or presence of transiently coexpressed YFP-tagged POT1ΔOB (YFP-POT1ΔOB). Expression of YFP-POT1ΔOB was confirmed by immunoblotting using an anti-green fluorescent protein antibody (Fig. 2B). F-POT1, F-RAP1, or myc-TIN2 was immunoprecipitated by virtue of the FLAG or myc epitopes, as appropriate, followed by immunoblotting with an anti-TRF2 antibody to assess the relative amount of coimmunoprecipitated endogenous TRF2. As previously reported (3, 42, 56, 57, 75), while empty vector failed to do so, ectopic F-POT1, F-RAP1, and myc-TIN2 all coimmunoprecipitated with TRF2. Importantly, the amount of endogenous TRF2 which coimmunoprecipitated with each of these three binding partners (F-POT1, F-RAP1, and myc-TIN2) was decreased upon expression of YFP-POT1ΔOB (Fig. 2B). Notably, previous reports demonstrated that expression of POT1ΔOB does not result in the loss of endogenous TRF2 from telomeres, as assessed by chromatin immunoprecipitation (51), suggesting that the reduction in the interaction of endogenous TRF2 with POT1, RAP1, and TIN2 is not due to mislocalization of TRF2. Thus, we conclude that POT1ΔOB robustly associates with endogenous TRF2 and disrupts its interaction with POT1, RAP1, and TIN2.
POT1 suppresses telomere elongation induced by POT1ΔOB.
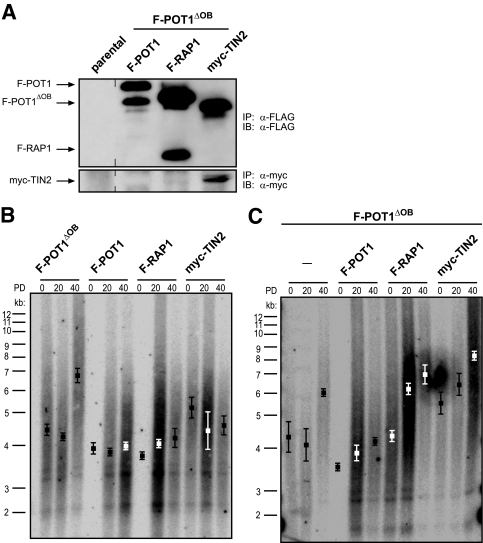
We next determined which of the TRF2 protein interactions disrupted by POT1ΔOB were critical in the regulation of telomere length. Specifically, we reasoned that if POT1ΔOB induced telomere elongation by disrupting the interaction of TRF2 with POT1, RAP1, or TIN2, such elongation may be suppressed by overexpressing the essential displaced protein. To test this hypothesis, telomere length was determined at regular intervals by Southern hybridization in immortal HA1 cells stably expressing F-POT1, F-RAP1, or myc-TIN2 in the absence or presence of stably expressed F-POT1ΔOB. Expression of the ectopic proteins was assessed by immunoprecipitation and immunoblotting using an anti-FLAG or anti-myc antibody, as appropriate (Fig. 1A and 3A).
FIG. 3.
POT1 suppresses telomere elongation induced by POT1ΔOB. (A) Detection of the indicated FLAG- or myc-tagged proteins stably expressed in HA1 cells by immunoprecipitation (IP) and immunoblotting (IB) with an anti-FLAG or anti-myc antibody as indicated. Vertical lines denote where the image has been cut. (B and C) Telomere length, as assessed by Southern hybridization with a 32P-labeled telomeric probe, of HA1 cells stably expressing the indicated transgenes at PDs 0, 20, and 40. Boxes indicate mean telomere length ± standard deviation calculated from two independently derived sets of cell lines.
