Abstract
Disseminated disease by Aspergillus granulosus has been reported only once previously in a cardiac transplant recipient. We report a fatal central nervous system infection in a lung transplant recipient. Key features of this species in the section Usti include growth at 37°C and large, randomly spaced aggregates of variably shaped Hülle cells.
CASE REPORT
An 18-year-old male with bronchiolitis obliterans following a bone marrow transplant and graft-versus-host disease underwent en-bloc double-lung transplantation with a tracheal anastomosis with bronchial artery revascularization in October 2006. Ten years previously he had been diagnosed with pre-B-cell acute lymphocytic leukemia. He had undergone three courses of chemotherapy with three subsequent relapses. One year prior to transplant, he underwent allogeneic bone marrow transplantation. Post-bone marrow transplant, the patient developed cytomegalovirus infection, Aspergillus terreus pneumonia, Mycobacterium avium complex (MAC) infection, and influenza A. He received antifungal treatment with voriconazole, amphotericin B lipid complex (ABLC), caspofungin, and posaconazole. MAC was treated with ethambutol, azithromycin, and levofloxacin. At the time of lung transplant, he had no known active infections.
Donor lung culture revealed methicillin-resistant Staphylococcus aureus, beta Streptococcus group F, and alpha Streptococcus. He was extubated, weaned from oxygen, and discharged on day 9 posttransplant (PT). He was readmitted on day 10 PT for seizures. A computed tomography scan of the brain revealed a subtle hypodensity in the left frontal lobe and the middle frontal gyrus, which was thought possibly to be an artifact versus an old ischemia or contusion. The seizure was attributed to tacrolimus toxicity, as his tacrolimus trough blood level was elevated at 34.8 ng/ml. During this admission, he developed respiratory distress and was intubated. Bronchoscopy on day 13 PT revealed mucosal sloughing and accumulated mucus, debris, and old blood. Bronchoalveolar lavage was sent for bacterial, viral, and fungal cultures with isolation of a Bipolaris species, which was not considered significant. After debridement, the patient was breathing easily, extubated, and discharged home the same day.
Following discharge, he developed headaches and subsequent seizures without loss of consciousness despite seizure prophylaxis. A fundoscopic exam on day 14 PT showed no signs of increased intracranial pressure. He underwent elective flexible bronchoscopy on day 16 PT, and bacterial, mycobacterium, viral, and fungal cultures from bronchoalveolar lavage fluid were unremarkable (10,000 oropharyngeal flora). On day 17 PT, he developed a low-grade fever and altered mental status. A lumbar puncture revealed 1,110 white blood cells/mm3 with 79% neutrophils, a cerebrospinal fluid (CSF) protein level of 109 mg/dl, and a CSF glucose level of 41 mg/dl, with a serum glucose level of 122 mg/dl. He was hospitalized and started on empirical intravenous vancomycin, cefotaxime, metronidazole, ABLC, and oral voriconazole. The initial CSF culture, sent for bacterial culture, India ink stain, and cryptococcal antigen, grew Staphylococcus warnari. Magnetic resonance imaging scan of his brain showed multifocal ring-enhancing lesions at the gray-white junction of both cerebral hemispheres and early ventriculitis. The initial magnetic resonance angiography showed only a slight irregularity of the vessels near some of the lesions, and the magnetic resonance venography was normal. The size and the location of the lesions did not lend themselves to an accessible brain biopsy. A computed tomography scan of the chest showed two small nodules in the left lower lobe, which were in the region of previous transbronchial biopsy sites. Septic emboli of infectious origin were suspected.
