Abstract
This report describes the molecular epidemiology, in vitro susceptibility, colonial and microscopic morphologies, and biochemical features of Trichosporon mycotoxinivorans, a newly recognized pathogen that appears to have a propensity for patients with cystic fibrosis. The index patient died with histologically documented Trichosporon pneumonia complicating cystic fibrosis. This is also the first report of disease caused by a Trichosporon species in a nontransplant patient with cystic fibrosis. As T. mycotoxinivorans has not previously been recognized as a respiratory pathogen, the significance of its recovery from sputum samples was not initially appreciated. Genetic analysis of archived clinical samples found three additional cases of T. mycotoxinivorans infection which had previously been identified as other members of the genus. An additional isolate of T. mycotoxinivorans was identified from a clinical sample on initial testing. Three of these four cases were also patients with cystic fibrosis. All isolates had MICs at 48 h of amphotericin B of ≥1 μg/ml and of echinocandins of ≥16 μg/ml, but they displayed various susceptibilities to the triazoles. In summary, Trichosporon mycotoxinivorans is a newly recognized human pathogen that is associated with cystic fibrosis.
Trichosporon species are uncommon but potentially life-threatening causes of localized and disseminated infections in immunocompromised patients. The most common species causing deep-seeded disseminated infection that has been recognized is Trichosporon asahii (11). Other species of Trichosporon are seldom reported as causes of deep-seeded infections. Herein, we describe the first case of human disease caused by Trichosporon mycotoxinivorans. Previously recognized as an industrial source for detoxification of mycotoxins, T. mycotoxinivorans has not been reported to cause disease in humans (20, 28). Additional testing of archived isolates of the genus Trichosporon at the University of Texas San Antonio Fungus Testing Laboratory (FTL) revealed three additional cases, including two in patients with cystic fibrosis. These isolates had previously been classified under other species of Trichosporon on the basis of their biochemical profiles alone. A newly acquired sample in the FTL collection, from a cystic fibrosis patient, was also analyzed and identified as T. mycotoxinivorans. This report describes the molecular epidemiology, in vitro susceptibility, colonial and microscopic morphologies, and biochemical features of T. mycotoxinivorans, a newly recognized pathogen that appears to have a propensity for patients with cystic fibrosis.
Index case.
A 20-year-old male with a history of cystic fibrosis presented to the emergency department complaining of acute onset of dyspnea. He was found to have a right-sided pneumothorax. He was treated with supplemental oxygen, a chest tube was placed, and he was then admitted to the ward for supportive care. His recent medical history was notable for receiving home therapy with piperacillin-tazobactam and trimethoprim-sulfamethoxazole, initiated 1 week earlier as an inpatient for an exacerbation of his underlying chronic lung infection from cystic fibrosis. His most recent preadmission sputum cultures were positive for methicillin-resistant Staphylococcus aureus and Candida albicans. His history showed that a sputum sample had grown multidrug-resistant Burkholderia cepacia. His chronic medications included nebulized tobramycin, nebulized dornase alpha, nebulized DNase (pulmozyme), oral pancrelipase, oral azithromycin, and inhaled fluticasone propionate (250 μg) plus salmeterol (50 μg) as one inhalation twice daily. During the year leading up to this admission his forced expiratory volume in the first second (FEV1) had declined from a baseline of 58% of that predicted (14) to 47% of that predicted at 3 months prior to admission and to 24% of that predicted at the most recent admission. Due to the severe drop in FEV1, in addition to antibiotics, he was treated with prednisone at 80 mg once daily for 3 days leading up to the final admission. The patient's nutritional status had been stable but compromised in recent years, and his body mass index was less than the third percentile for his age.
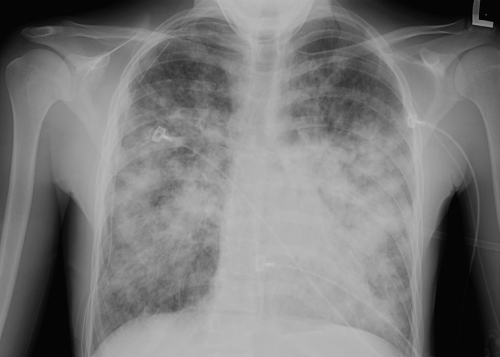
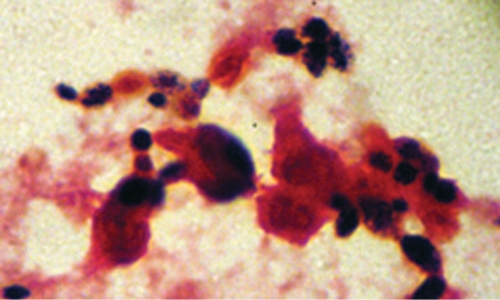
Initial management for the readmission focused on pulmonary toilet, and his medications were not changed. His pulmonary function worsened over the next 2 days, as evidenced by increasing supplementary oxygen requirements, and he was transferred to the pediatric intensive care unit. Vancomycin and intravenous tobramycin were added to the regimen, and trimethoprim-sulfamethoxazole was discontinued. Chest radiographs during this time worsened from the small residual right-sided pneumothorax status noted after placement of the chest tube to infiltrates in the right middle and right lower lobes and the left lower lung fields (Fig. 1). On the following day, his condition worsened and he required intubation. He subsequently became febrile and had multiple fevers each day, with his temperatures ranging from 38.3°C to 40°C. Gram stains of sputum from the time of admission were observed by the use of a high-powered microscope under conditions of oil immersion (Fig. 2) and showed no bacteria and >30 budding yeasts and >30 polymorphonuclear leukocytes per field. The microbiology culture results from the respiratory tract over the course of the hospitalization are summarized in Table 1.
