Abstract
This study was designed to determine the strength of the association between the isolation of nonfermentative gram-negative bacilli (NFGNB) from tap water faucet aerators and the prevalence of colonization or infection of patients in intensive care units (ICUs). Surveillance cultures were obtained during a 4-month period from 162 faucet aerators located in seven different ICUs. The prevalence of colonization or infection of ICU patients with NFGNB was determined by prospective surveillance during the same period. Fifty four (33%) of the faucet aerators contained NFGNB. Among the 66 NFGNB isolated from faucet aerators, the most frequently encountered ones were Sphingomonas paucimobili (26 isolates), Pseudomonas aeruginosa (14 isolates), Chryseobacterium meningosepticum (13 isolates), Achromobacter xylosoxidans (6 isolates), Burkholderia cepacia (4 isolates), and Stenotrophomonas maltophilia (3 isolates). Acinetobacter baumannii was not recovered. The most common NFGNB isolated from ICU patients were P. aeruginosa and A. baumannii. There was a significant correlation between the overall prevalence of NFGNB in faucet aerators and their prevalence in exposed ICU patients (Spearman r = 0.821, P = 0.02). There was also a significant correlation between the prevalence of C. meningosepticum in faucet aerators and its prevalence among ICU patients (Spearman r = 0.847, P = 0.016). The electrokaryotypes of four clinical isolates of C. meningosepticum were similar to those of faucet isolates. Measures directed at making the water supply safe may prevent infection by C. meningosepticum and other waterborne pathogens.
Nosocomial infections caused by nonfermentative gram-negative bacilli (NFGNB) are increasing in intensive care units (ICUs) (19, 20, 27). Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia account for most endemic infections. Outbreaks by other NFGNB such as Chryseobacterium spp. and Sphingomonas spp. are particularly troublesome (19, 20, 27).
Hospital water has been shown to be a significant source of health care-associated infections caused P. aeruginosa in ICUs (4, 15, 17, 18, 25, 26). Trautmann et al. (24) reported that P. aeruginosa could be frequently recovered from tap water outlets in ICUs and that the same genotypes were recovered from colonized or infected patients (24). Anaissie et al. estimated that 1,400 deaths occur each year in the United States as a result of waterborne nosocomial pneumonias caused by P. aeruginosa (1). Chlorinated tap water in hospitals often contains low levels of Legionella, P. aeruginosa, other NFGNB, mycobacteria, and Aspergillus (2, 7, 23, 24, 29). Assurance of a safe water supply is therefore an essential component of infection control in ICUs.
During a recent investigation to determine the source of an outbreak of multidrug-resistant A. baumannii in an ICU, we cultured tap water faucets. P. aeruginosa and numerous other NFGNB, including Chryseobacterium meningosepticum, A. xylosoxidans, and Sphingomonas paucimobilis, were recovered. A. baumannii could not be isolated. This led us to conduct a comparative study in all our ICUs to determine the frequency of NFGNB species in tap water and the strength of the association between the prevalence of NFGNB species in tap water and colonization or infection of patients by these microorganisms.
MATERIALS AND METHODS
ICUs.
The National Taiwan University Hospital (NTUH) is a 2,200-bed hospital located in Taipei, Taiwan. Seven ICUs were surveyed. These included a neurosurgical ICU, a surgical ICU (SICU), a cardiac SICU (CSICU), a pediatric ICU (PICU), two medical ICUs (MICUs), and a respiratory care unit. The ICUs were located on three campuses supported by three different central water supply systems. The sinks and faucets were located close to patient beds (within 2 m). More than half of the patients received ventilator support.
Water systems.
The potable water supply was provided by the Taipei municipal water system. Water samples collected from the central water supplies did not contain waterborne NFGNB. The central water systems of each campus were cleaned and maintained every 3 to 6 months. Cooling tower water was cultured for Legionella every 6 months.
Microbiological methods.