On day 55 PT, he started experiencing transient ischemic attacks, which manifested as acute aphasia and right-sided hemiparesis. Over the following month, he gradually became more neurologically impaired. As his disease progressed, posaconazole was added. Serial magnetic resonance imagings and magnetic resonance angiographies showed progression of vascular occlusion, particularly in the left internal carotid artery and distally in the left middle cerebral and posterior cerebral arteries but no significant change in the size and number of the ring-enhancing lesions. Cerebral arteriograms confirmed occlusion of the left internal carotid artery in addition to a sagittal sinus thrombus and evidence of collateral formation. As his vascular disease progressed, he developed ophthalmoplegia, significant tremor, aphasia, and worsening comprehension and communication capabilities. The occlusion of his cerebral vasculature continued to progress despite maximal antimicrobial therapy. He developed multiple areas of cerebral infarction, including the left medial temporal lobe, left caudate, and left internal capsule. An empirical course of ethambutol, clarithromycin, and levofloxacin was started day 76 PT for treatment of MAC. He had temporary halting of disease progression with courses of intravenous immunoglobulin for hypogammaglobulimemia, but this effect dissipated with subsequent doses. Antibiotics for the empirical treatment of MAC were stopped on day 83 PT. ABLC and posaconazole were continued until he was made “do not resuscitate” day 85 PT. The patient was transferred to a hospice facility in the community and died peacefully 2 days after hospital discharge on day 87 PT.
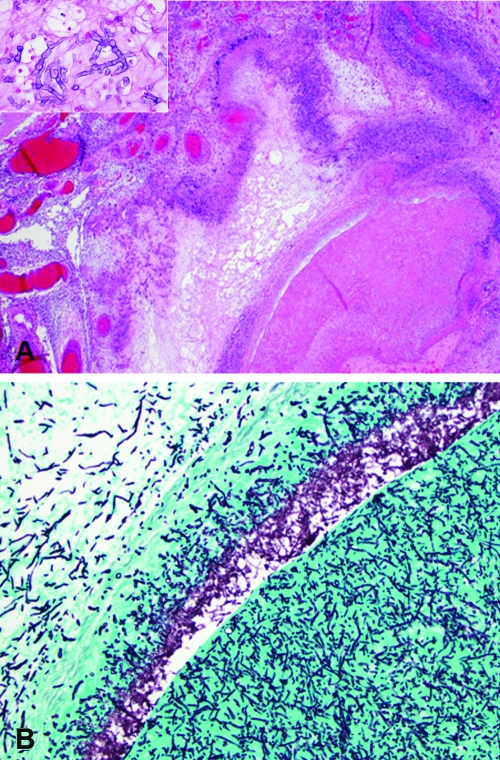
Autopsy examination of the brain showed an organizing exudate over the base of the brain and brain stem, with occlusive thrombosis of the basilar artery and left middle cerebral artery. There was left hemispheric infarction involving the frontotemporal and parietal lobes, left basal ganglia, and left thalamus, as well as severe cerebral edema with bilateral uncal herniation. The arterial thromboses were caused by an angioinvasive fungus, which extended through the arterial wall and elicited a necrotizing granulomatous reaction in the surrounding meninges (Fig. 1A and B). The nonpigmented fungal hyphae branched at acute angles and showed frequent septations, typical of Aspergillus and/or several other species (Fig. 1A, inset). Autopsy of the lungs revealed Enterobacter cloacae but no evidence of rejection or the presence of any fungal organisms, including Aspergillus terreus.
FIG. 1.
(A) The thrombosed left middle cerebral artery was surrounded by granulomatous inflammation in the meninges (hematoxylin and eosin; original magnification, ×20). (Inset) The fungal hyphae were nonpigmented and septate (hematoxylin and eosin; original magnification, ×400). (B) Silver stain highlights the fungal hyphae within the thrombus and extending through the arterial wall (Gomori methenamine silver; 100× original magnification).
Morphological and molecular characterization.