FIG. 1.
Hospital day 3 chest X ray showing residual right-sided pneumothorax and bilateral pneumonia.
FIG. 2.
Gram stain of sputum showing budding yeast and polymorphonuclear cells.
TABLE 1.
Respiratory microbiology results for index case
| Hospital day | Organism(s) isolateda | Source |
|---|---|---|
| 1 | 4+ Trichosporon | Sputum |
| 2 | 4+ Trichosporon, 2+ MRSA | Sputum |
| 2 | Influenza virus/parainfluenza virus/adenovirus/respiratory syncytial virus culture negative | Nasopharyngeal wash |
| 3 | 4+ Trichosporon | Bronchoalveolar lavage |
| 5 | 4+ Trichosporon | Tracheal aspirate |
| 6 | 4+ Trichosporon, 3+ C. albicans | Tracheal aspirate |
| 10 | 4+ Trichosporon, 3+ MRSA | Tracheal aspirate |
| 10 | AFB negative | Tracheal aspirate |
| 11 | 4+ Trichosporon, 3+ MRSA | Tracheal aspirate |
| 12 | 3+ Trichosporon | Right lower lung, autopsy |
| 12 | 4+ Trichosporon | Left lower lung, autopsy |
| 12 | 4+ Trichosporon | Right middle lung, autopsy |
| 12 | Negative bacterial, AFB, and viral cultures | All lung fields, autopsy |
MRSA, methicillin-resistant Staphylococcus aureus; AFB, acid-fast bacillus; 2+, >10 colonies observed in quadrant 1 of the culture plate; 3+, >10 colonies observed in quadrant 2 of the culture plate; 4+, >10 colonies observed in quadrant 3 of the culture plate.
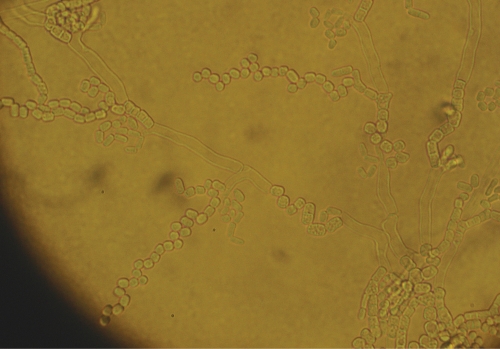
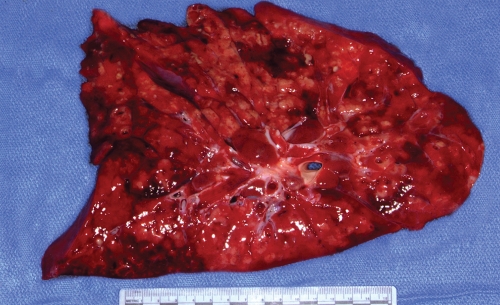
Liposomal amphotericin B at a dosage of 5 mg/kg of body weight/day was initiated on hospital day 4. On hospital day 6, the yeast was identified as a Trichosporon species on the basis of microscopic morphology and the biochemical testing results obtained with the API 20 C AUX system (API code, 6747776). Isolates grown on cornmeal agar consisted of dry white colonies that microscopically demonstrated true hyphae and consecutive arthroconidia (Fig. 3). “Hockey stick” arthroconidia suggestive of Geotrichum species were not seen. Fusiform giant cells also were not observed. The liposomal amphotericin B therapy was stopped, and voriconazole was started due to concerns of intrinsic drug resistance. His course in the intensive care unit was further complicated by tension pneumothorax and cardiac arrest. Because a poor functional outcome was predicted by serial neurological examinations, imaging of the central nervous system, and electroencephalography findings, the family withdrew medical support on hospital day 11. A partial autopsy was performed and revealed diffuse hemorrhagic and suppurative consolidation of the lungs with chronic bronchiectatic changes (Fig. 4). Cultures of tissue from each lobe collected postmortem grew Trichosporon species, and histology demonstrated diffuse infiltration of the lung parenchyma with budding yeasts (Fig. 5). A reference laboratory identified the organism as Trichosporon loubieri on the basis of morphology and growth characteristics. Additional cultures found no evidence of acid-fast organisms, respiratory viruses, or bacteria. A PCR for human immunodeficiency virus RNA was negative.
