Abstract
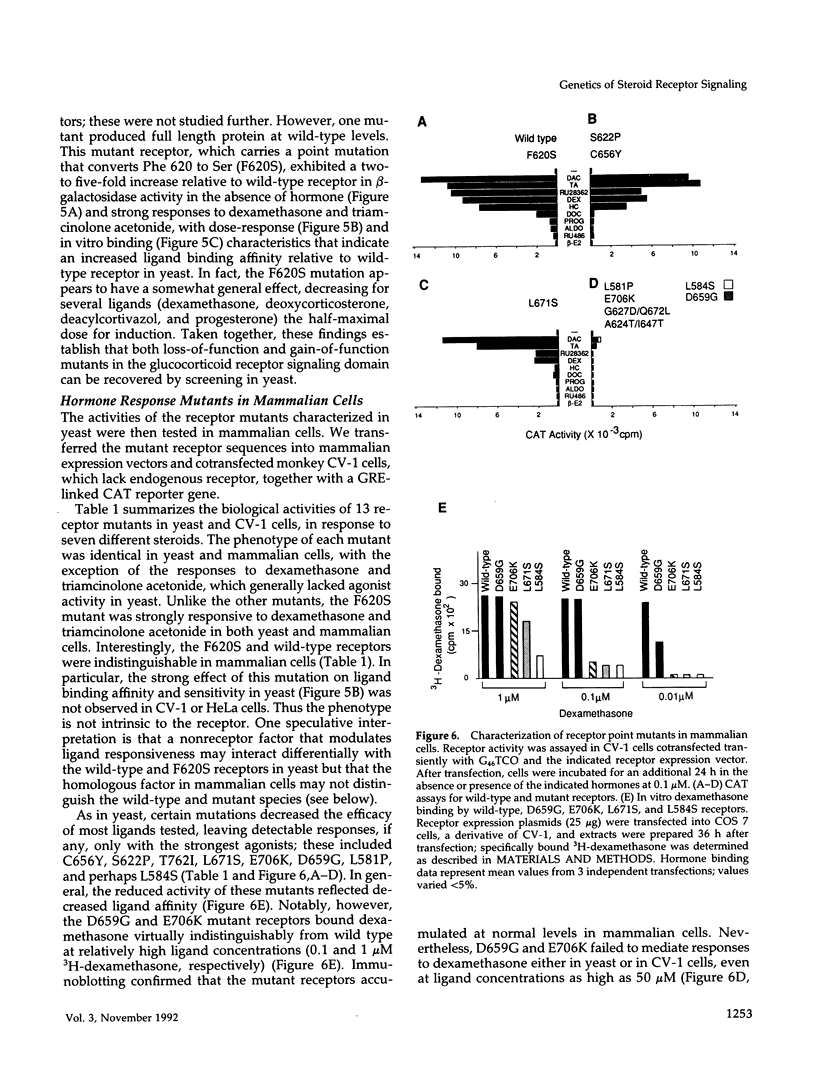
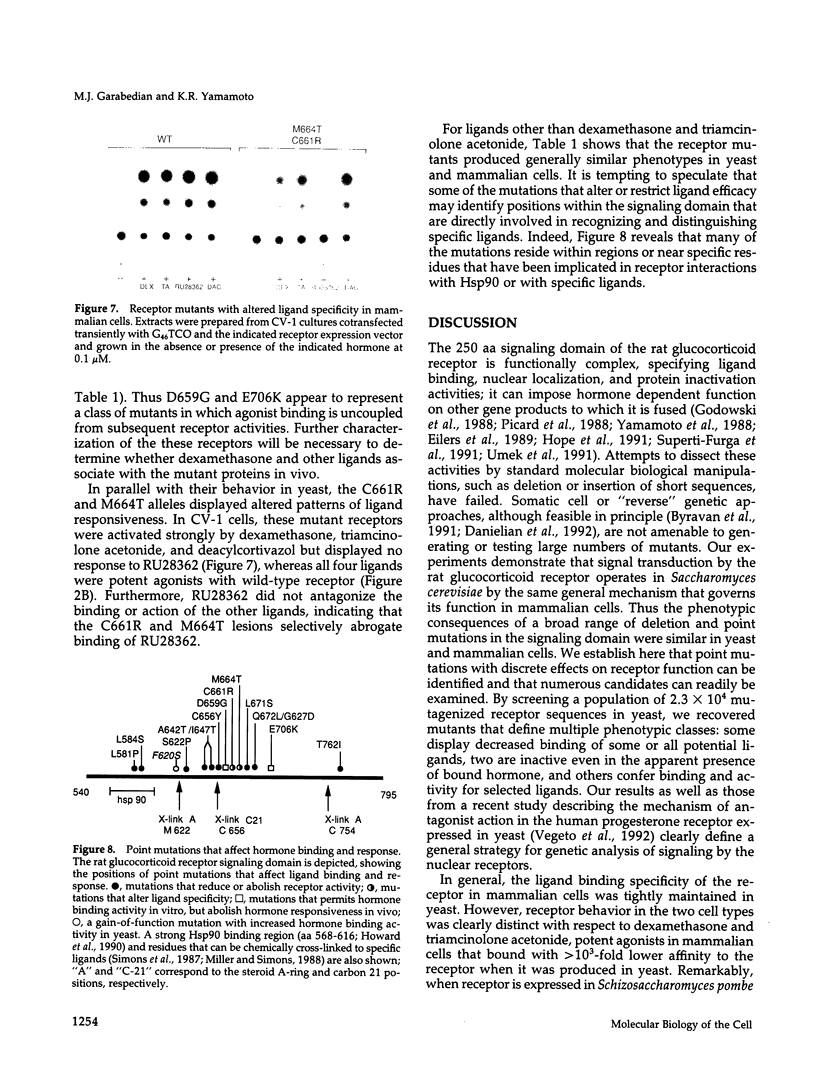
The mechanism of signal transduction by steroid receptor proteins is complex and not yet understood. We describe here a facile genetic strategy for dissection of the rat glucocorticoid receptor "signaling domain," a region of the protein that binds and transduces the hormonal signal. We found that the characteristics of signal transduction by the receptor expressed in yeast were similar to those of endogenous receptors in mammalian cells. Interestingly, the rank order of particular ligands differed between species with respect to receptor binding and biological efficacy. This suggests that factors in addition to the receptor alone must determine or influence ligand efficacy in vivo. To obtain a collection of receptors with distinct defects in signal transduction, we screened in yeast an extensive series of random point mutations introduced in that region in vitro. Three phenotypic classes were obtained: one group failed to bind hormone, a second displayed altered ligand specificity, and a third bound hormone but lacked regulatory activity. Our results demonstrate that analysis of glucocorticoid receptor action in yeast provides a general approach for analyzing the mechanism of signaling by the nuclear receptor family and may facilitate identification of non-receptor factors that participate in this process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Fikes J. D., Guarente L. A cDNA encoding a human CCAAT-binding protein cloned by functional complementation in yeast. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1968–1972. doi: 10.1073/pnas.88.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Dalman F. C., Sanchez E. R., Pratt W. B. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989 Mar 25;264(9):4992–4997. [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Sharp P. A., Guarente L. Function of a yeast TATA element-binding protein in a mammalian transcription system. Nature. 1988 Jul 7;334(6177):37–42. doi: 10.1038/334037a0. [DOI] [PubMed] [Google Scholar]
- Byravan S., Milhon J., Rabindran S. K., Olinger B., Garabedian M. J., Danielsen M., Stallcup M. R. Two point mutations in the hormone-binding domain of the mouse glucocorticoid receptor that dramatically reduce its function. Mol Endocrinol. 1991 Jun;5(6):752–758. doi: 10.1210/mend-5-6-752. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J., Strömstedt P. E., Persson B., Cederlund E., Gustafsson J. A., Jörnvall H. Identification of hormone-interacting amino acid residues within the steroid-binding domain of the glucocorticoid receptor in relation to other steroid hormone receptors. J Biol Chem. 1988 May 15;263(14):6842–6846. [PubMed] [Google Scholar]