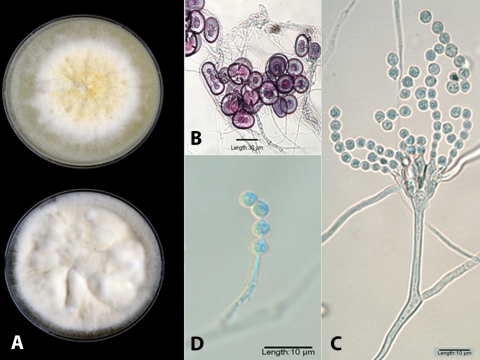
An isolate recovered from the autopsy brain stem was forwarded to the Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center at San Antonio, and accessioned into their stock collection as UTHSC 07-462. An initial tease of the isolate, tan and granular, revealed many globose to elongate Hülle cells but no fruiting heads of Aspergillus. The isolate was subcultured onto potato flakes agar (PFA), carnation leaf agar, and Czapek Dox agar (CZA), prepared in-house, and incubated at 25°C for demonstration of macroscopic and microscopic features. Slide culture preparations and temperature studies (25, 35, 40, and 45°C) were conducted on PFA. At 2 weeks (PFA at 25°C), colonies were cream to white and floccose at the periphery and buff to yellowish tan centrally, with a very slight clear exudate (Fig. 2A, top). Colonies on CZA were mostly cream, more irregularly furrowed, and exhibited reduced conidiation (Fig. 2A, bottom). The central granular areas on PFA consisted of colorless masses of thick-walled (6 to 8 μm), predominately globose but also oval to elongate to irregularly shaped Hülle cells with individual, mature cells ranging from 20 to 40 μm. The slide culture was also significant for profuse clusters of Hülle cells (Fig. 2B). Robust Aspergillus fruiting heads were sparse and were borne on long (200 to 480 μm), subhyaline to brown, smooth, thick-walled conidiophores terminating in small (12 to 18 μm wide by 15 to 25 μm long), oval to elliptical vesicles. Metulae and bottle-shaped phialides of almost equal length (3.5 to 5.5 μm) covered most of the surface of the vesicle (Fig. 2C). Conidia were pale green in mass, globose, and finely echinulate, measuring 3.5 to 5.5 μm in diameter (Fig. 2C and D). More commonly, fruiting structures were reduced in size with small, Penicillium-like vesicles or were single chains of conidia borne from solitary phialides (Fig. 2D). The isolate grew well at 25, 35, and 40°C but failed to grow at 45°C. On the basis of these features, it was tentatively identified as Aspergillus granulosus (2, 3, 8, 15, 21) and was deposited into the University of Alberta Mold Herbarium under accession no. UAMH 10935. The isolate was then further characterized by molecular sequencing under accession no. R-3921.
FIG. 2.
(A) PFA (top) and CZA (bottom) plates incubated for 14 days at 25°C. (B) Clusters of Hülle cells on the slide culture in lacto-fuchsin mounting medium. (C) Slide culture mounted in lactophenol cotton blue showing a more robust, biseriate fruiting head demonstrating metulae and bottle-shaped phialides of almost equal length. (D) Globose, finely echinulate conidia borne from a solitary phialide (lactophenol cotton blue mount).
DNA was prepared from the subcultures of R-3921 and the control type cultures, Aspergillus granulosus (NRRL 1932) and Aspergillus ustus (NRRL 275). These isolates were obtained from Clete Kurtzman, Agricultural Research Service Culture Collection, National Center for Agricultural Utilization Research, Peoria, IL. DNA isolated for each sample was prepared for PCR as previously described by Grant et al. (7).
DNA amplification by PCR was performed in 50-μl total volume reactions using 3 μl of the Prepman supernatant as template. Each reaction was performed using high-fidelity Pfx 50 DNA polymerase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Internal transcribed spacer (ITS) and D1/D2 amplicons were obtained using primers ITS1 and ITS4 and NL1 and NL4, respectively (11, 16, 24). PCR amplification was performed in a PTC-100 thermocycler (MJ Research, Watertown, MA), and a standard three-step protocol was used for all reactions, including 30 cycles for each reaction with an annealing temperature of 60°C and a 1-min extension time. To confirm PCR amplification, a 15-μl aliquot of each PCR was run on a 0.7% agarose gel and stained with ethidium bromide. The remaining PCR template was purified with the QIAquick PCR purification kit (Qiagen, Valencia, CA) and then sequenced at the UTHSCSA Advanced Nucleic Acids Core Facility. Each purified template was sequenced on both strands, and the sequences obtained were then used to perform nucleotide-nucleotide searches utilizing the BLASTn database at the NCBI website (http://www.ncbi.nlm.nih.gov/BLAST/).