FIG. 3.
Trichosporon species on cornmeal agar.
FIG. 4.
Postmortem specimen of lung showing diffuse hemorrhagic and suppurative consolidation with bronchiectatic changes suggestive of an acute infection superimposed on a chronic infection and cystic fibrosis-related changes.
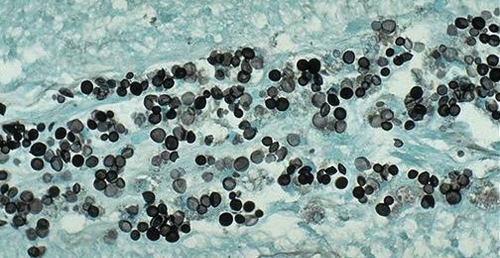
FIG. 5.
Postmortem lung biopsy specimen showing fungal elements. Gomori-methenamine silver stain. Magnification, ×100.
MATERIALS AND METHODS
Patients and specimens.
The same case isolate originally identified as T. loubieri was subsequently referred to FTL, Department of Pathology, University of Texas Health Science Center at San Antonio, for further characterization and was accessioned into its culture collection as UTHSC R-3912. Five archived clinical isolates of the genus Trichosporon which had been previously categorized as other members of the genus on the basis of their biochemical profiles and a subsequently acquired sample in the FTL collection were also recovered and analyzed.
Morphological identification.
Routine identification of isolates consisted of determination of the assimilation patterns by using the API 20C yeast identification system (bioMérieux, St. Louis, MO) and determination of microscopic morphology on cornmeal agar (Difco, Sparks, MD), temperature tolerance, and susceptibility to cycloheximide (Mycobiotic agar; Remel, Lenexa, KS).
Molecular identification.
All cultures were grown and maintained on YPD agar (2% dextrose, 2% peptone, 1% yeast extract, 2% agar) until they were used for analysis. Approximately 1 × 106 yeast cells were obtained from a YPD agar plate grown for 24 h at 30°C and inoculated into 50 μl of Prepman Ultra reagent (Applied Biosystems, Inc., Foster City, CA). The cells were then lysed, according to the manufacturer's instructions. PCR was performed in a 0.5-ml microcentrifuge tube with 5 μl of the Prepman supernatant. A 50-μl reaction volume was prepared with Triple Master Taq DNA polymerase (Eppendorf/Brinkmann Instruments, Inc., Westbury, NY), according to the manufacturer's instructions. Two primer pairs were used to amplify two regions of the ribosomal repeat from the genomic DNA template. The internal transcribed spacer (ITS) region (ITS1-5.8S-ITS2) was amplified by using primer ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and primer ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), as described previously (33) The D1/D2 region of the large ribosomal DNA subunit was amplified with primers NL-1 (5′-GCATATCAATAAGCGGAGGAAAAG-3) and NL-4 (5′-GGTCCGTGTTTCAAGACGG-3), as described previously (17). All PCRs were performed in a PTC-100 thermocycler (MJ Research, Watertown, MA) by using the preprogrammed three-step protocol as the standard program for all reactions. The annealing temperature for both primer pairs was 58°C, and 30 cycles with a 1-min extension time were used to prepare the amplicons. The PCR products were electrophoresed through a 0.7% agarose gel to confirm amplification. The remaining template DNA was then cleaned with a QIAquick PCR purification kit (Qiagen, Inc., Valencia, CA). Sequencing of both strands was performed at the University of Texas Health Science Center at San Antonio Advanced Nucleic Acids Core Facility with all four PCR primers as the sequencing primers. Both the ITS and the D1/D2 sequences were used to perform nucleotide-nucleotide searches by use of the BLASTn algorithm at the NCBI website (http://www.ncbi.nlm.nih.gov/BLAST/). Identifications were made when the BLAST searches yielded ≥98% identity.
In vitro susceptibility studies.
Antifungal susceptibility testing was accomplished by the method outlined in NCCLS document M27-A2 (23). Amphotericin B (Bristol-Myers Squibb, New York, NY), caspofungin (Merck, Rahway, NJ), anidulafungin (Pfizer, Inc., New York, NY), and micafungin (Astellas, Deerfield, IL) were tested in antibiotic medium 3 (Difco), while flucytosine (Valient, Irvine, CA), fluconazole (Pfizer, Inc.), itraconazole and ketoconazole (Janssen Pharmaceutica, Piscataway, NJ), posaconazole (Schering Plough, Galloping Hill, NJ), and voriconazole (Pfizer, Inc.) were tested in RPMI 1640 medium (Hardy Diagnostics, Santa Maria, CA). The microtiter plates were incubated at 35°C, and the MICs were read at both 24 and 48 h. The endpoint for amphotericin and the echinocandins was the lowest concentration that inhibited visual growth, while the endpoint for flucytosine and the azoles was the lowest concentration that resulted in a 50% or greater reduction in turbidity compared to that of the drug-free control tube.
Nucleotide sequence accession numbers.