As expected (2, 43, 47, 50, 51, 56), overexpression of F-POT1, F-RAP1, or myc-TIN2 alone induced subtle changes in telomere length, whereas the mean telomere length in cells expressing F-POT1ΔOB was increased to ∼7 kb over 40 PDs of observation (Fig. 3B). Coexpression of F-RAP1 or myc-TIN2 failed to suppress the telomere elongation induced by F-POT1ΔOB, as evidenced by the fact that telomeres of cells coexpressing F-RAP1 or myc-TIN2 with F-POT1ΔOB reached mean lengths of ∼7 to 8 kb, similar to those of control cells expressing F-POT1ΔOB alone (Fig. 3C). Thus, simply overexpressing any TRF2-interacting protein does not suppress POT1ΔOB-mediated elongation of telomeres. Importantly, expression of F-POT1 did suppress the telomere elongation induced by F-POT1ΔOB. Specifically, the telomeres of cells coexpressing F-POT1 and F-POT1ΔOB reached a mean length of ∼4.1 kb, ∼3 kb shorter than that observed in cells coexpressing F-POT1ΔOB and F-RAP1 or myc-TIN2 (Fig. 3C), similar to telomere lengths in cells expressing empty vector (Fig. 1B). These results were reproducible, as similar telomere dynamics were observed in an additional independently derived set of cell lines (Fig. 3B and C). Since F-POT1ΔOB disrupts the association of TRF2 with F-POT1, F-RAP1, and myc-TIN2 (Fig. 2B), but of these proteins only expression of F-POT1 suppressed the telomere elongation induced by F-POT1ΔOB (Fig. 3C), we surmise that disruption of the specific association between POT1 and TRF2 underlies the ability of POT1ΔOB to induce telomere elongation.
POT1 association with telomeric ssDNA and TRF2 regulates telomere length.
We have demonstrated that POT1ΔOB disrupts the association of TRF2 with POT1 and induces telomere elongation in a dominant-negative fashion (Fig. 1B, 2B, and 3C). We thus tested whether disrupting the interaction of POT1 and TRF2 through a different means, specifically by expressing the POT1ΔTRF2 mutant defective in associating with TRF2, would similarly induce telomere elongation. To this end, immortal HA1 cells were engineered to stably express empty vector, F-POT1, F-POT1ΔTPP1 or F-POT1ΔOB as controls or F-POT1ΔTRF2 (see Fig. S1 in the supplemental material). Expression of the ectopic proteins was assessed by immunoprecipitation and immunoblotting using an anti-FLAG antibody (Fig. 4A), and telomere length in these cells was determined at regular intervals by Southern hybridization. Vector control cells maintained a mean telomere length of ∼3.8 kb, and telomeres in cells stably expressing F-POT1ΔOB exhibited extensive elongation to a final length in excess of 12 kb (Fig. 4B). As expected (2, 50), expression of F-POT1 resulted in a small elongation of telomeres to a final mean length of ∼5 kb. Notably, telomeres in HA1 cells stably expressing F-POT1ΔTPP1 or F-POT1ΔTRF2 maintained a mean length of ∼3.9 kb, similar to those in vector control cells (Fig. 4B). Such results were reproducible, as similar telomere dynamics were observed in two additional independently derived sets of cell lines (Fig. 4B). Thus, unlike POT1ΔOB, POT1ΔTRF2 does not act in a dominant-negative fashion to promote telomere elongation.
