Abstract
Differentiating true coagulase-negative staphylococcal infection from contamination has an important impact on therapeutic implications. Time to positivity reflects bacterial density and may help in the interpretation of blood cultures. We retrospectively reviewed the records of 272 patients from June 2005 to January 2008 for clinical characteristics, microbiological data, and therapeutic outcome. Four groups were identified. The first three groups, as follows, included patients with one positive quantitative blood culture: the low-colony-count group (<10 CFU/ml), the moderate-colony-count group (30 to 100 CFU/ml), and the high-colony-count group (>100 CFU/ml). The control group included patients with two positive quantitative blood cultures and definite coagulase-negative staphylococcal bloodstream infection. The high-colony-count group had shorter time to positivity (≤16 h) than did the low-colony-count group (P < 0.0001). The low-colony-count group had a significantly longer time to positivity, >20 h (P = 0.001), than did the moderate-colony-count group. Even though antibiotics were not provided in 71% of cases and central venous catheter was retained in 83%, the low-colony-count group had a favorable outcome, suggesting that <10 CFU/ml represents contamination. The high-colony-count group, similar to the positive control group, required antibiotics in 81% of cases and central venous catheter removal in 51% (P = 0.001). A time to positivity of ≤16 h reflects high-grade bacteremia with CFU of >100. Similar to the positive control group, these patients required an active therapeutic approach. A time to positivity of >20 h indicates possible contamination with a CFU of <10, and active therapy may not be required.
Coagulase-negative staphylococci (CoNS) are the most commonly isolated contaminants from blood cultures, yet they are the most frequent cause of true bloodstream infections (9). This distinction has practical importance because of its therapeutic implications in terms of unnecessary use of antibiotics and emergence of resistance (6). Furthermore, failure to recognize and treat true bacteremia can lead to higher rates of morbidity and mortality, especially in the critically ill or immunocompromised cancer patients (15). Interpretation of the significance of CoNS-positive blood cultures is aided by the number of positive cultures, but this assessment is difficult when only a single culture sample is obtained before the initiation of antibiotic therapy (10). Quantitative blood cultures (QBCs) can aid interpretation but are not widely available in most laboratories. On the other hand, the time-to-positivity (TP) method has been suggested as a surrogate test for bacterial density and may also help in the interpretation of positive blood cultures, as QBCs do (1).
We postulated that patients with QBCs positive for a low colony count correlate with a prolonged TP and may reflect CoNS contamination. As a consequence, those patients require no therapeutic approach, whereas patients with QBCs positive for a high colony count correlate with a shorter TP and may reflect true CoNS bacteremia. Those patients require adequate therapeutic approach to achieve clinical and microbiologic resolution.
MATERIALS AND METHODS
Patient eligibility and selection.
A retrospective study was conducted at The University of Texas M. D. Anderson Cancer Center (MDACC) from June 2005 until January 2008. A waiver of informed consent and a waiver of authorization were requested and provided by our Institutional Review Board. We identified all patients aged 8 years and older with one or two CoNS-positive QBCs collected from a peripheral vein or a central venous catheter (CVC) or both. We categorized patients into four groups, as follows. (i) Patients with one CoNS-positive QBC from a CVC or a peripheral vein and <10 CFU/ml were included in the low-colony-count group. (ii) Patients with one CoNS-positive QBC and 30 to 100 CFU/ml were included in the moderate-colony-count group. (iii) Patients with one CoNS-positive QBC and >100 CFU/ml were included in the high-colony-count group. (iv) Patients with two simultaneous CoNS-positive QBCs that met the Centers for Disease Control criteria for true CoNS bloodstream infection were included in the two-positive-blood-culture group. This group was considered to be the positive control group for true bacteremia. TP was recorded for each patient along with CFU.
Data collection.
Two-hundred seventy-two patients with CoNS-positive QBC were identified. Extracted data included the following elements: demographic characteristics (age, sex, weight, and height), medical history, comorbidities, cancer history, predisposing factors, exposure to a health care institution for >72 h within 180 days of hospital admission, receipt of antibiotics in the 30 days prior to the first positive QBC or index QBC collection, length of hospitalization, clinical characteristics (fever, chills, and hypotension), neutropenic status, microbiologic results (time of draw, quantitative culture, and TP), antibiotic treatment data (date, time, dosing regimen, and duration), as well as CVC type, site, and dates of insertion and removal. Patients had been followed up for up to 3 months after the index QBC to determine outcome, relapse, and complications.
Microbiologic methods.
We collected and assessed QBCs as follows. After rigorous antiseptic cleansing of the skin and hub with 70% alcohol, we drew 20 ml of blood and divided the sample into two portions. Each blood culture set consisted of 10 ml of whole blood collected into the Isolator 10 tube, and 10 ml was added to a Bactec 26 Plus resin bottle (14). Short draws of <8 ml were excluded.