- Chakraborti P. K., Garabedian M. J., Yamamoto K. R., Simons S. S., Jr Creation of "super" glucocorticoid receptors by point mutations in the steroid binding domain. J Biol Chem. 1991 Nov 25;266(33):22075–22078. [PubMed] [Google Scholar]
- Cormack B. P., Strubin M., Ponticelli A. S., Struhl K. Functional differences between yeast and human TFIID are localized to the highly conserved region. Cell. 1991 Apr 19;65(2):341–348. doi: 10.1016/0092-8674(91)90167-w. [DOI] [PubMed] [Google Scholar]
- Dalman F. C., Bresnick E. H., Patel P. D., Perdew G. H., Watson S. J., Jr, Pratt W. B. Direct evidence that the glucocorticoid receptor binds to hsp90 at or near the termination of receptor translation in vitro. J Biol Chem. 1989 Nov 25;264(33):19815–19821. [PubMed] [Google Scholar]
- Dalman F. C., Scherrer L. C., Taylor L. P., Akil H., Pratt W. B. Localization of the 90-kDa heat shock protein-binding site within the hormone-binding domain of the glucocorticoid receptor by peptide competition. J Biol Chem. 1991 Feb 25;266(6):3482–3490. [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Picard D., Yamamoto K. R., Bishop J. M. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989 Jul 6;340(6228):66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gametchu B., Harrison R. W. Characterization of a monoclonal antibody to the rat liver glucocorticoid receptor. Endocrinology. 1984 Jan;114(1):274–279. doi: 10.1210/endo-114-1-274. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Gill G., Tjian R. A highly conserved domain of TFIID displays species specificity in vivo. Cell. 1991 Apr 19;65(2):333–340. doi: 10.1016/0092-8674(91)90166-v. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Picard D. Steroid receptors. How to be both a receptor and a transcription factor. Biochem Pharmacol. 1989 Oct 1;38(19):3135–3143. doi: 10.1016/0006-2952(89)90605-9. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Picard D., Yamamoto K. R. Signal transduction and transcriptional regulation by glucocorticoid receptor-LexA fusion proteins. Science. 1988 Aug 12;241(4867):812–816. doi: 10.1126/science.3043662. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Rusconi S., Miesfeld R., Yamamoto K. R. Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature. 1987 Jan 22;325(6102):365–368. doi: 10.1038/325365a0. [DOI] [PubMed] [Google Scholar]
- Hope T. J., Huang X. J., McDonald D., Parslow T. G. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: mapping cryptic functions of the arginine-rich motif. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7787–7791. doi: 10.1073/pnas.87.19.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K. J., Distelhorst C. W. Evidence for intracellular association of the glucocorticoid receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Mar 5;263(7):3474–3481. [PubMed] [Google Scholar]