Microbial surveillance of NFGNB in ICU tap water faucets was conducted from August through November 2006. A cotton swab was used to sample the inner surface of the faucet aerators. The swabs were immediately inoculated onto sheep blood agar plates and incubated at 37°C for 3 to 5 days. Representative colonies were identified by standard biochemical methods (21). Clinical isolates of NFGNB were stored at −70°C until final identification.
Molecular typing.
The electrokaryotypes of faucet and clinical isolates were analyzed by pulsed-field gel electrophoresis (PFGE). Bacterial genomic DNA was prepared for restriction endonuclease (ApaI, SpeI, and XbaI) digestion in agarose plugs as described previously (16). Electrokaryotypes of environmental and clinical isolates were determined in a CHEF-MAPPER (Bio-Rad) apparatus at 14°C for 22 to 24 h at 6 V/cm with pulse intervals ranging from 1 to 40 s, at an angle of 120°. A Staphylococcus aureus NCTC8325 chromosomal DNA size standard (Bio-Rad) was used as a reference marker and was run in each gel in order to normalize the gel image. Preliminary qualitative analysis of the karyotypes obtained from the same gel was performed by visual inspection of photographs of ethidium bromide-stained gels. Isolates were considered similar if the banding patterns varied by three or fewer readily detectable bands. Isolates were considered different if they varied by four or more bands. To compare the band patterns of aggregated data, the GelCompar software package (version 4.0; Applied Maths, Kortrijk, Belgium) was used. Strains showing 80% or greater banding pattern similarity were considered to have similar or identical electrokaryotypes.
Data collection and definition.
Microbial surveillance of colonization and infection of ICU patients with NFGNB was conducted simultaneously with the sampling of water faucets from August through November 2006. Prospective, hospital-wide on-site surveillance of nosocomial infections utilizing a standardized protocol and case report forms has been conducted at the NTUH since 1981 (9). Infection control nurses visit hospital services routinely and review charts to identify cases of nosocomial infection. The prevalence rate of suspected pathogens in each unit during the study period was expressed as the number of patients with a pathogen-specific colonization/infection rate per 1,000 patient days. The contamination rate of faucet aerators was defined as the number of faucets culture positive for any NFGNB divided by the number of faucets surveyed during the study period.
Data analysis.
The faucet contamination rates for each NFGNB in each unit were compared to the prevalence rates of each NFGNB in the ICU patients. Differences in proportions were compared by χ2 tests. Correlations between two proportions were determined by the Spearman method. All statistical tests were considered two tailed and were significant at P < 0.05.
Institutional approval.
This study was approved by the Institutional Ethics Review Board of the NTUH.
RESULTS
Cultures were obtained from the inner surfaces of 162 tap water faucet aerators. Of these, 136 were located close to patient care beds and 26 were at nursing stations. Fifty-four (33%) of the faucets were contaminated with NFGNB. The NFGNB contamination rates in the ICUs varied from 13.6 to 50%. Sixty-six NFGNB isolates were obtained from faucets. The predominant pathogen was S. paucimobili (26 isolates), followed by P. aeruginosa (14 isolates), C. meningosepticum (13 isolates), Achromobacter xylosoxidans (6 isolates), Burkholderia cepacia (4 isolates), and S. maltophilia (3 isolates). A. baumannii was not recovered from any of the faucets. The faucet contamination rate in the SICU and CSICU was significantly lower than that in the two MICUs (19 versus 46%, P = 0.005).
The most common NFGNB isolated in the ICUs during the study period were P. aeruginosa (1,776 isolates), A. baumannii (1,230 isolates) S. maltophilia (848 isolates), B. cepacia (147 isolates), C. meningosepticum (133 isolates), and S. paucimobilis (131 isolates). The prevalence rates per 1,000 patient days for the five leading NFGNB were as follows: P. aeruginosa, 1.96; A. baumannii, 1.39; S. maltophilia, 0.46; B. cepacia, 0.19; C. meningosepticum, 0.15. The infection/colonization prevalence rates of NFGNB among the ICUs varied from 14.7 to 49.6% (Table 1).