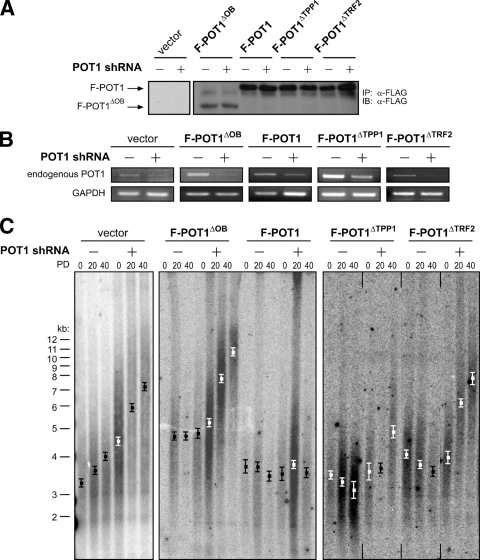
One caveat for the POT1ΔTRF2 mutant is that it does not efficiently colocalize with telomeric chromatin, as assessed by immunofluorescence and chromatin immunoprecipitation (3). However, expression of POT1ΔTRF2 reduces the number of telomere dysfunction-induced foci and the amount of the ssDNA-binding complex RPA localizing to telomeres upon knockdown of endogenous POT1 (3). These data suggest that POT1ΔTRF2 associates with telomeres, but at a reduced capacity. Thus, perhaps POT1ΔTRF2 fails to displace enough endogenous POT1 from critical telomeric binding sites to affect telomere length in a dominant-negative fashion, potentially because the endogenous POT1 protein is bound more strongly or localized more efficiently to telomeres through its association with endogenous TRF2. We therefore tested whether replacing endogenous POT1 with POT1ΔTRF2 could induce telomere elongation. Specifically, immortal HA1 cells were engineered to stably express empty vector as a negative control, RNAi-resistant F-POT1 as a positive control, RNAi-resistant F-POT1ΔOB, F-POT1ΔTPP1, or F-POT1ΔTRF2. These cells were subsequently stably infected with retrovirus encoding either empty vector as a negative control or POT1 shRNA to reduce endogenous POT1 expression (3, 71). Ectopic wild-type or mutant F-POT1 expression was confirmed by immunoprecipitation and immunoblotting with an anti-FLAG antibody (Fig. 5A), and appropriate knockdown of endogenous POT1 was validated by RT-PCR (Fig. 5B). Telomere length in the resultant cell lines was then assessed by Southern hybridization at regular intervals over 40 PDs.
FIG. 5.
POT1 association with telomeric ssDNA and TRF2 regulates telomere length. (A) Detection of the indicated RNAi-resistant FLAG-tagged POT1 (F-POT1) proteins stably expressed in HA1 cells by immunoprecipitation (IP) and immunoblotting (IB) with an anti-FLAG antibody. (B) Detection of endogenous POT1 mRNA, or GAPDH mRNA as a loading control, by RT-PCR in the indicated HA1 cells. (C) Telomere length, as assessed by Southern hybridization with a 32P-labeled telomeric probe, of HA1 cells stably expressing the indicated F-POT1 proteins in the absence (−) or presence (+) of POT1 shRNA at PDs 0, 20, and 40. Boxes indicate mean telomere length ± standard deviation calculated from two independently derived sets of cell lines. Vertical lines denote where the image has been cut.
Knockdown of endogenous POT1 in vector control cells increased the mean length of telomeres in these cells by ∼3.0 kb, and this effect was rescued by overexpression of F-POT1 (Fig. 5C). Such telomere elongation was not rescued by overexpression of F-POT1ΔOB. In fact, cells in which endogenous POT1 was knocked down and replaced by F-POT1ΔOB displayed longer telomeres than cells in which F-POT1ΔOB was expressed in the presence of endogenous POT1 (∼10.5 kb) (Fig. 5C). Such data highlight the critical role of the ssDNA-binding activity of POT1 in regulating telomere length. These results were reproducible, as similar telomere dynamics were observed in an independently derived set of cell lines (Fig. 5C). F-POT1ΔOB did not induce the robust telomere elongation previously observed (Fig. 5C versus 1B, 3B, 3C, and 4B). This is due to the lower expression of F-POT1ΔOB from the pBabe-hygro-F-POT1ΔOB plasmid (used in this experiment to accommodate puromycin selection for the POT1shRNA vector) than from the pBabe-puro-F-POT1ΔOB plasmid (used in previous experiments). Indeed, HA1 cells stably infected with pBabe-hygro-F-POT1ΔOB express lower levels of F-POT1ΔOB and more subtle elongation of telomeres than the same cells stably infected with Babe-puro-F-POT1ΔOB (see Fig. S2 in the supplemental material). Nevertheless, F-POT1ΔOB expressed from the pBabe-hygro vector induced longer telomeres compared to vector control cells (Fig. 5C; also see Fig. S2 in the supplemental material).