The ITS and the D1/D2 sequences were submitted to GenBank under accession numbers EU118973 to EU118984 and FJ416595 to FJ416596, respectively.
RESULTS
Morphological identification.
The morphological and molecular identities of seven isolates from five patients are summarized in Table 2 and are most consistent with T. mycotoxinivorans. All isolates assimilated glucose, glycerol, 2-keto-d-gluconate, arabinose, xylose, galactose, inositol, methyl-d-glucoside, N-acetyl-glucosamine, maltose, sucrose, trehalose, and raffinose. All but one isolate assimilated lactose, and one isolate assimilated cellobiose. All isolates were resistant to cycloheximide, as evidenced by growth on mycobiotic agar. The case isolate has been deposited in the University of Alberta Mold Herbarium, Edmonton, Alberta, Canada, and is accessioned as UAMH 10833.
TABLE 2.
Morphological and molecular identification of seven isolates from five patients
| Patient no. | FTL no. | GenBank accession no. | API code | Mycosel result | Growth at 42°C | Molecular identification |
|---|---|---|---|---|---|---|
| 1a,b | R-3912 | EU118979 | 6745776 | Posc | Pos | T. mycotoxinivorans |
| 2b | 07-301 | EU118980 | 6747776 | Pos | Pos | T. mycotoxinivorans |
| 2b | 07-302 | EU118981 | 6745776 | Pos | Pos | T. mycotoxinivorans |
| 2b | 07-303 | EU118982 | 6745376 | Pos | Negd | T. mycotoxinivorans |
| 3 | 07-384 | EU118983 | 6745376 | Pos | Weake | T. mycotoxinivorans |
| 4b | 07-497 | EU118984 | 6747776 | Pos | Weak | T. mycotoxinivorans |
| 5b | 08-288 | FJ416596 | 6747776 | Pos | Pos | T. mycotoxinivorans |
From a patient with cystic fibrosis.
From the index case patient.
Pos, positive.
Neg, negative.
Weak, weakly positive.
Molecular identification.
The BLASTn search results suggested that the isolates were most closely related to Trichosporon mycotoxinivorans (Table 2). The ITS sequences of all isolates were most closely related to the ITS sequences of two Trichosporon mycotoxinivorans isolates (strains HB 1175 and TU-GM8, GenBank accession nos. AJ601389 and DQ325457, respectively) at 99% identity and an isolate of T. loubieri (strain CBS 7065, GenBank accession no. AB018027) at 98% identity. The D1/D2 sequences of the isolates were most closely related to the D1/D2 sequence of an isolate of T. mycotoxinivorans (strains HB 1175, GenBank accession no. AJ601388) at 99% identity and two isolates of T. loubieri (strains CBS 7065 and IFM 53857, GenBank accession nos. AF075522 and AB186489, respectively) at 98% identity. Strain HB 1175 is the type culture from which the original description of the species T. mycotoxinivorans was described (20).
In vitro susceptibility.
Retrospective in vitro antifungal susceptibility testing data for the index case isolate and the six additional strains identified as T. mycotoxinivorans against 10 antifungal agents are displayed in Table 3. The MICs at 48 h of amphotericin B ranged from 1 to 4 μg/ml, while those for the echinocandins caspofungin, micafungin, and anidulafungin were ≥16 μg/ml; but the isolates displayed various susceptibilities to the triazoles.
TABLE 3.
MIC and time of MIC calculation
| FTL no. | MIC (μg/ml) at the indicated times (h) of MIC calculationc
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB
|
CAS
|
MICA
|
ANID
|
5FC
|
FLU
|
ITRA
|
KETO
|
VORI
|
POSA
|
|||||||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| R-3912a,b | 0.25 | 1 | 16 | 16 | >16 | >16 | >16 | >16 | 4 | 8 | 0.5 | 0.5 | 0.03 | 0.06 | 0.25 | 0.5 | 0.03 | 0.03 | <0.015 | <0.015 |
| 07-301b | 0.5 | 4 | 16 | >16 | >16 | >16 | >16 | >16 | 32 | >64 | 64 | >64 | 0.125 | 1 | 2 | 8 | >8 | >8 | 0.25 | 0.5 |
| 07-302b | 0.25 | 1 | 16 | 16 | >16 | >16 | >16 | >16 | 8 | 32 | 32 | 32 | 0.125 | 1 | 4 | 8 | 4 | 8 | 0.25 | 1 |
| 07-303b | 0.5 | 2 | 8 | 16 | >16 | >16 | 16 | 16 | 16 | 64 | 64 | 64 | 0.03 | 0.06 | 0.125 | 0.5 | 2 | 2 | 0.03 | 0.06 |
| 07-384 | 0.5 | 2 | 16 | 16 | >16 | >16 | >16 | >16 | 16 | 32 | 1 | 1 | 0.06 | 0.06 | 0.25 | 0.25 | 0.06 | 0.06 | <0.015 | <0.015 |
| 07-497b | 0.5 | 2 | 16 | >16 | >16 | >16 | >16 | >16 | 16 | 64 | 2 | 2 | 0.06 | 0.5 | 0.25 | 0.5 | 0.06 | 0.125 | <0.015 | 0.125 |
| 08-2888b | 0.125 | 1 | 16 | >16 | >16 | >16 | >16 | >16 | 8 | 32 | 0.5 | 2 | 0.06 | 0.25 | 0.5 | 0.5 | 0.03 | 0.06 | ≤0.015 | 0.125 |
From a patient with cystic fibrosis.