TABLE 1.
Distribution of NFGNB contamination rates in faucet aerators of tap water systems and prevalence rates of patient colonization or infection in ICUs
| Study unit or parameter | Water supply system | No. of faucets surveyed | Faucet contamination rate (%), infection prevalence rate/1,000 patient days
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| NFGNBa | S. paucimobilis | C. meningosepticum | P. aeruginosa | A. xylosoxidans | B. cepacia | S. maltophilia | |||
| MICU a | I | 33 | 42.4, 49.6 | 12.1, 3.4 | 21.2, 3.4 | 6.1, 24.9 | 18.2, 1.3 | 0, 3 | 0, 13.7 |
| MICU b | II | 30 | 50, 42.7 | 36.7, 2.9 | 10, 2.7 | 16.7, 19.1 | 0, 1 | 0, 3.6 | 10, 13.5 |
| NICUb | II | 18 | 27.8, 21.8 | 0, 1.6 | 5.6, 1.2 | 22.2, 11.7 | 0, 0.4 | 0, 1 | 0, 6 |
| SICU | II | 20 | 25, 27.7 | 15, 1.2 | 4.5, 1.4 | 0, 16.3 | 0, 0.3 | 10, 0.8 | 0, 8.2 |
| PICU | II | 10 | 20, 19 | 20, 2 | 0, 1.2 | 0, 10.4 | 0, 0 | 0, 0.6 | 0, 4.9 |
| CSICU | II | 22 | 13.6, 14.7 | 0, 1.2 | 0, 1 | 0, 7.1 | 0, 0 | 9.1, 0.2 | 0, 5.2 |
| RCUc | III | 29 | 34.5, 19.4 | 20.7, 0.3 | 3.4, 0.6 | 10.3, 10.3 | 0, 0.5 | 0, 1.6 | 0, 6 |
| Correlation coefficient | 0.821 | 0.018 | 0.847 | 0.296 | 0.618 | −0.579 | 0.408 | ||
| P value | 0.02 | 0.818 | 0.016 | 0.518 | 0.139 | 0.173 | 0.363 | ||
A. baumannii was not found in the faucet survey. Thus, NFGNB contamination and prevalence rates include the aforementioned six NFGNB and do not include A. baumannii.
NICU, neurosurgical ICU.
RCU, respiratory care unit.
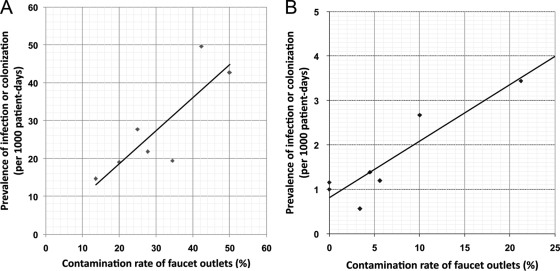
The faucet contamination rates for the six most common NFGNB were compared to the colonization or infection prevalence rates among patients in each ICU (Table 1). There was a significant correlation between the prevalence rates of NFGNB in faucets and ICU patients (r = 0.821, P = 0.02, Fig. 1A). There was also a significant correlation between the presence of C. meningosepticum in faucets and the colonization or infection prevalence rate in ICU patients (r = 0.847, P = 0.016, Fig. 1B). There was no significant correlation for other NFGNB (Table 1).
FIG. 1.
Correlation between faucet aerator contamination rates and infection or colonization prevalence rates in patients in different ICUs due to (A) NFGNB described in Table 1 (correlation coefficient r = 0.821, P = 0.02) and (B) C. meningosepticum (r = 0.847, P = 0.016).