BLAST outputs were sorted based on maximum identity, and identifications were made when BLAST searches yielded ≥98% identity for sequences that displayed at least 90% query coverage. β-Tubulin amplicons were obtained using primers bt2a (5′-GGTAACCAAATCGGTGCTGCTTTC-3′) and bt2b (5′-ACCCTCAGTGTAGTGACCCTTGGC-3′) under the conditions described by Glass and Donaldson (6) and with a PTC-100 thermocycler (MJ Research, Watertown, MA). PCR products were visualized, cleaned, and sequenced as described above using the PCR primers as sequencing primers. Sequences were then used to perform individual nucleotide-nucleotide searches using the BLASTn algorithm at the NCBI website (http://www.ncbi.nlm.nih.gov/BLAST/). Following the classification scheme of Houbraken et al. (8) for Aspergillus, the section Usti includes eight species: A. ustus, A. puniceus, A. granulosus, A. pseudodeflectus, A. calidoustus, A. insuetus, and A. keveii. A. granulosus and A. ustus, in particular, are morphologically very closely related. A. ustus is a variable species, due to the fact that isolates may vary in their colony color from mud brown to slate gray, with colony reverse colors from uncolored through yellow to dark brown (8). Therefore, we examined NRRL 275, NRRL 1932, and R-3921 using sequence analysis of the ITS region and the 28S rRNA gene (D1/D2) of the rRNA gene cluster. A summary of results obtained from the BLASTn searches for the three Aspergillus isolates is shown in Table 1.
TABLE 1.
Summary of BLASTn results for the ITS, D1/D2, and β-tubulin regions
| Subculture ID | BLASTn ID for ITS region | GenBank accession no. of top 3 ITS hits | % Identity | BLASTn ID for D1/D2 region | GenBank accession no. of top 3 D1/D2 hits | % Identity | BLASTn ID for β-tubulin gene | GenBank accession no. of top 3 β-tubulin hits | % Identity | Identity based on sequence analysis of all 3 regions |
|---|---|---|---|---|---|---|---|---|---|---|
| A. ustus reference | A. ustus | EF652492.1 | 100 | A. puniceus | EF652498.1 | 100 | A. ustus | EF591727.1 | 100 | A. ustus |
| isolate (NRRL 275) | A. ustus | EF652455.1 | 100 | A. puniceus | EF652469.1 | 100 | A. ustus | EF591722.1 | 99 | |
| A. ustus | AY373877.1 | 100 | A. ustus | EF652455.1 | 100 | A. ustus | EF591726.1 | 99 | ||
| A. granulosus reference | A. granulosus | EF652430.1 | 100 | A. granulosus | EF652430.1 | 100 | A. granulosus | EF591734.1 | 99 | A. granulosus |
| isolate (NRRL 1932) | A. granulosus | EF652429.1 | 100 | A. granulosus | EF652429.1 | 100 | A. granulosus | EF591733.1 | 99 | |
| A. granulosus | EF591737.1 | 100 | A. pseudodeflectus | EF652507.1 | 99 | A. granulosus | EF591735.1 | 98 | ||
| R-3921 | A. granulosus | EF652430.1 | 99 | A. granulosus | EF652430.1 | 100 | A. granulosus | EF591734.1 | 99 | A. granulosus |
| A. granulosus | EF652429.1 | 99 | A. granulosus | EF652429.1 | 100 | A. granulosus | EF591735.1 | 98 | ||
| A. granulosus | EF591737.1 | 99 | A. granulosus | AF433050.1 | 100 | A. granulosus | EF591733.1 | 98 |
The molecular identification on the top three BLASTn identities resulted in R-3921 being classified as A. granulosus. When the BLAST hits were reviewed with a 98% or higher identity as the cutoff point, A. ustus was very close, with 99% identity in the D1/D2 region, demonstrating the close relationship of A. ustus to A. granulosus at the sequence level for the D1/D2 locus. For the ITS region, however, no A. ustus sequence showed a percent identity greater than 97%. Nonetheless, in order to confirm the molecular data we obtained using the ITS and D1/D2 regions, the β-tubulin gene was also examined for further identity confirmation of the Aspergillus species. The closest A. ustus sequence for R-3921 displayed 87% identity by β-tubulin gene sequencing. Based on the sequence identities using ITS, D1/D2, and β-tubulin regions, R-3921 was identified as A. granulosus. Comparison of the ITS and D1/D2 regions along with the β-tubulin gene proved to be satisfactorily discriminatory for the accurate identification of A. granulosus and A. ustus species.