The Isolator tubes were taken to the laboratory within 4 hours and processed according to the manufacturer's recommendations. The sample tubes were centrifuged at 3,000 × g for 30 min and then moved to a laminar flow hood. The tubes were punctured, the supernatant was discarded, and the concentrate was removed by pipette and planted onto two blood agar and two chocolate agar plates. Plates were incubated at 35°C with 5% CO2 and observed once per day for 4 days. Colony growth was evaluated using Gram staining and Staphaurex, l-pyrolidonyl-p-naphthylamide, optochin susceptibility, oxidase, and motility as necessary and identified using the Vitek AMS instrument (bioMérieux, Inc., Durham, NC) (6).
Blood culture bottles were taken promptly to the microbiology laboratory and placed in an automatic culture detector (Bactec 9240, Bactec Plus aerobic/F; Becton Dickinson DIS, Sparks, MD), which records culture positivity according to fluorescence changes related to microbial growth. Those records are printed every 15 min. We used the record printout to document TP in our study. The machine is monitored by lab personnel every 24 h for removal of positive culture. All patients with polymicrobial blood cultures that yielded other organisms in conjunction with CoNS were excluded.
Study design and analysis.
Our analysis consisted of two parts. In part one, we sought to establish a significant inverse correlation between TP and CFU/ml of patients with a single positive QBC. We also identified different TP cutoff points that can be used to differentiate between patients with QBC in the low-colony-count group and patients with QBC in the high-colony-count groups.
In part two, we compared outcomes among patients with QBC in the low-colony-count group, patients with QBC in the high-colony-count group, and patients in the two-positive-blood-culture group. Outcome comparison between those groups was performed to demonstrate the difference in therapeutic approach between contamination (QBC with <10 CFU/ml) and true bloodstream infection or high-grade bacteremia (QBC with >100 CFU/ml).
The therapeutic approach was considered adequate when active antibiotic administration and/or catheter removal or exchange was dictated by the sole presence of CoNS-positive blood cultures. All patients with polymicrobial bacteremia or evidence of concomitant infection other than the CoNS bloodstream infection were excluded from the outcome analysis. Antibiotics included vancomycin, linezolid, daptomycin, quinupristin-dalfopristin, clindamycin, or tigecycline and were considered active when the isolated organism was susceptible.
Definitions.
Neutropenia was defined as an absolute neutrophil count of <500/microliter. Fever of >38°C or 100.4°F at the time of the positive index QBC was recorded for all patients. Response was defined as a resolution of clinical signs and symptoms and/or eradication of the bacteremia within 72 h from the date that active therapy was administered. Relapse was defined as the recurrence of bacteremia with the same organism (CoNS bacteria with similar antibiograms) after an initial clinical and/or microbiologic response within 3 months of the last positive QBC.
All deaths within 2 weeks of the index QBC were recorded.
Statistical analysis.
We compared the four groups according to their clinical outcomes. Kruskal-Wallis tests were used to compare continuous variables. If a significant result was detected, we used Wilcoxon rank-sum tests for pairwise comparisons. Chi-square or Fisher's exact tests were used for categorical data analyses and the subsequent pairwise comparisons if a significant result was found. The α levels of all post-hoc pairwise comparisons were adjusted using a sequential Bonferroni adjustment to control type I errors. For two-group comparisons, chi-square or Fisher's exact tests were used for categorical data analyses, and Wilcoxon rank-sum tests were used for continuous variables. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
From July 2005 to January 2008, we identified 272 patients with CoNS-positive QBCs. Seventy-two patients were included in the two-positive-blood-culture group, 56 were in the low-colony-count group, 55 were in the moderate-colony-count group, and 89 were in the high-colony-count group.
Since QBC is reported as a wide range of CFU, patients with 10 to 30 CFU/ml were not included in the analysis, in an attempt to decrease the high rate of overlap between the low (<10 CFU/ml)- and possible moderate (10 to 100 CFU/ml)-colony-count groups.
Relationship between TP and CFU.
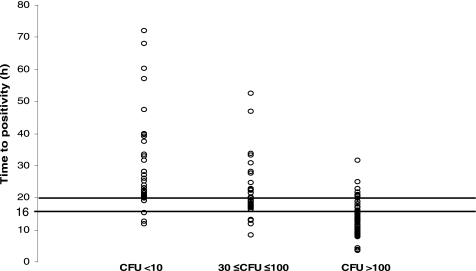
Among patients with a single CoNS-positive QBC, we found a statistically significant inverse correlation between TP and CFU, as illustrated in Fig. 1. TP was significantly different among the three groups with a single CoNS-positive QBC (median for low-colony-count group, 20.9 h [range, 10.4 to 72.0 h]; median for moderate-colony-count group, 17.7 h [range, 5.7 to 52.4 h]; median for high-colony-count group, 11.6 h [range, 2.5 to 31.6 h]; P < 0.0001). The midpoint between the two extreme TP medians (11.6 h for high-colony-count group and 20.9 h for the low-colony-count group) was 16.2 h.
FIG. 1.
TP (hours) distribution of different CFU groups.