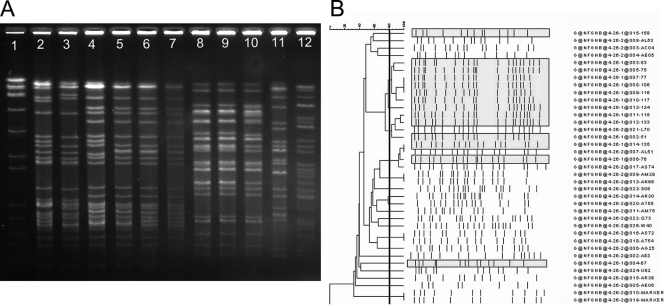
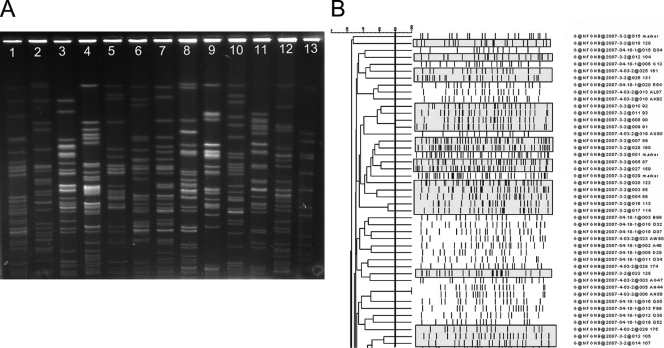
The electrokaryotypes of the 66 faucet isolates and 212 representative clinical isolates were determined by PFGE. Clinical isolates included P. aeruginosa (90 isolates), S. maltophilia (47 isolates), S. paucimobilis (35 isolates), C. meningosepticum (30 isolates); B. cepacia (7 isolates), and A. xylosoxidans (3 isolates). The electrokaryotypes of clinical isolates of C. meningosepticum from four patients located in different units were similar to those of faucet cultures (Fig. 2A). The majority of the faucet isolates of C. meningosepticum clustered together with a similarity of 80% or more (Fig. 2B). The electrokaryotypes of representative samples of P. aeruginosa obtained from faucet cultures and clinical isolates are shown in Fig. 3A. Both faucet isolates and clinical isolates were heterogeneous, with a similarity of less than 80% (Fig. 3B). Other NFGNB isolated in the faucet survey were polyclonal (data not shown) and were unrelated to the clinical isolates.
FIG. 2.
(A) Electrokaryotypes of C. meningosepticum isolates digested by the restriction enzyme ApaI and analyzed by PFGE for 22 h with pulse intervals ranging from 1 to 15 s. Lane 1, DNA size marker; lanes 2, 4, 8, 9, 11, and 12, clinical isolates (isolates P80, L70, AL51, AS74, P81, and L39 in panel B); lanes 3, 5, 6,7, and 10, faucet isolates. The karyotypes of four clinical isolates (lanes 2, 4, 8, and 9) were similar to those of faucet isolates (lanes 3, 5, 6, and 7, respectively). (B) Phylogenetic tree of C. meningosepticum isolates collected from the faucet survey and from patients. The karyotypes of faucet isolates are shaded in gray.
FIG. 3.
(A) Electrokaryotypes of P. aeruginosa isolates digested with the restriction enzyme SpeI and analyzed by PFGE for 24 h with pulse intervals ranging from 0.2 to 40 s. The faucet isolates (lanes 1 to 6) and clinical isolates (lanes 7 to 12) were different. Lane 13 was a DNA size marker. (B) Phylogenetic tree of P. aeruginosa isolates collected in the faucet survey and from patients. The pulsotypes of faucet isolates are shaded in gray.