The type strain of Aspergillus granulosus Raper and Thom 1944, isolated in 1942 from soil in Fayetteville, AR, was originally classified in the A. ustus group. The assignment of Aspergillus species to groups was an early method used to categorize like strains with similar morphological features (19, 20). In 1965, Raper and Fennell (17) transferred the species to the A. versicolor group, which was expanded to accommodate species that were thought to be related but were somewhat dissimilar. They did, however, acknowledge that except for the difference in Hülle cells, A. granulosus bore a close resemblance to A. ustus. In 1985 (4), groups were assigned a taxonomic hierarchy as sections with the placement of A. granulosus in the now obsolete section Versicolores (15). In 1989, species were further categorized on the basis of their conidial ornamentation as seen with scanning electron microscopy (10), and A. granulosus was described as having a lobate-reticulate pattern. More recently, a polyphasic approach has been used to clarify the taxonomic placement of Aspergillus species. This has included molecular characterization by multilocus sequence analysis (primarily the ITS region and parts of the β-tubulin, calmodulin, and actin genes) combined with analysis of extrolite profiles and morphological features (8, 15). Although the lineage numbers for section Usti differ somewhat between Houbraken et al. (8) and Peterson (15), these analyses have resulted in the unequivocal placement of A. granulosus in the Aspergillus section Usti. It is of interest to speculate that this organism, first described from soil in Arkansas and subsequently recovered in other temperate areas (Texas, Arizona, Costa Rica, and Liberia) (17), may be more prevalent in warmer climates, as the isolates recovered thus far in transplant recipients appear to have been from residents of these areas. The first case reported in New York was at a Veterans' Affairs Medical Center, and the authors do not speculate about where the infection may have been acquired. The ability of the organism to grow at 40°C also suggests its neurotropic potential, as was borne out in this case. Aspergillus granulosus is distinguished from A. calidoustus, the most common clinically significant species in the section Usti and formerly called A. ustus (21), by its buff to pale brownish rather than grayish yellow to grayish brown colony, by conidia that are only slight roughened (finely echinulate or verruculose) rather than coarsely roughened to echinulate, by sparse conidial production in some isolates, by a negative Ehrlich reaction (21), and by the preponderance of colorless aggregates of globose to elongate Hülle cells at maturity, giving a granular appearance to the colony. This species also differs significantly in its in vitro antifungal susceptibility pattern, appearing considerably more susceptible than A. calidoustus, which demonstrates resistance to several classes of antifungal agents (5, 9, 12-14, 18, 22, 23, 25). In vitro antifungal susceptibility testing of the case isolate according to the previously published Clinical and Laboratory Standards Institute M38-A document for filamentous fungi (1) demonstrated the following MICs: amphotericin B, 0.5 μg/ml; caspofungin (reported as the minimum effective concentration), 0.125 μg/ml; voriconazole, 4 μg/ml; and posaconazole, 1 μg/ml. Two additional isolates from lung transplant recipients (Texas and California) received in the Fungus Testing Laboratory for identification and antifungal susceptibility testing were also confirmed as A. granulosus. While these patients are neither part of this case report nor are any clinical details available, Table 2 provides the in vitro susceptibility data for them as well as for the first reported isolate in a cardiac transplant recipient (3). Although no defined breakpoints exist for this organism, in vitro data for most antifungal agents tested for these three isolates would suggest clinical efficacy based upon achievable serum drug concentrations using standard dosing regimens.