In regard to the ability of POT1 to associate with TRF2, cells expressing F-POT1ΔTPP1 or F-POT1ΔTRF2 in the presence of endogenous POT1 maintained an approximately constant mean telomere length of ∼3 to 4 kb, similar to vector control cells (Fig. 1B and 5C). Knocking down endogenous POT1 with shRNA and replacing this with F-POT1ΔTPP1 induced only a small ∼1.4-kb increase in mean telomere length, to a final length of ∼5 kb. Conversely, replacement of endogenous POT1 with F-POT1ΔTRF2 resulted in greater elongation of ∼3.9 kb, to a final mean telomere length of ∼8 kb (Fig. 5C), suggesting that loss of an association with TRF2 is more detrimental in regard to the regulation of telomere length. Given that in the presence of endogenous POT1 the overexpression of F-POT1ΔTPP1 resulted in slightly smaller telomeres than overexpression of F-POT1ΔTRF2, it is notable that the difference between mean telomere lengths induced by expressing these mutants in the absence of endogenous POT1 is greater (3.0 kb; P = 0.003) than the difference in mean telomere lengths observed due to their expression in the presence of endogenous POT1 (0.4 kb; P = 0.099). These results were reproducible, as similar telomere dynamics were observed in an independently derived set of cell lines (Fig. 5C). Thus, we conclude that inhibiting the ability of POT1 to associate with TRF2 induced telomere elongation, consistent with the notion that an interaction between POT1 and TRF2 is required to regulate telomere length.
DISCUSSION
We propose that POT1 suppresses telomere elongation when simultaneously bound to telomeric ssDNA and TRF2. This notion is supported by the following: (i) expression of a POT1 mutant that is unable to bind ssDNA (POT1ΔOB) induced telomere elongation (2, 50, 51, 74) (Fig. 1B, 3B, 3C, and 4B), an effect that was enhanced if endogenous POT1 was replaced with POT1ΔOB (Fig. 5C); (ii) telomere elongation induced by POT1ΔOB in a dominant-negative fashion was abrogated by introduction of a mutation that reduced association with TRF2 (Fig. 1B); (iii) POT1ΔOB associated with TRF2 and displaced POT1, RAP1, and TIN2 (Fig. 2B), but of these proteins, only ectopically expressed POT1 suppressed telomere elongation induced by POT1ΔOB (Fig. 3C); and (iv) replacing endogenous POT1 with a mutant defective in association with TRF2 resulted in telomere elongation (Fig. 5C).
POT1 associates with both TPP1 and TRF2, yet we find that it is primarily the latter interaction that affects telomere length. This does not discount a role for TPP1 in POT1 function. Binding to TPP1 enhances the association of POT1 with telomeric ssDNA, at least in vitro (72, 73), and binding to TPP1, but not TRF2, prevents telomere uncapping, as assessed by telomere dysfunction-induced foci and RPA localization at telomeres (3, 24, 29, 37, 73). With regard to telomere length, mutations that inhibit the ability of POT1ΔOB to promote telomere elongation can reduce TPP1 binding (50), although these mutations may also reduce the association with TRF2, given that one such mutation resides in the region disrupted in POT1ΔOBΔTRF2 (3). Knockdown of POT1 and replacement with POT1ΔTPP1 also caused a small increase in telomere length, although this was much less than that observed when POT1 was replaced with POT1ΔTRF2 (Fig. 5C). In sum, an interaction with TPP1 may have some influence on the ability of POT1 to regulate telomere length, although this association appears to play a more prominent role in the capping function of POT1. Overall, POT1 appears to perform different functions at telomeres through specific proteins: TPP1 to cap telomeres and TRF2 to regulate telomere length.