DISCUSSION
There is considerable evidence from epidemiological and molecular studies that there is a close correlation between nosocomial infections caused by Legionella (3, 10, 12, 14) and P. aeruginosa (4, 17, 18, 20, 25, 26) and contaminated tap water in ICUs. The present study focused on whether this link includes other NFGNB as well. We found that one-third of the ICU faucet aerators sampled were contaminated with other waterborne NFGNB in addition to P. aeruginosa. We found a highly significant association between all of the NFGNB studied and colonization and infection of ICU patients. The only species-specific association was found with C. meningosepticum. This microorganism is of particular importance in Taiwan, where it has been responsible for outbreaks of meningitis and sepsis in newborns and immunocompromised patients (5). The electrokaryotypes of four clinical isolates of C. meningosepticum were similar to those of faucet isolates. S. paucimobilis and C. meningosepticum were the leading non-Pseudomonas NFGNB that contaminated faucet aerators. This is in contrast to the findings of others that emphasize the importance of contamination by P. aeruginosa (4, 17, 18, 20, 25, 26). Some outbreak of non-Pseudomonas NFGNB were reported to be associated with tap water or faucet aerators (6, 7, 11, 13, 28).
We were unable to demonstrate a direct relationship between contamination of faucets and colonization or infection of patients with S. paucimobilis, but this species may have been missed in clinical specimens because of the difficulty in detecting small colonies after 24 h of incubation and contamination of specimens with other microorganisms (21).
We were also unable to demonstrate a similarity by molecular methods between P. aeruginosa, S. maltophilia, B. cepacia, and A. xylosoxidans isolates recovered from faucets and the corresponding species from clinical isolates. A. baumannii was the most common nosocomial NFGNB in ICU patients, but it was not recovered from faucets. This suggests that the major mode of transmission of these microorganisms was by means other than contaminated tap water.
Transmission of waterborne NFGNB from contaminated tap water and faucet aerators can be prevented by rinsing medical respiratory equipment, including nebulizers, with sterile water (7, 22-24). We noted by on-site audit that some hospital staff continued to rinse single-patient-use nebulizers with tap water. This break in technique was immediately corrected.
There was an uneven distribution of NFGNB among the faucets in the various ICUs. This could not be explained by differences in the central water supply systems, because these sources were not contaminated by NFGNB (data not shown). The present observations support the concept that tap water faucets become contaminated within the health care environment at the point of use (7, 24).
The U.S. CDC recommends routine testing for Legionella in the water systems of health care facility areas housing high-risk patients (22). In Germany and France, environmental surveillance of water systems is an integral part of the infection control programs used (7). Routine sampling for Pseudomonas is recommended in patient care areas occupied by burn and other high-risk patients (8). Based on our present experience, we recommend that water be sampled if confirmed C. meningosepticum infections are documented.
This study has several limitations. First, surveillance cultures of the ICU faucet aerators were obtained only once. It is quite possible that we missed potential dynamic changes in microbial ecology. Second, some species of waterborne NFGNB may have been underdetected in clinical isolates because of slow growth rates and mixtures with other microbes. Third, we may have underestimated the molecular relationship of the clinical isolates with the faucet isolates because of inadequate sample size and the polyclonality of waterborne NFGNB.
In conclusion, this study demonstrated a strong positive correlation between tap water system contamination and the prevalence rate of waterborne NFGNB in ICU patients. A key finding was the molecular link between C. meningosepticum in faucet aerators with clinical isolates in high-risk patients. This study emphases the need to insist on the use of sterile water in ICUs, detect breaks in techniques by hospital staff, and include water surveillance in outbreak investigations.
Acknowledgments
We are grateful to members of the Center for Infection Control and Engineering Affairs Office at the NTUH for their important contributions to this study. We are indebted to Calvin Kunin for critically reviewing the manuscript. We appreciate the technical support of Yu-Huei Chen.
J. L. Wang was supported by grant NSC 95-2314-B-002-097 from the National Science Council, Taiwan, and Y. C. Chen was supported by grant DOH96-DC-1010 from the Center for Disease Control, Department of Health, Taiwan.