- Howard K. J., Holley S. J., Yamamoto K. R., Distelhorst C. W. Mapping the HSP90 binding region of the glucocorticoid receptor. J Biol Chem. 1990 Jul 15;265(20):11928–11935. [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Léopold P., O'Farrell P. H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991 Sep 20;66(6):1207–1216. doi: 10.1016/0092-8674(91)90043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak P., McDonnell D. P., Weigel N. L., Schrader W. T., O'Malley B. W. Expression of functional chicken oviduct progesterone receptors in yeast (Saccharomyces cerevisiae). J Biol Chem. 1989 Dec 25;264(36):21613–21618. [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- Miller N. R., Simons S. S., Jr Steroid binding to hepatoma tissue culture cell glucocorticoid receptors involves at least two sulfhydryl groups. J Biol Chem. 1988 Oct 15;263(29):15217–15225. [PubMed] [Google Scholar]
- Moguilewsky M., Raynaud J. P. Evidence for a specific mineralocorticoid receptor in rat pituitary and brain. J Steroid Biochem. 1980 Jan;12:309–314. doi: 10.1016/0022-4731(80)90285-x. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lerman L. S., Maniatis T. A general method for saturation mutagenesis of cloned DNA fragments. Science. 1985 Jul 19;229(4710):242–247. doi: 10.1126/science.2990046. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Denis M., Gustafsson J. A. The transformed glucocorticoid receptor has a lower steroid-binding affinity than the nontransformed receptor. Biochemistry. 1990 Feb 20;29(7):1880–1886. doi: 10.1021/bi00459a031. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Picard D., Schena M., Yamamoto K. R. An inducible expression vector for both fission and budding yeast. Gene. 1990 Feb 14;86(2):257–261. doi: 10.1016/0378-1119(90)90287-2. [DOI] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt W. B., Jolly D. J., Pratt D. V., Hollenberg S. M., Giguere V., Cadepond F. M., Schweizer-Groyer G., Catelli M. G., Evans R. M., Baulieu E. E. A region in the steroid binding domain determines formation of the non-DNA-binding, 9 S glucocorticoid receptor complex. J Biol Chem. 1988 Jan 5;263(1):267–273. [PubMed] [Google Scholar]
- Privalsky M. L., Sharif M., Yamamoto K. R. The viral erbA oncogene protein, a constitutive repressor in animal cells, is a hormone-regulated activator in yeast. Cell. 1990 Dec 21;63(6):1277–1286. doi: 10.1016/0092-8674(90)90423-c. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Schmit J. P. Structure-activity relationships for glucocorticoids-I. Determination of receptor binding and biological activity. J Steroid Biochem. 1977 Sep;8(9):911–919. doi: 10.1016/0022-4731(77)90187-x. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Yamamoto K. R. Functional dissection of the hormone and DNA binding activities of the glucocorticoid receptor. EMBO J. 1987 May;6(5):1309–1315. doi: 10.1002/j.1460-2075.1987.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Schena M., Freedman L. P., Yamamoto K. R. Mutations in the glucocorticoid receptor zinc finger region that distinguish interdigitated DNA binding and transcriptional enhancement activities. Genes Dev. 1989 Oct;3(10):1590–1601. doi: 10.1101/gad.3.10.1590. [DOI] [PubMed] [Google Scholar]