Mechanistically, it remains to be resolved how POT1 regulates telomere length through its association with telomeric ssDNA and TRF2. One possibility is that the connection of telomeric ssDNA (via the OB folds of POT1) with telomeric dsDNA (via association with telomeric dsDNA by TRF2) may promote the formation of higher-order chromatin structures that physically block access of telomerase. At face value, the finding that POT1ΔTRF2 did not promote telomere elongation in a dominant-negative fashion seems inconsistent with this model. However, the lack of dominant-negative activity may simply reflect a reduction in the ability of POT1ΔTRF2 to associate with telomere chromatin (3), and moreover, POT1ΔTRF2 does induce telomere elongation in the absence of endogenous POT1. The idea of POT1-TRF2 regulating telomere length is also supported by the finding that fusing POT1 to the TRF2 paralog, TRF1, both abolishes the telomere elongation observed when POT1 and TRF1 are expressed in trans and induces the in vitro formation of t-loops (25), structures found on telomeres (28) that could inhibit access of telomerase to telomere ends (6, 23). Additionally, Pot1 can be recruited to telomeres via an association with telomeric dsDNA-binding protein Taz1 in fission yeast. Moreover, it has been suggested that at long telomeres Pot1 associates with the Taz1 complex and this inhibits telomerase by stabilizing a closed conformation of telomeres, while at short telomeres this protein mediated-association of telomeric ssDNA and dsDNA decreases and exposes telomeres to elongation by telomerase (53). POT1 and TRF2 are found together in larger protein complexes at the telomere (49, 57). Reducing the amount of one of the proteins in such complexes, TRF1, has been reported to decrease POT1 association with telomeric chromatin (51), and knockdown of many telomeric proteins results in telomere elongation (10, 38, 43, 47, 51, 56, 66, 69, 71, 74, 77). Thus, regulation of telomere length by POT1 may yet involve other proteins in addition to TRF2. Nevertheless, TRF2 plays a dominant role in the ability of POT1 to regulate telomere length. In summary, we demonstrate that the interaction of POT1 with telomeric ssDNA and TRF2 suppresses telomere elongation, indicating a critical role for these interactions in telomere length homeostasis.
Supplementary Material
Acknowledgments
We thank J. Campisi and Z. Songyang for constructs encoding TIN2 and RAP1, respectively, and members of the Counter lab for helpful discussions.
This research is supported by NIH grant CA082481 and the Edward Spiegel Fund of the Lymphoma Foundation.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 3 August 2009.
REFERENCES
- 1.Allsopp, R. C., and C. B. Harley. 1995. Evidence for a critical telomere length in senescent human fibroblasts. Exp. Cell Res. 219:130-136. [DOI] [PubMed] [Google Scholar]
- 2.Armbruster, B. N., C. M. Linardic, T. Veldman, N. P. Bansal, D. L. Downie, and C. M. Counter. 2004. Rescue of an hTERT mutant defective in telomere elongation by fusion with hPot1. Mol. Cell. Biol. 24:3552-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrientos, K. S., M. F. Kendellen, B. D. Freibaum, B. N. Armbruster, K. T. Etheridge, and C. M. Counter. 2008. Distinct functions of POT1 at telomeres. Mol. Cell. Biol. 28:5251-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann, P., and T. R. Cech. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292:1171-1175. [DOI] [PubMed] [Google Scholar]
- 5.Baumann, P., E. Podell, and T. R. Cech. 2002. Human Pot1 (protection of telomeres) protein: cytolocalization, gene structure, and alternative splicing. Mol. Cell. Biol. 22:8079-8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn, E. H. 2000. Telomere states and cell fates. Nature 408:53-56. [DOI] [PubMed] [Google Scholar]
- 7.Blasco, M. A. 2007. Telomere length, stem cells and aging. Nat. Chem. Biol. 3:640-649. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 9.Broccoli, D., A. Smogorzewska, L. Chong, and T. de Lange. 1997. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17:231-235. [DOI] [PubMed] [Google Scholar]
