Abstract
A case of chronic sinus disease due to morphologically atypical Aspergillus flavus is described. Multiple fungal isolates sporulated poorly or not at all, displaying unusual color and microscopic morphology, including the absence of typical vesicles and phialides, which caused the isolates to resemble several other fungal genera superficially. The patient received multiple antifungal therapies over at least 10 years with various azole drugs, including voriconazole, itraconazole, and posaconazole. We speculate that this lengthy exposure to azole antifungal drugs may have caused or promoted the atypical morphology seen in these isolates.
CASE REPORT
A 47-year-old immunocompetent African American female presented to the Infectious Disease and Otolaryngology Clinics in July 2008 with a complaint of swelling over her left eye. She had a long-standing history of fungal sinusitis and left supraorbital fungal abscess that had been treated extensively in the past with multiple surgical interventions and antifungal therapy, including itraconazole, voriconazole, and posaconazole. This most recent exacerbation of symptoms began within a few weeks of discontinuing a 6-month treatment with posaconazole for a previous fungal abscess. On examination, the patient had a firm, nontender, 1.5-cm mass over the left orbital ridge that was nonerythematous and did not appear to extend into the orbital soft tissue. On nasal endoscopy, she was also found to have bilateral mucosal edema with areas of green crusting on the mucosal surface. The supraorbital lesion was aspirated, and 1.5 ml of tan, purulent fluid was sent to the microbiology laboratory for bacterial and fungal cultures.
The patient had a complicated medical history beginning with multiple medical and surgical treatments for nasal polyps and a deviated septum in the mid 1980s to the early 1990s. She had documented granulomatous sinusitis dating back to 1996 that was complicated by a mucocele of the left frontal sinus with bone erosion that extended into the soft tissue of the forehead. Since that time, she has undergone multiple procedures for debridement of the sinuses, reconstruction of a bony defect of the forehead, and drainage of the recurrent left supraorbital abscess. Wound cultures dating back to January 2000 from either sinus debridement or abscess material had consistently demonstrated apparently typical-appearing Aspergillus flavus. A recent portion of her complicated history is summarized here.
In July 2006, the patient complained of sinus symptoms and was found to have nasal crusting. She was given a course of prednisone for exacerbation of her allergic fungal sinusitis. In November 2006, the patient was seen for swelling of the left supraorbital lesion. A 4- to 6-month treatment with itraconazole was begun in December 2006. Her symptoms did not resolve, and in March 2007, the abscess cavity was resected surgically. By May 2007, the patient was complaining again of sinus symptoms and was treated with prednisone. In July 2007, she experienced an exacerbation of the left supraorbital abscess, which was aspirated in September 2007 and the patient was started on 6 months of posaconazole. Her symptoms recurred within weeks of finishing the drug. She returned to the clinic in July 2008 for aspiration of a supraorbital abscess and was started on posaconazole again in September 2008. In January 2009, the eyelid was aspirated again.
Detailed records of fungal cultures from the microbiology laboratory were available beginning in July 2005, when fungi were recovered from two wound specimens from the left forehead/supraorbital lesion. The isolate from the first specimen was described as a cottony white colony with a cream reverse that failed to sporulate, yielding only sterile, branching, septate hyphae. The second specimen also produced a cottony mold with an olive green center, white edges, and a cream reverse. A tease preparation of this isolate demonstrated septate hyphae with rare conidiophores consistent with A. flavus. In June 2006, a round, white, compact fungal isolate was recovered from a sample obtained during the removal of a reconstructive implant of the forehead secondary to infection. On microscopy, the isolate showed minimal sporulation consisting of globose conidial heads with tapering phialides and chains of round conidia reminiscent of Aspergillus species. In November 2006, the sample yielded a flat gray mold with branching septate hyphae and a complete absence of sporulation, which was sent to the Centers for Disease Control and Prevention (CDC) Fungus Reference Unit for identification. A mold with similar colonial morphology was recovered from the September 2007 aspirate culture. Sporulation consisting of chains of smooth, globose conidia arising from single conidiophores was achieved after subculture in tap water agar; it was felt to be most consistent with Scopulariopsis species. This isolate is no longer available for examination. The July 2008 aspirate culture yielded a white mold with septate hyphae and no sporulation, which was sent to the CDC for identification. The January 2009 eyelid aspirate yielded another fungal isolate, which was also sent to the CDC.