- Schena M., Picard D., Yamamoto K. R. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 1991;194:389–398. doi: 10.1016/0076-6879(91)94029-c. [DOI] [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Scherrer L. C., Dalman F. C., Massa E., Meshinchi S., Pratt W. B. Structural and functional reconstitution of the glucocorticoid receptor-hsp90 complex. J Biol Chem. 1990 Dec 15;265(35):21397–21400. [PubMed] [Google Scholar]
- Simons S. S., Jr, Pumphrey J. G., Rudikoff S., Eisen H. J. Identification of cysteine 656 as the amino acid of hepatoma tissue culture cell glucocorticoid receptors that is covalently labeled by dexamethasone 21-mesylate. J Biol Chem. 1987 Jul 15;262(20):9676–9680. [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B., Johnson D. F. Anti-inflammatory pyrazolo-steroids: potent glucocorticoids containing bulky A-ring substituents and no C3-carbonyl. Biochem Biophys Res Commun. 1979 Feb 14;86(3):792–800. doi: 10.1016/0006-291x(79)91782-0. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G., Bergers G., Picard D., Busslinger M. Hormone-dependent transcriptional regulation and cellular transformation by Fos-steroid receptor fusion proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5114–5118. doi: 10.1073/pnas.88.12.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Vanderbilt J. N., Miesfeld R., Maler B. A., Yamamoto K. R. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987 Jan;1(1):68–74. doi: 10.1210/mend-1-1-68. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Allan G. F., Schrader W. T., Tsai M. J., McDonnell D. P., O'Malley B. W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992 May 15;69(4):703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- Wright A. P., Carlstedt-Duke J., Gustafsson J. A. Ligand-specific transactivation of gene expression by a derivative of the human glucocorticoid receptor expressed in yeast. J Biol Chem. 1990 Sep 5;265(25):14763–14769. [PubMed] [Google Scholar]
- Yamamoto K. R., Godowski P. J., Picard D. Ligand-regulated nonspecific inactivation of receptor function: a versatile mechanism for signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):803–811. doi: 10.1101/sqb.1988.053.01.091. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S. K., Yamamoto K. R. Signaling and regulation by a mammalian glucocorticoid receptor in Drosophila cells. Mol Endocrinol. 1991 Jun;5(6):844–853. doi: 10.1210/mend-5-6-844. [DOI] [PubMed] [Google Scholar]