- 10.Bunch, J. T., N. S. Bae, J. Leonardi, and P. Baumann. 2005. Distinct requirements for pot1 in limiting telomere length and maintaining chromosome stability. Mol. Cell. Biol. 25:5567-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calado, R. T., and N. S. Young. 2008. Telomere maintenance and human bone marrow failure. Blood 111:4446-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campisi, J., S. H. Kim, C. S. Lim, and M. Rubio. 2001. Cellular senescence, cancer and aging: the telomere connection. Exp. Gerontol. 36:1619-1637. [DOI] [PubMed] [Google Scholar]
- 13.Canudas, S., B. R. Houghtaling, J. Y. Kim, J. N. Dynek, W. G. Chang, and S. Smith. 2007. Protein requirements for sister telomere association in human cells. EMBO J. 26:4867-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra, A., T. R. Hughes, C. I. Nugent, and V. Lundblad. 2001. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 15:404-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, Y., Y. Yang, M. van Overbeek, J. R. Donigian, P. Baciu, T. de Lange, and M. Lei. 2008. A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins. Science 319:1092-1096. [DOI] [PubMed] [Google Scholar]
- 16.Cook, B. D., J. N. Dynek, W. Chang, G. Shostak, and S. Smith. 2002. Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol. Cell. Biol. 22:332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Counter, C. M., A. A. Avilion, C. E. LeFeuvre, N. G. Stewart, C. W. Greider, C. B. Harley, and S. Bacchetti. 1992. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11:1921-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Counter, C. M., F. M. Botelho, P. Wang, C. B. Harley, and S. Bacchetti. 1994. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J. Virol. 68:3410-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Counter, C. M., W. C. Hahn, W. Wei, S. D. Caddle, R. L. Beijersbergen, P. M. Lansdorp, J. M. Sedivy, and R. A. Weinberg. 1998. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 95:14723-14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Counter, C. M., M. Meyerson, E. N. Eaton, L. W. Ellisen, S. D. Caddle, D. A. Haber, and R. A. Weinberg. 1998. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 16:1217-1222. [DOI] [PubMed] [Google Scholar]
- 21.d'Adda di Fagagna, F., P. M. Reaper, L. Clay-Farrace, H. Fiegler, P. Carr, T. Von Zglinicki, G. Saretzki, N. P. Carter, and S. P. Jackson. 2003. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194-198. [DOI] [PubMed] [Google Scholar]
- 22.d'Adda di Fagagna, F., S. H. Teo, and S. P. Jackson. 2004. Functional links between telomeres and proteins of the DNA-damage response. Genes Dev. 18:1781-1799. [DOI] [PubMed] [Google Scholar]
- 23.de Lange, T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19:2100-2110. [DOI] [PubMed] [Google Scholar]
- 24.Denchi, E. L., and T. de Lange. 2007. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448:1068-1071. [DOI] [PubMed] [Google Scholar]
- 25.Etheridge, K. T., S. A. Compton, K. S. Barrientos, S. Ozgur, J. D. Griffith, and C. M. Counter. 2008. Tethering telomeric double- and single-stranded DNA-binding proteins inhibits telomere elongation. J. Biol. Chem. 283:6935-6941. [DOI] [PubMed] [Google Scholar]
- 25a.Freibaum, B. D., and C. M. Counter. 2006. hSnm1B is a novel telomere-associated protein. J. Biol. Chem. 281:15033-15036. [DOI] [PubMed] [Google Scholar]
- 26.Grant, J. D., D. Broccoli, M. Muquit, F. J. Manion, J. Tisdall, and M. F. Ochs. 2001. Telometric: a tool providing simplified, reproducible measurements of telomeric DNA from constant field agarose gels. BioTechniques 31:1314-. 6:1318. [DOI] [PubMed] [Google Scholar]
- 27.Greider, C. W., and E. H. Blackburn. 1985. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405-413. [DOI] [PubMed] [Google Scholar]
- 28.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 29.Guo, X., Y. Deng, Y. Lin, W. Cosme-Blanco, S. Chan, H. He, G. Yuan, E. J. Brown, and S. Chang. 2007. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J. 26:4709-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn, W. C., S. A. Stewart, M. W. Brooks, S. G. York, E. Eaton, A. Kurachi, R. L. Beijersbergen, J. H. Knoll, M. Meyerson, and R. A. Weinberg. 1999. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 5:1164-1170. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 32.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458-460. [DOI] [PubMed] [Google Scholar]