Mycological studies.
At the CDC, all three isolates (2006, 2008, and 2009) grew at 30, 37, and 40°C on Sabouraud dextrose agar and Czapek's agar as a downy white organism with a gray reverse. They were partially inhibited on cycloheximide-containing media and did not grow on benomyl agar. When cultured on Czapek's agar at 25°C in the presence of visible light, the colony color changed to grayish green with white edges after 14 days of incubation, but sporulation was not induced under these conditions. The 2008 isolate produced asexual conidia spontaneously when plated on potato dextrose agar at 25°C for 2 weeks, but the 2006 and 2009 isolates did not sporulate until they were plated on malt agar for 2 weeks at 37°C. All three slide cultures showed basipetal chains of conidia arising from diminutive vesicles (Fig. 1B). The microscopic features and the dark colony color were thought to resemble Scopulariopsis species, although the morphology was recognized to be atypical for this genus. Genomic DNA was prepared, and the ribosomal internal transcribed spacer region was sequenced with primers ITS1 and ITS4 as previously described (3). All three isolates displayed identical sequences. A BLAST search of the GenBank database showed that the entire ∼600-nucleotide submitted sequence gave a 100% match to A. flavus NRRL 4998 and A. oryzae NRRL 447 sequences (GenBank accession no. EF661566 and EF661560, respectively). To provide further resolution at the species level, the mitochondrial cytochrome b locus was sequenced according to previously published methods (12) and a BLAST analysis of these sequences revealed that they displayed a 100% match to A. flavus IFM 48054 (GenBank accession no. AB022837). Antifungal susceptibility testing was attempted, but insufficient numbers of conidia could be collected.
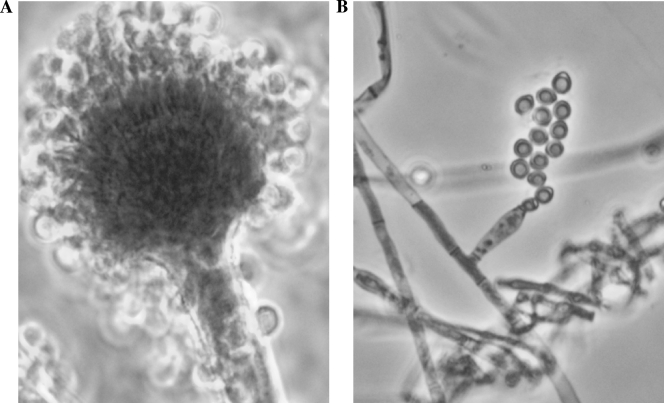
FIG. 1.
(A) Slide culture of typical A. flavus showing conidia arising from biseriate phialides (magnification, ×200). (B) Slide culture of an A. flavus patient isolate showing atypical morphology (magnification, ×400).
Conclusions.
The atypical morphology of these isolates and the recognition of their clinical significance prompted the pursuit of an identification by DNA sequencing methods. Comparative sequence analysis revealed that these isolates were A. flavus. Aspergillus species are well-known causes of sinusitis, as well as a spectrum of lower respiratory diseases. In particular, chronic invasive aspergillosis has been described in immunocompetent hosts as a syndrome where fungi invade the sinus bone or surrounding tissue of the orbit or central nervous system (1, 5, 7). Typical isolates of A. flavus have a distinctive yellow-green colony color and microscopic morphology, with biseriate (two rows of) phialides arising from a conidiophore vesicle with a rough-walled stipe (Fig. 1A). Identification to the species level is achieved by recognition of these characteristic structures in the clinical laboratory. All three isolates of A. flavus recovered from this patient over the course of infection sporulated poorly, rendering morphological methods ineffective for identification.
