Abstract
Mycobacterium bolletii is a rapidly growing nontuberculous mycobacterium first characterized in 2006. Here, we report a case of disseminated infection caused by M. bolletii in a young adult patient. To our knowledge, this is the first case of disseminated M. bolletii infection in an otherwise healthy young adult.
CASE REPORT
A previously healthy 23-year-old man presented with a cough, sputum symptoms, and multiple enlarged lymph nodes in the neck. Three months before, he had been diagnosed with smear-negative pulmonary tuberculosis and had received first-line antituberculosis drugs. Despite 1 month of antituberculosis treatment, the patient failed to show any clinical improvement. After then, he was referred to Samsung Medical Center. A physical examination showed that the patient was 184.6 cm tall and weighed 64.5 kg. There were multiple enlarged lymph nodes in the bilateral neck and anticubital fossa of the right forearm. Mild pectus excavatum was noted.
The leukocyte count was 6,300/μl, with a differential of 66% neutrophils and 19% lymphocytes. The erythrocyte sedimentation rate was 50 mm/h, and the cyclic AMP receptor protein level increased to 2.88 mg/dl. A human immunodeficiency virus antibody test result was negative. The CD4-positive-T-lymphocyte count (CD4 count) in the peripheral blood cells was lower (260/mm3) than normal (reference values of 430 to 1,010/mm3).
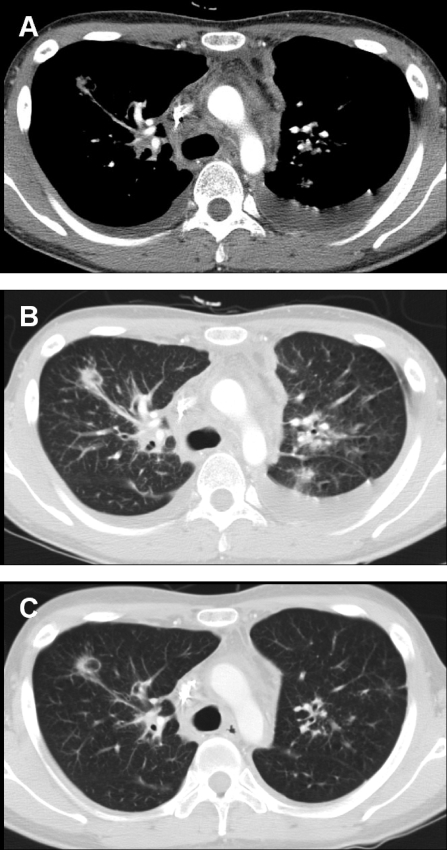
A chest computed tomography (CT) scan revealed bilateral mediastinal and hilar lymphadenopathy, with axial interstial thickening and multiple parenchymal nodules in both lungs. Bilateral pleural effusions and pericardial effusion were also noticed (Fig. 1A and B). An abdominal CT scan showed mild hepatospelonomegaly, with multiple small nodules in the liver and spleen. Multiple intra-abdominal lymph node enlargements were associated.
FIG. 1.
Twenty-three-year-old man with disseminated Mycobacterium bolletii infection. (A) A contrast-enhanced chest CT scan obtained at the level of the carina by using the mediastinal window setting shows extensive mediastinal lymphadenopathy. Note the bilateral pleural effusion. (B) A chest CT scan obtained using the lung window setting shows multiple pulmonary nodules. (C) After 4 weeks of antibiotic therapy, the chest lesions, including the mediastinal lymphadenopathy and parenchymal nodules, were improved. Also note the disappearance of the bilateral pleural effusion.
Bronchoscopic examination revealed multiple small endobronchial nodules, and a bronchial mucosal biopsy showed chronic granulomatous inflammation. An excisional biopsy of the lymph node at the right forearm also showed chronic granulomatous inflammation, with many multinucleated giant cells. The aspirated pleural fluid contained many white blood cells, with a differential of 94% lymphocytes. The level of adenosine deaminase in the effusion was elevated (65.1 IU/liter; reference values of 0 to 40 IU/liter).
Acid-fast-bacillus stains in multiple sputum and bronchial washing fluid specimens were negative. Two weeks later, however, nonpigmented mycobacterial isolates (Samsung 194) were cultured on egg-based Lowenstein-Jensen slants from multiple sputum specimens. The optimal growth temperature of this isolate was 30°C.