- 33.Hastie, N. D., M. Dempster, M. G. Dunlop, A. M. Thompson, D. K. Green, and R. C. Allshire. 1990. Telomere reduction in human colorectal carcinoma and with ageing. Nature 346:866-868. [DOI] [PubMed] [Google Scholar]
- 34.Hayflick, L. 1965. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37:614-636. [DOI] [PubMed] [Google Scholar]
- 35.Hayflick, L. 1997. Mortality and immortality at the cellular level. A review. Biochemistry (Moscow) 62:1180-1190. [PubMed] [Google Scholar]
- 36.Herbert, B., A. E. Pitts, S. I. Baker, S. E. Hamilton, W. E. Wright, J. W. Shay, and D. R. Corey. 1999. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc. Natl. Acad. Sci. USA 96:14276-14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hockemeyer, D., W. Palm, T. Else, J. P. Daniels, K. K. Takai, J. Z. Ye, C. E. Keegan, T. de Lange, and G. D. Hammer. 2007. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nat. Struct. Mol. Biol. 14:754-761. [DOI] [PubMed] [Google Scholar]
- 38.Hockemeyer, D., A. J. Sfeir, J. W. Shay, W. E. Wright, and T. de Lange. 2005. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 24:2667-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houghtaling, B. R., L. Cuttonaro, W. Chang, and S. Smith. 2004. A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr. Biol. 14:1621-1631. [DOI] [PubMed] [Google Scholar]
- 40.Kelleher, C., I. Kurth, and J. Lingner. 2005. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol. Cell. Biol. 25:808-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 42.Kim, S. H., C. Beausejour, A. R. Davalos, P. Kaminker, S. J. Heo, and J. Campisi. 2004. TIN2 mediates functions of TRF2 at human telomeres. J. Biol. Chem. 279:43799-43804. [DOI] [PubMed] [Google Scholar]
- 43.Kim, S. H., P. Kaminker, and J. Campisi. 1999. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 23:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei, M., E. R. Podell, and T. R. Cech. 2004. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat. Struct. Mol. Biol. 11:1223-1229. [DOI] [PubMed] [Google Scholar]
- 45.Lei, M., A. J. Zaug, E. R. Podell, and T. R. Cech. 2005. Switching human telomerase on and off with hPOT1 protein in vitro. J. Biol. Chem. 280:20449-20456. [DOI] [PubMed] [Google Scholar]
- 46.Lenain, C., S. Bauwens, S. Amiard, M. Brunori, M. J. Giraud-Panis, and E. Gilson. 2006. The Apollo 5′ exonuclease functions together with TRF2 to protect telomeres from DNA repair. Curr. Biol. 16:1303-1310. [DOI] [PubMed] [Google Scholar]
- 47.Li, B., and T. de Lange. 2003. Rap1 affects the length and heterogeneity of human telomeres. Mol. Biol. Cell 14:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, B., S. Oestreich, and T. de Lange. 2000. Identification of human Rap1: implications for telomere evolution. Cell 101:471-483. [DOI] [PubMed] [Google Scholar]
- 49.Liu, D., M. S. O'Connor, J. Qin, and Z. Songyang. 2004. Telosome—a mammalian telomere associated complex formed by multiple telomeric proteins. J. Biol. Chem. 279:51338-51342. [DOI] [PubMed] [Google Scholar]
- 50.Liu, D., A. Safari, M. S. O'Connor, D. W. Chan, A. Laegeler, J. Qin, and Z. Songyang. 2004. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 6:673-680. [DOI] [PubMed] [Google Scholar]
- 51.Loayza, D., and T. De Lange. 2003. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423:1013-1018. [DOI] [PubMed] [Google Scholar]
- 52.Loayza, D., H. Parsons, J. Donigian, K. Hoke, and T. de Lange. 2004. DNA binding features of human POT1: a nonamer 5′-TAGGGTTAG-3′ minimal binding site, sequence specificity, and internal binding to multimeric sites. J. Biol. Chem. 279:13241-13248. [DOI] [PubMed] [Google Scholar]
- 53.Miyoshi, T., J. Kanoh, M. Saito, and F. Ishikawa. 2008. Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 320:1341-1344. [DOI] [PubMed] [Google Scholar]
- 54.Morin, G. B. 1989. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 59:521-529. [DOI] [PubMed] [Google Scholar]
- 55.Nugent, C. I., T. R. Hughes, N. F. Lue, and V. Lundblad. 1996. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274:249-252. [DOI] [PubMed] [Google Scholar]