Several recent reports demonstrate the recovery of nonsporulating fungi that have been identified by comparative sequence-based methodology (2, 3, 8). In the past 5 years, the CDC fungus reference laboratory has received four Aspergillus isolates that were colorless (nonpigmented), failed to sporulate altogether, produced typical sporulation only after extended incubation on malt extract agar at 37°C, or sporulated readily but failed to produce the vesicles and phialides characteristic of Aspergillus species, as was seen in this case. The situation can usually be resolved by using DNA sequencing to identify the isolate. In several past cases where CDC has provided consultation, the referring laboratories had suspected that these white (colorless) cultures were dimorphic fungi, usually Histoplasma capsulatum, Blastomyces dermatitidis, or Coccidioides species, and these situations had generated great anxiety for reasons of laboratory biosafety before it was determined that these were actually Aspergillus isolates. It is also important to rule out the identification as a filamentous basidiomycete. Within this group, Schizophyllum commune is known to cause sinus and brain infections, but a number of other filamentous basidiomycetes are common commensals of the lower respiratory tract. They can be recognized as white, flocculent, generally nonsporulating isolates that fail to grow on media containing cycloheximide but that grow well on media containing benomyl (9).
CDC has conducted broad multisite surveillance for fungal infections in transplant recipients (2001 to 2006) and population-based surveillance for fungal infections (1991 to 1993) but failed to recover any Aspergillus isolates with such atypical color or sporulation. The study conducted in Associated Regional and University Pathologists laboratories in 2005 and 2006 also failed to detect any Aspergillus species among isolates identified as nonsporulating molds (8). The receipt of four such isolates in the past 5 years in our reference laboratory suggests that surveillance studies should be designed to determine if the prevalence of this phenomenon is increasing.
A. flavus was consistently recovered as the etiologic agent throughout the course of this patient's disease. In 2005, when the organism was first recovered, it was a sporulating fungus with morphology characteristic of A. flavus. Subsequently, in 2007, 2008, and 2009, the organism was recovered in culture as a poorly sporulating or nonsporulating mold. The patient's clinical history revealed that she had received multiple treatments with fungistatic azole drugs, primarily itraconazole but also including voriconazole and posaconazole, over a lengthy period of chronic disease. These drugs inhibited but did not eradicate the organism. Interestingly, a prior study demonstrated that when grown in the presence of the azole antifungal voriconazole, A. fumigatus and A. flavus developed as atypically white, poorly sporulating colonies without pigmentation (11). These authors hypothesized that triazole antifungal drugs may interfere with the gene(s) required for the early development of conidia in Aspergillus. The conidiation process in Aspergillus is a highly genetically regulated process involving hundreds of genes. Many classic studies of these pathways in A. nidulans have shown that inhibition of late gene regulators results in the production of partially developed or malformed conidiophores and conidia (4). Mutations in genes in the sporulation pathway have resulted in mutants with aberrant conidiophores and/or poor sporulation phenotypes in A. fumigatus as well (6, 10). In the present study, A. flavus was first recovered as a robustly sporulating culture in the early stages of disease in 2005 but was later isolated with poorly sporulating morphology in 2007 to 2009. Since the patient received many repetitions of azole therapy during this period, it is tempting to speculate that chronic in vivo azole exposure may have resulted in such an aberrant phenotype in this A. flavus isolate.
Clinical mycologists should be aware that such isolates may increase in prevalence as more and more patients receive multiple rounds of azole antifungal therapy over long periods of time. It is not clear whether such isolates will readily revert to more typical color and sporulation when subcultured in the absence of drug. The isolates described here never regained the typical yellow-green A. flavus colony color or typical microscopic conidial structures, even after approximately 10 passages over several months, although in the presence of light they did darken somewhat in color.
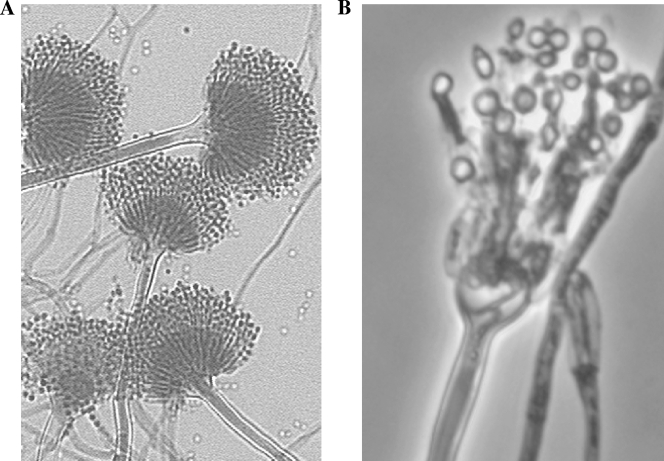
Clinical mycologists examining white (colorless), poorly sporulating isolates should be aware that these could be atypical aspergilli and should inquire whether the patient has a history of azole antifungal administration, particularly if the patient has received multiple courses of therapy for fungal sinusitis or another chronic fungal infection. Two previous isolates obtained from this patient in 2007 and 2008 were initially thought to be atypical Scopulariopsis. Laboratory staff should be aware that atypical aspergilli can superficially resemble a number of other genera, including Penicillium and Scopulariopsis, and can be wrongly discarded as contaminants if the patient's history is not investigated. The CDC reference laboratory has examined white, misshapen, poorly sporulating isolates that we have identified as A. terreus (Fig. 2) and A. fumigatus, as well as A. flavus. Although the inhibitory effect of triazole drugs has not been formally tested on A. terreus, we presume that this species can also be affected. DNA-based methods should be considered as a means to obtain a rapid identification of such isolates when they are clinically significant.