From the index case patient.
AMB, amphotericin B, CAS, caspofungin; MICA, micafungin; ANID, anidulafungin; 5-FC, fluconazole; FLU, flucytosine; ITRA, itraconazole; KETO, ketoconazole; VORI, voriconazole; POSA, posaconazole.
DISCUSSION
The case described here represents the first reported case of T. mycotoxinivorans disease in a human and is the first case of Trichosporon species pneumonia in a nontransplant cystic fibrosis patient. Trichosporon is a known cause of pneumonia in immunocompromised patients (32). This patient had histologically documented Trichosporon pneumonia from postmortem examination, hence fulfilling the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria for proven invasive fungal infection (6). That the isolate was initially identified as T. loubieri prompted further investigation to include the genetic analysis, which revealed its true identity. A review of the archives of FTL and five isolates from clinical specimens most closely resembling Trichosporon ovoides but with some significant variations in morphology and physiologic features unexpectedly revealed that all were T. mycotoxinivorans when they were characterized by ITS and D1/D2 sequencing. Because only a single IGS1 (intergenic spacer 1 region) GenBank record existed for both T. mycotoxinivorans and T. loubieri, we considered that the use of the more common ITS and D1/D2 regions would be more accurate. Although the match cutoff was >98% identity in order for a sequence to be considered conspecific and two species in the ITS and D1/D2 outputs that met this criterion were found (T. mycotoxinivorans and T. loubieri), there were multiple matches and they were consistently divided by species, with the T. mycotoxinivorans records consistently showing the higher degree of identity (99% versus 98%). Importantly, the sequences from the type culture also showed a higher level of identity correlating with the sequences from the isolates described in this report for both loci.
Four of these isolates (three from the same patient, each of which displayed different macroscopic morphologies) were from cystic fibrosis patients. An additional isolate from a cystic fibrosis patient, analyzed for the first time, was also identified as T. mycotoxinivorans. Clinical information from these three patients with cystic fibrosis was not available for review. A comment on T. loubieri bears note. T. loubieri is an uncommon pathogen that has previously been reported as a cause of human disease in only two other cases. The first case, reported by Padhye et al., describes a 45-year-old man with a history of polycystic kidney disease and end-stage renal disease (24). Cultures of tissue from the left kidney following nephrectomy were performed, and the cultures grew T. loubieri. He was successfully treated with a combination of amphotericin B and oral itraconazole. The second case, reported by Marty et al., describes a 56-year-old woman with pre-B-cell lymphoblastic leukemia who developed disseminated T. loubieri infection following induction chemotherapy (19). She was treated with oral fluconazole; however, she was unable to proceed with consolidation chemotherapy and soon died. Identification of the organism in both cases was confirmed by ribosomal DNA analysis. Mention of the isolation of T. loubieri from the sputum of a cystic fibrosis patient, with unclear clinical relevance, was made in the Newsletter of the Australasian Federation of Medical and Veterinary Mycology, but no further clinical details were provided (34). The rarity of this organism and its potential association with other cystic fibrosis patients prompted the more detailed investigation described here.
The taxonomy of the genus Trichosporon underwent a significant revision in 1992 on the basis of the molecular analysis of reference isolates, resulting in a schema that included six species, T. asahii, T. mucoides, T. inkin, T. ovoides, T. cutaneum, and T. asteroides, believed to be medically relevant (12). The first two species in this list were grouped into the ecological niche of isolates causing systemic human infections, while the last four species were given the designation of isolates causing superficial infections. Invasive Trichosporon species, predominantly known in earlier literature as T. beigelli, have most commonly been identified as causes of disease in immunocompromised patients receiving chemotherapy or chronic systemic corticosteroids; they have also occasionally been isolated from burn patients and premature neonates. As reflected by more recently published reports, the preponderance of these isolates likely represent T. asahii and T. mucoides, which are associated with invasive disease in immunocompromised and, rarely, immunocompetent patients (2, 7, 13, 21). In contrast, T. ovoides, T. cutaneum, T. inkin, and T. asteroides (now called T. jirovici) are more commonly associated with superficial infections such as white piedra of the scalp or pubic hair (T. inkin and T. ovoides) and dermatophytic infections (T. inkin and T. jirovici) and are only rarely associated with central venous line infections or disseminated disease in the immunocompromised host (5, 16). T. pullulans bears morphological similarities to the other Trichosporon species; however, it is phylogenetically distinct and should be classified elsewhere. Furthermore, some experts believe that the inability to grow at temperatures greater than 20°C functionally excludes its role as a pathogen and that reported cases (18, 22, 29) of T. pullulans disease are in fact caused by other species that were misidentified due to a reliance on morphology and biochemical testing alone and not genetic testing (3).