TABLE 2.
In vitro antifungal susceptibility data for A. granulosus
| Isolate (reference) | Antifungal susceptibility (μg/ml) to:
|
||||
|---|---|---|---|---|---|
| Amphotericin B (MIC) | Caspofungin (MEC)a | Itraconazole (MIC) | Voriconazole (MIC) | Posaconazole (MIC) | |
| Case isolate | 0.5 | 0.125 | NTb | 4 | 1 |
| Texas lung transplant | 1 | 0.25 | 0.5 | 2 | NT |
| California lung transplant | NT | 0.5 | NT | 0.25 | 0.5 |
| Cardiac transplant (3) | 0.29 | NT | 1.25 | NT | NT |
MEC, minimum effective concentration.
NT, not tested.
A. granulosus may be an underreported species in the section Usti, particularly in lung transplant recipients, as it may be difficult to identify morphologically due to poor conidiation. Profuse Hülle cell production, however, would suggest a species in the genus Aspergillus or Emericella. The ability of some isolates to grow at 40°C, as evidenced by this case, also highlights its neurotropic potential.
Nucleotide sequence accession numbers.
GenBank accession numbers are as follows: FJ771045 for the 18S rRNA gene (partial sequence), ITS1, 5.8S rRNA gene, ITS2 (complete sequence), and 28S rRNA gene (partial sequence); FJ771046 for the 28S rRNA gene (partial sequence); and FJ771047 for the β-tubulin gene (partial sequence).
Acknowledgments
B.L.W. is supported by grant PR054228 from the U.S. Army Medical Research and Material Command, Office of Congressionally Directed Medical Research Programs. A.M.R. is supported by NIDCR grant DE14318 (CO STAR).
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. NCCLS M38-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed., p. 478-479. Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
- 3.Fakih, M. G., G. E. Bareden, C. A. Oakes, and C. S. Berenson. 1995. First reported case of Aspergillus granulosus infection in a cardiac transplant patient. J. Clin. Microbiol. 33:471-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gams, W., M. Christensen, A. H. S. Onions, J. I. Pitt, and R. A. Samson. 1985. Infrageneric taxa of Aspergillus, p. 55-62. In R. A. Samson and J. I. Pitt (ed.), Advances in Aspergillus and Penicillium systematics. Plenum Press, New York, NY.
- 5.Gené, J., A. Azón-Masoliver, J. Guarro, G. De Febrer, A. Martinez, C. Grau, M. Ortoneda, and F. Ballester. 2001. Cutaneous infections caused by Aspergillus ustus, an emerging opportunistic fungus in immunosuppressed patients. J. Clin. Microbiol. 39:1134-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass, N. L., and G. C. Donaldson. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant, D. C., D. A. Sutton, C. A. Sandberg, R. D. Tyler, Jr., E. H. Thompson, A. M. Romanelli, and B. C. Wickes. 2009. Disseminated Geosmithia argillacea infection in a German Shepherd dog. Med. Mycol. 47:221-226. [DOI] [PubMed] [Google Scholar]
- 8.Houbraken, J., M. Due, J. Varge, M. Meijer, J. C. Frisvad, and R. A. Samson. 2007. Polyphasic taxonomy of Aspergillus section Usti. Stud. Mycol. 59:107-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwen, P. C., M. E. Rupp, M. R. Bishop, M. G. Rinaldi, D. A. Sutton, S. Tarantolo, and S. H. Hinrichs. 1998. Disseminated aspergillosis caused by Aspergillus ustus in a patient following allogeneic peripheral stem cell transplantation. J. Clin. Microbiol. 36:3713-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozakiewicz, Z. 1989. Aspergillus species on stored products. Mycol. Papers 161:1-188. [Google Scholar]