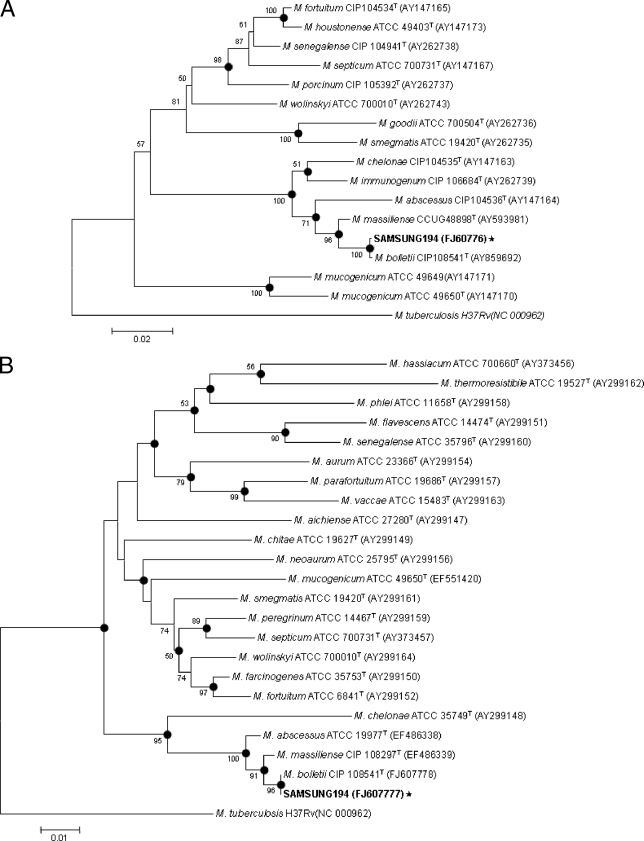
Isolate Samsung 194 was initially identified as Mycobacterium abscessus by using a PCR-restriction fragment length polymorphism method, based on the rpoB gene (7). However, the direct sequencing protocol targeting the nearly complete 16S rRNA gene (1,440 bp) of isolate Samsung 194 (GenBank accession no. FJ607775) showed 100% identity with M. abscessus (GenBank accession no. AY457071), Mycobacterium bolletii (GenBank accession no. AY859681), Mycobacterium chelonae (GenBank accession no. AY457082), and Mycobacterium massiliense (GenBank accession no. AY593980), suggesting that targeting of the 16S rRNA gene is not useful for differentiation of rapidly growing mycobacteria (RGM). For exact species determination of isolate Samsung 194, a 723-bp fragment of the RNA polymerase β subunit (rpoB) gene (GenBank accession no. FJ607776) and a 603-bp hsp65 gene (GenBank accession no. FJ607777) were determined (1, 5). Phylogenetic analysis based on the partial rpoB sequences showed that this strain belonged to M. bolletii, with only one base mismatch (99.9% similarity) to the type strain (GenBank accession no. AY859692) (Fig. 2A). Furthermore, hsp65 sequences of the strain showed 100% similarity with the M. bolletii type strain (GenBank accession no. FJ607778) (Fig. 2B).
FIG. 2.
Phylogenetic positions of isolate Samsung 194 and RGM species, based on the partial RNA polymerase ß subunit gene (rpoB) (A) and the partial heat shock protein 65 gene (hsp65) (B). This tree was constructed using the neighbor-joining method. The percentages indicated at the nodes represent bootstrap levels supported by 1,000 resampled data sets. Bootstrap values of <50% are not shown. The solid circle indicates that the corresponding nodes (groupings) were also recovered in the maximum-parsimony trees. Mycobacterium tuberculosis was used as the outgroup species. Scale bars represent differences in nucleotide sequences.
Antimicrobial susceptibility testing was performed according to the guidelines set forth by the Clinical and Laboratory Standards Institute (CLSI) (formerly the National Committee for Clinical Laboratory Standards [NCCLS]) (9, 10). The MICs of all tested drugs were determined by the broth microdilution method and interpreted according to the guidelines described in the 2003 NCCLS document M24-A (10). The MIC of moxi-floxacin for this RGM has not yet been described by the CLSI. In its place, we followed the recommendation for aerobic organisms in NCCLS M100-S11, from 2001 (9). The test revealed that the isolate Samsung 194 was resistant to imipenem, tobramycin, clarithromycin, ciprofloxacin, and doxycycline. The isolate was sensitive to amikacin and intermediate to cefoxitin and moxifloxacin (Table 1).
TABLE 1.
Susceptibility testing results for antimicrobial agents against the isolate Samsung 194 and MICs
| Drug | MIC (μg/ml) for categorya
|
MIC (μg/ml) for isolate Samsung 194 | ||
|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | ||
| Amikacin | ≤16 | 32 | ≥64 | 8 |
| Cefoxitin | ≤16 | 32-64 | ≥128 | 32 |
| Imipenem | ≤4 | 8 | ≥16 | 16 |
| Tobramycin | ≤4 | 8 | ≥16 | 32 |
| Clarithromycin | ≤2 | 4 | ≥8 | 32 |
| Ciprofloxacin | ≤1 | 2 | ≥4 | 4 |
| Moxifloxacin | ≤1 | 2 | ≥4 | 2 |
| Doxycycline | ≤1 | 2-8 | ≥16 | >32 |
The patient was treated with clarithromycin (1,000 mg/day), ciprofloxacin (1,000 mg/day), amikacin (15 mg/kg of body weight/day), and cefoxitin (200 mg/kg/day) for the initial 4 weeks. After 4 weeks of therapy, his lesions, including the lymphadenopathy, pleural effusion, and pericardial effusion, were greatly improved (Fig. 1C). The patient continued to receive treatment with clarithromycin and ciprofloxacin for more than 12 months, and the level of CD4+ T cells in the peripheral blood cells became normal (620/mm3). However, follow-up mycobacterial sputum cultures have been persistently positive, although he remained well.