Trichosporon mycotoxinivorans was first described in 2004 by Molnar et al. after isolation of the yeast from the hind gut of a termite (20). Genetic analysis revealed that its ITS sequence had 99.3% homology with the ITS sequence of T. loubieri; however, for the IGS1 sequences, the degree of homology was 66.7%. Further study of this yeast revealed that it has the ability to detoxify mycotoxins, such as ochratoxin A and zearalenone, hence the species name, which derives from Latin and which means “mycotoxin devouring.” Molnar et al. speculated that this ability would have application in veterinary feeds as a way of detoxifying contaminating mycotoxins (20). This hypothesis was tested by challenging chicks fed a diet containing T. mycotoxinivorans with ochratoxin A. Those chicks avoided the immunosuppressive effects experienced by the chicks in the control group and had an improved feed-to-weight gain ratio (25). In vitro studies with a pig intestine model and ochratoxin A challenge demonstrated that treatment with T. mycotoxinivorans resulted in the clearance of mycotoxin at a rate comparable to or faster than that of the bacterial comparators Stenotrophomonas sp. (90% deactivation) and Eubacterium sp. (70% deactivation) at 6 h of incubation (28).
While molecular diagnostic methods, as detailed above, are able to accurately delineate the taxonomic identity of this organism, the availability and timeliness of these resources are limited.
For clinical microbiologists, growth characteristics and microscopic features can help suggest the identity. On glucose-yeast extract-peptone medium, T. mycotoxinivorans forms cream to tan mucoid colonies with few white aerial mycelia (20). It forms true hyphae which disarticulate into arthroconidia. Cells range in size from 3 to 7 by 4 to 29 μm. By comparison, T. loubieri is characterized by the formation of fusiform giant cells. Although enlarged cells were noted in this isolate that separated it from other Trichosporon species, the cells were notably smaller than those seen for T. loubieri.
T. mycotoxinivorans is urease positive, grows on cycloheximide medium, and reliably assimilates a variety of carbohydrates, including rhamnose, which helps distinguish it from some of the other Trichosporon species and the genus Geotrichum (10, 11) but not from T. loubieri. Growth characteristics which differentiate the species from T. loubieri, as originally described by Molnar et al., are the inability to grow at or above 40°C and assimilation of inulin and galactitol (20). In reviewing our isolates, however, the growth temperature maximums appeared to be variable, as four isolates grew well at 42°C, including the case isolate, two demonstrated weak growth at this temperature, and one was negative for growth at this temperature (Table 2). Inulin and galactitol are not included in the API 20C systems, and so tests for the assimilation of inulin and galactitol were not performed.
This case highlights several potential pitfalls for clinicians and laboratory personnel confronting a similar case. First, correct identification of the organism is complicated by inconsistent results on standard biochemical testing and the various morphologies of species within the genus (26). Indeed, the extent to which the organism might be evaluated can be called into question, particularly if light growth is identified, given that Candida species are commonly cultured from the sputum of cystic fibrosis patients, particularly those receiving inhaled aminoglycosides, but are generally not thought to be pathogenic (4). Molds, particularly Aspergillus spp., are commonly recovered from patients with cystic fibrosis, but they are of significance only in specific clinical situations, such as after transplantation or in patients with allergic bronchopulmonary aspergillosis (27). Although the cystic fibrosis patient described here was receiving chronic inhaled steroids supplemented by a short 3-day systemic course, in the absence of a central line or past solid organ transplant, this patient would not typically fall into a category considered at risk of invasive fungal disease. The combination of a low prediagnosis suspicion and the intrinsic delay in establishing a genus-specific diagnosis can result in a clinically significant delay in both the initiation of pathogen-specific antifungal therapy and the empirical selection of effective agents. The interaction of the effects of corticosteroid treatment, chronically impaired pulmonary function, and malnutrition on the pathogenicity of this organism bears further study. Each of these factors likely played an important role in the patient's demise. Nevertheless, the overwhelming involvement of lung tissue (documented histologically) indicates that the likely primary cause of death was Trichosporon pneumonia.
Amphotericin B and lipid formulations as well as the echinocandins have limited efficacy against Trichosporon species. Triazoles are considered the therapeutic class of choice on the basis of in vitro data, animal models, and individual case descriptions (1, 2, 8, 9, 15, 30, 31). Among the limited options among the antifungal triazoles, voriconazole may be preferred on the basis of its intravenous and oral formulations, as well as its bioavailability. Fluconazole is another option; however, the maximum concentration in plasma/MIC ratio of voriconazole appears to be higher than that of fluconazole. Itraconazole and posaconazole are other options, but their bioavailabilities in seriously ill patients may be uncertain.