We have no conflicts of interest to report.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Anaissie, E. J., S. R. Penzak, and M. C. Dignani. 2002. The hospital water supply as a source of nosocomial infections: a plea for action. Arch. Intern. Med. 162:1483-1492. [DOI] [PubMed] [Google Scholar]
- 2.Bert, F., E. Maubec, B. Bruneau, P. Berry, and N. Lambert-Zechovsky. 1998. Multi-resistant Pseudomonas aeruginosa outbreak associated with contaminated tap water in a neurosurgery intensive care unit. J. Hosp. Infect. 39:53-62. [DOI] [PubMed] [Google Scholar]
- 3.Best, M., V. L. Yu, J. Stout, A. Goetz, R. R. Muder, and F. Taylor. 1983. Legionellaceae in the hospital water-supply. Epidemiological link with disease and evaluation of a method for control of nosocomial Legionnaires’ disease and Pittsburgh pneumonia. Lancet 2:307-310. [DOI] [PubMed] [Google Scholar]
- 4.Blanc, D., I. Nahunana, C. Petgnat, A. Wenger, J. Bille, and P. Francioli. 2004. Faucets as a reservoir of endemic Pseudomonas aeruginosa colonization/infections in intensive care units. Intensive Care Med. 30:1964-1968. [DOI] [PubMed] [Google Scholar]
- 5.Chiu, C. H., M. Waddingdon, D. Greenberg, P. C. Schreckenberger, and A. M. Carnahan. 2000. Atypical Chryseobacterium meningosepticum and meningitis and sepsis in newborns and the immunocompromised, Taiwan. Emerg. Infect. Dis. 6:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane, L. R., L. C. Tagle, and W. A. Palutke. 1981. Outbreak of Pseudomonas paucimobilis in an intensive care facility. JAMA 246:985-987. [PubMed] [Google Scholar]
- 7.Exner, M., A. Kramer, L. Lajoie, J. Gebel, S. Engelhart, and P. Hartemann. 2005. Prevention and control of health care-associated waterborne infections in health care facilities. Am. J. Infect. Control 33:S26-S40. [DOI] [PubMed] [Google Scholar]
- 8.Freije, M. R. 2005. Formulating a risk reduction strategy for waterborne pathogens in hospital water systems. Am. J. Infect. Control 33:S50-S53. [DOI] [PubMed] [Google Scholar]
- 9.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 16:128-140. [DOI] [PubMed] [Google Scholar]
- 10.Goetz, A. M., J. E. Stout, S. L. Jacobs, M. A. Fisher, R. E. Ponzer, S. Drenning, and V. L. Yu. 1998. Nosocomial Legionnaires’ disease discovered in community hospitals following cultures of the water system: seek and ye shall find. Am. J. Infect. Control. 26:8-11. [DOI] [PubMed] [Google Scholar]
- 11.Hoque, S. N., J. Graham, M. E. Kaufmann, and S. Tabaqchali. 2001. Chryseobacterium (Flavobacterium) meningosepticum outbreak associated with colonization of water taps in a neonatal intensive care unit. J. Hosp. Infect. 47:188-192. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, J. T., V. L. Yu, M. G. Best, R. M. Vickers, A. Goetz, R. Wagner, H. Wicker, and A. Woo. 1985. Nosocomial legionellosis in surgical patients with head-and-neck cancer: implications for epidemiological reservoir and mode of transmission. Lancet 2:298-300. [DOI] [PubMed] [Google Scholar]
- 13.Kappstein, I., H. Grundmann, T. Hauer, and C. Niemeyer. 2000. Aerators as a reservoir of Acinetobacter junii: an outbreak of bacteraemia in paediatric oncology patients. J. Hosp. Infect. 44:27-30. [DOI] [PubMed] [Google Scholar]
- 14.Kool, J. L., D. Bergmire-Sweat, J. C. Butler, E. W. Brown, E. W., D. J. Peabody, D. S. Massi, J. C. Carpenter, J. M. Pruckler, R. F. Benson, and B. S. Fields. 1999. Hospital characteristics associated with colonization of water systems by Legionella and risk of nosocomial Legionnaires’ disease: a cohort study of 15 hospitals. Infect. Control. Hosp. Epidemiol. 20:798-805. [DOI] [PubMed] [Google Scholar]
- 15.Merlani, G. M., and P. Francioli. 2003. Established and emerging waterborne nosocomial infections. Curr. Opin. Infect. Dis. 16:343-347. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., R. J. Hollis, and H. S. Sader. 1992. Chromosomal restriction fragment analysis by pulsed-field gel electrophoresis, p. 10.5.c.1-10.5.c. 11. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, DC.