RGM, characterized by visible growth on solid media within 7 days, are ubiquitous environmental organisms increasingly recognized as human pathogens (4). The RGM species most commonly associated with human infections are Mycobacterium abscessus and Mycobacterium fortuitum (4). Mycobacterium bolletii was recognized as a species separate from the M. chelonae-M. abscessus group in 2006 (11). Here, we report the first case of a disseminated infection caused by M. bolletii in a young adult patient.
Infections caused by RGM have received greater clinical attention in recent years because of their increasing incidences in AIDS patients as well as in nonimmunocompromised patients (3). Disseminated infection with Mycobacterium abscessus in immunocompromised patients has been described to occur in a few cases (8, 12).
M. bolletii was validated as a species separate from the M. chelonae-M. abscessus group in 2006. M. bolletii is rarely isolated as a pathogen, and previous cases involved subcutaneous infections after mesotherapy, a procedure comprising multiple subcutaneous injections of pharmaceutical and/or homeopathic medications for cosmetic purposes (12), and respiratory tract infections (2). This is the first recognized case of M. bolletii causing disseminated infection, with a CD4 count in the peripheral blood cells that was lower than normal in the initial presentation time. However, the CD4 count was normalized after antibiotic treatment. Therefore, low CD4 count might be a consequence of the disseminated M. bolletii infection rather than the cause of the disseminated infection.
Additionally, a specific immunodeficiency was not found in this case. Phytohemagglutinin-induced gamma interferon production and lipopolysaccharide-induced production of tumor necrosis factor alpha and interleukin-12p40 were normal. In addition, genetic defects along the interleukin-12/gamma interferon pathway were not found (data not shown).
The limited reports regarding M. bolletii infection in the current medical literature make it difficult to find reliable information on antibiotic therapy. Given the phenotypic similarities to M. abscessus, a similar antibiotic therapy may be reasonable. However, M. bolletii has been known to be highly resistant to antimicrobial drugs, including clarithromycin, unlike other nontuberculous mycobacterial species (1, 2, 6). Although the isolates were resistant to clarithromycin (MIC < 32 μg/ml), the patient had been given clarithromycin (1,000 mg/day) for prolonged duration because of limited options for an oral antibiotic regimen. The optimal antibiotic therapy for M. bolletii infection should be established in future studies.
Given the one-base mismatch in the rpoB gene to the M. bolletii type stain (GenBank accession no. AY859692), this isolate may be a rather different variant of M. bolletii, providing a likely explanation for the higher-virulence phenotype of this isolate.
Footnotes
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Adekambi, T., P. Berger, D. Raoult, and M. Drancourt. 2006. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int. J. Syst. Evol. Microbiol. 56:133-143. [DOI] [PubMed] [Google Scholar]
- 2.Adekambi, T., and M. Drancourt. 2009. Mycobacterium bolletii respiratory infections. Emerg. Infect. Dis. 15:302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo, R. E., and K. N. Olivier. 2008. Diagnosis and treatment of infections caused by rapidly growing mycobacteria. Semin. Respir. Crit. Care Med. 29:577-588. [DOI] [PubMed] [Google Scholar]
- 5.Kim, H., S. H. Kim, T. S. Shim, M. N. Kim, G. H. Bai, Y. G. Park, S. H. Lee, G. T. Chae, C. Y. Cha, Y. H. Kook, and B. J. Kim. 2005. Differentiation of Mycobacterium species by analysis of the heat-shock protein 65 gene (hsp65). Int. J. Syst. Evol. Microbiol. 55:1649-1656. [DOI] [PubMed] [Google Scholar]
- 6.Kim, H. Y., Y. Kook, Y. J. Yun, C. G. Park, N. Y. Lee, T. S. Shim, B. J. Kim, and Y. H. Kook. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J. Clin. Microbiol. 46:3384-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, H., H. E. Bang, G. H. Bai, and S. N. Cho. 2003. Novel polymorphic region of the rpoB gene containing Mycobacterium species-specific sequences and its use in identification of mycobacteria. J. Clin. Microbiol. 41:2213-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller, P. S., and R. S. Edson. 2001. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn. Microbiol. Infect. Dis. 39:33-37. [DOI] [PubMed] [Google Scholar]
- 9.NCCLS. 2001. Performance standards for antimicrobial susceptibility testing; 11th informational supplement. Document no. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 10.NCCLS. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. Document no. M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed]
- 11.Viana-Niero, C., K. V. Lima, M. L. Lopes, M. C. Rabello, L. R. Marsola, V. C. Brilhante, A. M. Durham, and S. C. Leao. 2008. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J. Clin. Microbiol. 46:850-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu, V. C., K. D. Wu, C. C. Hung, S. L. Lin, J. W. Huang, and P. R. Hsueh. 2005. Disseminated Mycobacterium abscessus infection in a hemodialysis patient with acquired reactive perforating collagenosis—a case study and literature review. Clin. Nephrol. 63:57-60. [DOI] [PubMed] [Google Scholar]