The case described here demonstrates that cystic fibrosis patients, particularly those compromised by poor lung function, a high-risk nutritional-metabolic status, and, possibly, the use of corticosteroids, may be at risk of uncommon invasive fungal diseases, as exemplified by the T. mycotoxinivorans disease described here. Vigilance and a high index of suspicion should be maintained when fungal isolates from cultures of sputum from symptomatic cystic fibrosis patients are evaluated. Trichosporon mycotoxinivorans is an emerging pathogen with unique growth and morphological characteristics. To confirm its identity, however, DNA analysis may be required. The epidemiological link to cystic fibrosis patients requires further study. Patients suspected of deep infection with T. mycotoxinivorans, or any Trichosporon species for that matter, should receive empirical therapy with voriconazole pending the results of antifungal susceptibility testing. Further research into the ecological niche and the pathogen-host interactions of T. mycotoxinivorans is warranted.
Acknowledgments
The views expressed in this manuscript are those of the author and do not reflect the official policy of the U.S. Department of Army, the U.S. Department of Defense, or the U.S. government.
B.L.W. is supported by grant PR054228 from the U.S. Army Medical Research and Materiel Command, Office of Congressionally Directed Medical Research Programs. This work was supported in part by the intramural research program of the National Cancer Institute.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Anaissie, E., A. Gokaslan, R. Hachem, R. Rubin, G. Griffin, R. Robinson, J. Sobel, and G. Bodey. 1992. Azole therapy for trichosporonosis: clinical evaluation of eight patients, experimental therapy for murine infection, and review. Clin. Infect. Dis. 15:781-787. [DOI] [PubMed] [Google Scholar]
- 2.Antachopoulos, C., E. Papakonstantinou, J. Dotis, E. Bibashi, M. Tamiolaki, D. Koliouskas, and E. Roilides. 2005. Fungemia due to Trichosporon asahii in a neutropenic child refractory to amphotericin B: clearance with voriconazole. J. Pediatr. Hematol. Oncol. 27:283-285. [DOI] [PubMed] [Google Scholar]
- 3.Boekhout, T., and E. Gueho. 2003. Basidiomycetous yeasts, 2nd ed. Marcel Dekker, New York, NY.
- 4.Burns, J. L., J. M. Van Dalfsen, R. M. Shawar, K. L. Otto, R. L. Garber, J. M. Quan, A. B. Montgomery, G. M. Albers, B. W. Ramsey, and A. L. Smith. 1999. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J. Infect. Dis. 179:1190-1196. [DOI] [PubMed] [Google Scholar]
- 5.Davies, F., S. Logan, E. Johnson, and J. L. Klein. 2006. Sternal wound infection by Trichosporon inkin following cardiac surgery. J. Clin. Microbiol. 44:2657-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Pauw, B., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Munoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, and J. E. Bennett. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebright, J. R., M. R. Fairfax, and J. A. Vazquez. 2001. Trichosporon asahii, a non-Candida yeast that caused fatal septic shock in a patient without cancer or neutropenia. Clin. Infect. Dis. 33:E28-E30. [DOI] [PubMed] [Google Scholar]
- 8.Erer, B., M. Galimberti, G. Lucarelli, C. Giardini, P. Polchi, D. Baronciani, D. Gaziev, E. Angelucci, and G. Izzi. 2000. Trichosporon beigelii: a life-threatening pathogen in immunocompromised hosts. Bone Marrow Transplant. 25:745-749. [DOI] [PubMed] [Google Scholar]
- 9.Girmenia, C., L. Pagano, B. Martino, D. D'Antonio, R. Fanci, G. Specchia, L. Melillo, M. Buelli, G. Pizzarelli, M. Venditti, and P. Martino. 2005. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 43:1818-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gueho, E., G. S. de Hoog, and M. T. Smith. 1992. Neotypification of the genus Trichosporon. Antonie van Leeuwenhoek 61:285-288. [DOI] [PubMed] [Google Scholar]
- 11.Gueho, E., L. Improvisi, G. S. de Hoog, and B. Dupont. 1994. Trichosporon on humans: a practical account. Mycoses 37:3-10. [DOI] [PubMed] [Google Scholar]
- 12.Gueho, E., M. T. Smith, G. S. de Hoog, G. Billon-Grand, R. Christen, and W. H. Batenburg-van der Vegte. 1992. Contributions to a revision of the genus Trichosporon. Antonie van Leeuwenhoek 61:289-316. [DOI] [PubMed] [Google Scholar]
- 13.Kendirli, T., E. Ciftci, E. Ince, S. Oncel, N. Dalgic, H. Guriz, E. Unal, and U. Dogru. 2006. Successful treatment of Trichosporon mucoides infection with lipid complex amphotericin B and 5-fluorocytosine. Mycoses 49:251-253. [DOI] [PubMed] [Google Scholar]
- 14.Knudson, R. J., M. D. Lebowitz, C. J. Holberg, and B. Burrows. 1983. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am. Rev. Respir. Dis. 127:725-734. [DOI] [PubMed] [Google Scholar]