- 11.Kurtzman, C. P., and C. J. Robnett. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35:1216-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai, K., Y. Kanda, S. Mineishi, A. Hori, A. Chizuka, H. Niiya, T. Tanimoto, M. Ohnishi, M. Kami, A. Makimoto, R. Tanosaki, Y. Matsuno, N. Yamazaki, K. Tobinai, and Y. Takaue. 2002. Primary cutaneous aspergillosis caused by Aspergillus ustus following reduced-intensity stem cell transplantation. Ann. Hematol. 81:93-596. [DOI] [PubMed] [Google Scholar]
- 13.Panackal, A. A., A. Imhof, E. W. Hanley, and K. A. Marr. 2006. Aspergillus ustus infections among transplant recipients. Emerg. Infect. Dis. 12:403-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavie, J., C. Lacroix, D. G. Hermoso, M. Robin, C. Ferry, A. Bergeron, M. Feuilhade, F. Dromer, E. Gluckman, J.-M. Molina, and P. Ribaud. 2005. Breakthrough disseminated Aspergillus ustus infection in allogeneic hematopoietic stem cell transplant recipients receiving voriconazole or caspofungin prophylaxis. J. Clin. Microbiol. 43:4902-4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson, S. W. 2008. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100:205-222. [DOI] [PubMed] [Google Scholar]
- 16.Peterson, S. W., and C. P. Kurtzman. 1990. Phylogenetic relationships among species of the genus Issatchenkia Kudriavzev. Antonie van Leeuwenhoek 58:235-240. [DOI] [PubMed] [Google Scholar]
- 17.Raper, K. B., and D. I. Fennell. 1965. The genus Aspergillus, p. 460-462. Williams and Wilkins, Baltimore, MD.
- 18.Saracli, M. A., F. M. Mutlu, S. T. Yildiran, A. E. Kurekci, A. Gonlumm, Y. Uysal, U. Erdem, A. C. Basustaoglu, and D. A. Sutton. 2007. Clustering of invasive Aspergillus ustus eye infections in a tertiary care hospital: a molecular epidemiologic study of an uncommon species. Med. Mycol. 45:377-384. [DOI] [PubMed] [Google Scholar]
- 19.Thom, C., and M. B. Church. 1926. The Aspergilli. Williams and Wilkins Co., Baltimore, MD.
- 20.Thom, C., and K. R. Raper. 1945. A manual of Aspergilli, p. 175-178. Williams and Wilkins, Baltimore, MD.
- 21.Varga, J., J. Houbraken, H. A. L. Van Der Lee, P. E. Verweij, and R. A. Samson. 2008. Aspergillus calidoustus sp. nov., causative agent of human infections previously assigned to Aspergillus ustus. Eukaryot. Cell 7:630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verweij, P. E., M. F. Q. van den Bergh, P. M. Rath, B. E. de Pauw, A. Voss, and J. F. G. M. Meis. 1999. Invasive aspergillosis caused by Aspergillus ustus: case report and review. J. Clin. Microbiol. 37:1606-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss, L. M., and W. A. Thiemke. 1983. Disseminated Aspergillus ustus infection following cardiac surgery. Am. J. Clin. Pathol. 80:408-411. [DOI] [PubMed] [Google Scholar]
- 24.White, T. J., T. D. Bruns, S. B. Lee, and J. W. Taylor. 1990. Amplification and sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In N. Innis, D. Gelfand, J. Sninsky, and T. C. White (ed.), PCR protocols and applications. A laboratory manual. Academic Press, New York, NY.
- 25.Yildiran, S. T., F. M. Mutlu, M. A. Saracli, Y. Uysal, A. Gonlum, G. Sobaci, and D. A. Sutton. 2006. Fungal endophthalmitis caused by Aspergillus ustus in a patient following cataract surgery. Med. Mycol. 44:665-669. [DOI] [PubMed] [Google Scholar]