FIG. 2.
(A) Slide culture of typical A. terreus showing conidia arising from biseriate phialides (magnification, ×200). (B) Slide culture of a recent A. terreus clinical isolate showing atypical morphology (magnification, ×400).
Acknowledgments
We thank the staff of the Emory University Hospital mycology laboratory for outstanding cooperation and G. Marshall Lyon for reading the manuscript.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Alrajhi, A., M. Enani, Z. Mahasin, and K. Al-Omran. 2001. Chronic invasive aspergillosis of the paranasal sinuses in immunocompetent hosts from Saudi Arabia. Am. J. Trop. Med. Hyg. 65:83-86. [DOI] [PubMed] [Google Scholar]
- 2.Bagyalakshmi, R., K. L. Therese, S. Prasanna, and H. N Madhavan. 2008. Newer emerging pathogens of ocular non-sporulating molds (NSM) identified by polymerase chain reaction (PCR)-based DNA sequencing technique targeting internal transcribed spacer (ITS) region. Curr. Eye Res. 33:139-147. [DOI] [PubMed] [Google Scholar]
- 3.Balajee, S. A., M. D. Lindsley, N. Iqbal, J. Ito, P. G. Pappas, et al. 2007. Nonsporulating clinical isolate identified as Petromyces alliaceus (anamorph Aspergillus alliaceus) by morphological and sequence-based methods. J. Clin. Microbiol. 45:2701-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo, A. M., R. A. Wilson, J. W. Bok, and N. P. Keller. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy, C. J., and M. H. Nguyen. 1998. Invasive sinus aspergillosis in apparently immunocompetent hosts. J. Infect. 37:229-240. [DOI] [PubMed] [Google Scholar]
- 6.Mah, J. H., and J. H. Yu. 2006. Upstream and downstream regulation of asexual development in Aspergillus fumigatus. Eukaryot. Cell 5:1585-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyarzabal, M. F., B. E. Chevretton, and R. J. Hay. 2000. Semi-invasive allergic aspergillosis of the paranasal sinuses. J. Laryngol. Otol. 114:290-292. [DOI] [PubMed] [Google Scholar]
- 8.Pounder, J. I., K. E. Simmon, C. A. Barton, S. L. Hohmann, M. E. Brandt, et al. 2007. Discovering potential pathogens among fungi identified as nonsporulating molds. J. Clin. Microbiol. 45:568-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigler, L., and S. P. Abbott. 1997. Characterizing and conserving diversity of filamentous basidiomycetes from human sources. Microbiol. Cult. Coll. 13:21-27. [Google Scholar]
- 10.Twumasi-Boateng, K., Y. Yu, D. Chen, F. N. Gravelat, W. C. Nierman, et al. 2009. Transcriptional profiling identifies a role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 8:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varanasi, N. L., I. Baskaran, G. J. Alangaden, P. H. Chandrasekar, and E. K. Manavathu. 2004. Novel effect of voriconazole on conidiation of Aspergillus species. Int. J. Antimicrob. Agents 23:72-79. [DOI] [PubMed] [Google Scholar]
- 12.Wang, L., K. Yokoyama, H. Takahasi, N. Kase, Y. Hanya, et al. 2001. Identification of species in Aspergillus section Flavi based on sequencing of the mitochondrial cytochrome b gene. Int. J. Food Microbiol. 71:75-86. [DOI] [PubMed] [Google Scholar]