- 15.Kontoyiannis, D. P., H. A. Torres, M. Chagua, R. Hachem, J. J. Tarrand, G. P. Bodey, and I. I. Raad. 2004. Trichosporonosis in a tertiary care cancer center: risk factors, changing spectrum and determinants of outcome. Scand. J. Infect. Dis. 36:564-569. [DOI] [PubMed] [Google Scholar]
- 16.Koyanagi, T., N. Nishida, S. Osabe, Y. Imamura, S. Yamamoto, A. Shichiji, and Y. Nakamura. 2006. Autopsy case of disseminated Trichosporon inkin infection identified with molecular biological and biochemical methods. Pathol. Int. 56:738-743. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzman, C. P., and C. J. Robnett. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35:1216-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lestini, B. J., and J. A. Church. 2006. Trichosporon pullulans as a complication of chronic granulomatous disease in a patient undergoing immunosuppressive therapy for inflammatory bowel disease. Pediatr. Infect. Dis. J. 25:87-89. [DOI] [PubMed] [Google Scholar]
- 19.Marty, F. M., D. H. Barouch, E. P. Coakley, and L. R. Baden. 2003. Disseminated trichosporonosis caused by Trichosporon loubieri. J. Clin. Microbiol. 41:5317-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molnar, O., G. Schatzmayr, E. Fuchs, and H. Prillinger. 2004. Trichosporon mycotoxinivorans sp. nov., a new yeast species useful in biological detoxification of various mycotoxins. Syst. Appl. Microbiol. 27:661-671. [DOI] [PubMed] [Google Scholar]
- 21.Moretti-Branchini, M. L., K. Fukushima, A. Z. Schreiber, K. Nishimura, P. M. Papaiordanou, P. Trabasso, R. Tanaka, and M. Miyaji. 2001. Trichosporon species infection in bone marrow transplanted patients. Diagn. Microbiol. Infect. Dis. 39:161-164. [DOI] [PubMed] [Google Scholar]
- 22.Moylett, E. H., J. Chinen, and W. T. Shearer. 2003. Trichosporon pullulans infection in 2 patients with chronic granulomatous disease: an emerging pathogen and review of the literature. J. Allergy Clin. Immunol. 111:1370-1374. [DOI] [PubMed] [Google Scholar]
- 23.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. CLSI document M27-A2. NCCLS, Wayne, PA.
- 24.Padhye, A. A., S. Verghese, P. Ravichandran, G. Balamurugan, L. Hall, P. Padmaja, and M. C. Fernandez. 2003. Trichosporon loubieri infection in a patient with adult polycystic kidney disease. J. Clin. Microbiol. 41:479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Politis, I., K. Fegeros, S. Nitsch, G. Schatzmayr, and D. Kantas. 2005. Use of Trichosporon mycotoxinivorans to suppress the effects of ochratoxicosis on the immune system of broiler chicks. Br. Poult. Sci. 46:58-65. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Tudela, J. L., T. M. Diaz-Guerra, E. Mellado, V. Cano, C. Tapia, A. Perkins, A. Gomez-Lopez, L. Rodero, and M. Cuenca-Estrella. 2005. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob. Agents Chemother. 49:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saiman, L., and J. Siegel. 2004. Infection control in cystic fibrosis. Clin. Microbiol. Rev. 17:57-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schatzmayr, G., F. Zehner, M. Taubel, D. Schatzmayr, A. Klimitsch, A. P. Loibner, and E. M. Binder. 2006. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 50:543-551. [DOI] [PubMed] [Google Scholar]
- 29.Shigehara, K., K. Takahashi, K. Tsunematsu, H. Koba, S. Katoh, M. Asakawa, and A. Suzuki. 1991. A case of Trichosporon pullulans infection of the lung with adult T-cell leukemia. Jpn. J. Med. 30:135-137. [DOI] [PubMed] [Google Scholar]
- 30.Walsh, T. J., J. W. Lee, G. P. Melcher, E. Navarro, J. Bacher, D. Callender, K. D. Reed, T. Wu, G. Lopez-Berestein, and P. A. Pizzo. 1992. Experimental Trichosporon infection in persistently granulocytopenic rabbits: implications for pathogenesis, diagnosis, and treatment of an emerging opportunistic mycosis. J. Infect. Dis. 166:121-133. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, T. J., G. P. Melcher, M. G. Rinaldi, J. Lecciones, D. A. McGough, P. Kelly, J. Lee, D. Callender, M. Rubin, and P. A. Pizzo. 1990. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh, T. J., K. R. Newman, M. Moody, R. C. Wharton, and J. C. Wade. 1986. Trichosporonosis in patients with neoplastic disease. Medicine (Baltimore) 65:268-279. [DOI] [PubMed] [Google Scholar]
- 33.White, T. J., T. D. Bruns, S. B. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics., p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, NY.
- 34.Woodgyer, A. 2004. Trichosporon: recent developments. Mycoses Newsl. Official Newsl. Australasian Federation Med. Vet. Mycol. 9:18-21. [Google Scholar]