- 56.O'Connor, M. S., A. Safari, D. Liu, J. Qin, and Z. Songyang. 2004. The human Rap1 protein complex and modulation of telomere length. J. Biol. Chem. 279:28585-28591. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor, M. S., A. Safari, H. Xin, D. Liu, and Z. Songyang. 2006. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc. Natl. Acad. Sci. USA 103:11874-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Hayer, K. M., and C. M. Counter. 2006. A genetically defined normal human somatic cell system to study ras oncogenesis in vivo and in vitro. Methods Enzymol. 407:637-647. [DOI] [PubMed] [Google Scholar]
- 59.Pennock, E., K. Buckley, and V. Lundblad. 2001. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104:387-396. [DOI] [PubMed] [Google Scholar]
- 60.Raynaud, C. M., L. Sabatier, O. Philipot, K. A. Olaussen, and J. C. Soria. 2008. Telomere length, telomeric proteins and genomic instability during the multistep carcinogenic process. Crit. Rev. Oncol. Hematol. 66:99-117. [DOI] [PubMed] [Google Scholar]
- 61.Savage, S. A., and B. P. Alter. 2008. The role of telomere biology in bone marrow failure and other disorders. Mech. Ageing Dev. 129:35-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shay, J. W., and S. Bacchetti. 1997. A survey of telomerase activity in human cancer. Eur. J. Cancer 33:787-791. [DOI] [PubMed] [Google Scholar]
- 63.Smith, S., and T. de Lange. 2000. Tankyrase promotes telomere elongation in human cells. Curr. Biol. 10:1299-1302. [DOI] [PubMed] [Google Scholar]
- 64.Smith, S., I. Giriat, A. Schmitt, and T. de Lange. 1998. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282:1484-1487. [DOI] [PubMed] [Google Scholar]
- 65.Smogorzewska, A., and T. de Lange. 2004. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73:177-208. [DOI] [PubMed] [Google Scholar]
- 66.Smogorzewska, A., B. van Steensel, A. Bianchi, S. Oelmann, M. R. Schaefer, G. Schnapp, and T. de Lange. 2000. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song, K., D. Jung, Y. Jung, S. G. Lee, and I. Lee. 2000. Interaction of human Ku70 with TRF2. FEBS Lett. 481:81-85. [DOI] [PubMed] [Google Scholar]
- 68.van Overbeek, M., and T. de Lange. 2006. Apollo, an Artemis-related nuclease, interacts with TRF2 and protects human telomeres in S phase. Curr. Biol. 16:1295-1302. [DOI] [PubMed] [Google Scholar]
- 69.van Steensel, B., and T. de Lange. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740-743. [DOI] [PubMed] [Google Scholar]
- 70.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 71.Veldman, T., K. T. Etheridge, and C. M. Counter. 2004. Loss of hPot1 function leads to telomere instability and a cut-like phenotype. Curr. Biol. 14:2264-2270. [DOI] [PubMed] [Google Scholar]
- 72.Wang, F., E. R. Podell, A. J. Zaug, Y. Yang, P. Baciu, T. R. Cech, and M. Lei. 2007. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445:506-510. [DOI] [PubMed] [Google Scholar]
- 73.Xin, H., D. Liu, M. Wan, A. Safari, H. Kim, W. Sun, M. S. O'Connor, and Z. Songyang. 2007. TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature 445:559-562. [DOI] [PubMed] [Google Scholar]
- 74.Yang, Q., R. Zhang, I. Horikawa, K. Fujita, Y. Afshar, A. Kokko, P. Laiho, L. A. Aaltonen, and C. C. Harris. 2007. Functional diversity of human protection of telomeres 1 isoforms in telomere protection and cellular senescence. Cancer Res. 67:11677-11686. [DOI] [PubMed] [Google Scholar]
- 75.Yang, Q., Y. L. Zheng, and C. C. Harris. 2005. POT1 and TRF2 cooperate to maintain telomeric integrity. Mol. Cell. Biol. 25:1070-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye, J. Z., J. R. Donigian, M. van Overbeek, D. Loayza, Y. Luo, A. N. Krutchinsky, B. T. Chait, and T. de Lange. 2004. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J. Biol. Chem. 279:47264-47271. [DOI] [PubMed] [Google Scholar]
- 77.Ye, J. Z., D. Hockemeyer, A. N. Krutchinsky, D. Loayza, S. M. Hooper, B. T. Chait, and T. de Lange. 2004. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18:1649-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, X., V. Mar, W. Zhou, L. Harrington, and M. O. Robinson. 1999. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.