- 17.Reuter, S., A. Sigge, H. Wiedeck, and M. Trautmann. 2002. Analysis of transmission pathways of Pseudomonas aeruginosa between patients and tap water outlets. Crit. Care Med. 30:2222-2228. [DOI] [PubMed] [Google Scholar]
- 18.Rogues, A. M., H. Boulestreau, A. Lashéras, A. Boyer, D. Gruson, C. Merle, Y. Castaing, Y., C. M. Bébear, and J. P. Gachie. 2007. Contribution of tap water to patient colonisation with Pseudomonas aeruginosa in a medical intensive care unit. J. Hosp. Infect. 67:72-78. [DOI] [PubMed] [Google Scholar]
- 19.Rolston, K. V., D. P. Kontoyiannis, D. Yadegarynia, and I. I. Raad. 2005. Nonfermentative gram-negative bacilli in cancer patients: increasing frequency of infection and antimicrobial susceptibility of clinical isolates to fluoroquinolones. Diagn. Microbiol. Infect. Dis. 51:215-218. [DOI] [PubMed] [Google Scholar]
- 20.Sader, H. S., and R. N. Jones. 2005. Antimicrobial susceptibility of uncommonly isolated non-enteric gram-negative bacilli. Int. J. Antimicrob. Agents 25:95-109. [DOI] [PubMed] [Google Scholar]
- 21.Schreckenberger, P. C., M. I. Daneshvar, R. S. Weyant, and D. G. Hollis. 2003. Acinetobacter, Achromobacter, Chryseobacterium, Moraxella, and other nonfermentative gram-negative rods, p. 749-779. In P. R. Murray (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 22.Schulster, L., and R. Y. Chinn. 2003. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm. Rep. 52(RR-10):1-42. [PubMed] [Google Scholar]
- 23.Squier, C., V. L. Yu, and J. E. Stout. 2000. Waterborne nosocomial infections. Curr. Infect. Dis. Rep. 2:490-496. [DOI] [PubMed] [Google Scholar]
- 24.Trautmann, M., P. M. Lepper, and M. Haller. 2005. Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. Am. J. Infect. Control 33:S41-S49. [DOI] [PubMed] [Google Scholar]
- 25.Trautmann, M., T. Michalsky, H. Wiedeck, V. Radosavljevic, and M. Ruhnke. 2001. Tap water colonization with Pseudomonas aeruginosa in a surgical intensive care unit (ICU) and relation to Pseudomonas infections of ICU patients. Infect. Control Hosp. Epidemiol. 22:49-52. [DOI] [PubMed] [Google Scholar]
- 26.Vallés, J., D. Mariscal, P. Cortes, P. Coll, P., A. Villagrá, E. Díaz, A. Artigas, and J. Rello. 2004. Patterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumonia. Intensive Care Med. 30:1768-1775. [DOI] [PubMed] [Google Scholar]
- 27.Vidal, F., J. Mensa, M. Almela, M. Olona, J. A. Martínez, F. Marco, M. J. López, A. Soriano, J. P. Horcajada, J. M. Gatell, C. Richart, et al. 2003. Bacteraemia in adults due to glucose non-fermentative gram-negative bacilli other than P. aeruginosa. QJM 96:227-234. [DOI] [PubMed] [Google Scholar]
- 28.Weber, D. J., W. A. Rutala, C. N. Blanchet, M. Jordan, and M. F. Gergen. 1999. Faucet aerators: A source of patient colonization with Stenotrophomonas maltophilia. Am. J. Infect. Control 27:59-63. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. 2008. Guidelines for drinking-water quality. World Health Organization, Geneva, Switzerland. http://www.who.int/water_sanitation_health/dwq/gdwq0506_7.pdf