We performed a pairwise comparison using the Wilcoxon rank-sum test to determine a cutoff point for TP. Eighty-one percent of patients in the high-colony-count group had a TP of ≤16 h, compared with 27% of patients in the moderate-colony-count group. Only 10% of patients in the low-colony-count group had a TP of ≤16 h (P < 0.0001).
We established a reliable correlation between a colony count of >100 CFU/ml and a TP of ≤16 h, indicating that both parameters were in agreement (κ coefficient = 0.62). Therefore, a TP of ≤16 h was considered suggestive of high colony count and high-grade bacteremia.
Ninety-five percent of patients in the high-colony-count group had a TP of <20 h. Thirty percent of patients in the low-colony-count group had a TP of ≤20 h, while 70% of patients in the moderate-colony-count group had a TP of ≤20 h. Hence, a TP of ≥20 h was suggestive of low colony count or contamination (<10 CFU/ml). A TP of 16 to 20 h was suggestive of moderate colony count or low-grade bacteremia (30 to 100 CFU/ml).
Outcome analysis.
Patients with evidence of a concomitant infection (e.g., pneumonia, cellulitis, or a urinary tract infection) other than CoNS bloodstream infection were excluded from the outcome analysis in all groups. Clinical data suggestive of infection, including fever, chills, hypotension, neutropenia, antibiotic administration, and catheter management, were reviewed to evaluate the clinical significance of a single blood culture with different CFUs. Nine patients in the low-colony-count group displayed symptoms, but fever was attributed to febrile neutropenia, while 63% (49 of 78) of patients in high-colony-count group and 86% (62 of 72) of patients in the positive control group displayed evidence of infectious symptoms. Furthermore, we found statistically significant differences in therapeutic approach between patients in the low-colony-count group and patients in the high-colony-count group as well as patients in the two-positive-blood-culture group (P ≤ 0.01). Ninety-three percent (67 of 72) of patients in the two-positive-blood-culture group and 81% (63 of 78) of patients in the high-colony-count group received active antibiotic treatment, whereas only 29% (9 of 31) of patients in the low-colony-count group were given antibiotic therapy (P < 0.0001). All nine patients were febrile and had severe neutropenia (Table 1).
TABLE 1.
Outcome analysis among patients with a single positive blood culture in different colony count groups and the positive control group (excluding all patients with evidence of a concomitant infection from all groups)b
| Group | Clinical significance of the blood culture | No. (%) of patients with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms (fever, chills, hypotension) | Neutropenia | Active antibiotic therapy received | Catheter
|
Relapsea within 12 wk | Complications within 12 wkb | |||||
| Removed | Retained | Exchanged | Removed or exchanged within 7 days | |||||||
| Low-colony-count group (CFU < 10) (n = 31) | Contaminant | 9 (29) | 9 (29) | 9 (29) | 5/30 (17) | 25/30 (83) | 0/30 (0) | 4/30 (13) | 0 (0) | 0 (0) |
| Moderate-colony-count group (30 < CFU < 100) (n = 55) | Indeterminate | 24 (44) | 13 (24) | 35 (64) | 11/49 (22) | 38/49 (78) | 0/49 (0) | 10/49 (20) | 4 (7) | 0 (0) |
| High-colony-count group (CFU > 100) (n = 78) | Pathogen | 49 (6) | 19 (24) | 63 (81) | 36/71 (51) | 34/71 (48) | 1/71 (1) | 36/71 (51) | 1 (1) | 2 (3) |
| Control group with two positive blood cultures (n = 72) | Definite diagnosis of CoNS bacteremia | 62 (86) | 32 (44) | 67 (93) | 38/69 (55) | 27/69 (39) | 4/69 (6) | 38/68 (56) | 3 (4) | 0 (0) |
All complications were septic thrombophlebitis. Relapse, a repeat positive culture within 12 weeks was available and had the same antibiogram result as the index culture.
P < 0.001 for the following factors: symptoms, active antibiotic therapy received, and catheter-related factors. P = 0.03 for neutropenia, P = 0.22 for relapse within 12 weeks, and P = 0.5 for complications within 12 weeks.
Similarly, 61% (42 of 69) of patients in the two-positive-blood-culture group and 52% (37 of 71) of patients in the high-colony-count group had their catheter either removed or exchanged, compared to only 17% (5 of 30) of patients in the low-colony-count group (P < 0.001). All five patients were febrile and had severe neutropenia (Table 1).
We found no statistically significant differences in defervescence rate, microbiologic resolution within 72 h, relapse, and complication rate within 12 weeks or death within 2 weeks among all three single-QBC groups. Two patients in the high-colony-count group experienced septic thrombophlebitis of retained catheters secondary to CoNS. Another two patients in the same group died of causes unrelated to CoNS bacteremia.
These results demonstrate that the low-colony-count group shows evidence suggestive of contamination and achieves favorable outcome even though no active therapy was required, whereas the high-colony-count group seems to have true bacteremia and requires active therapeutic intervention, like the positive control patients in the two-positive-blood-culture group.
All outcome analyses were repeated, comparing the positive control group and patients with a single positive blood culture and TPs of >20 h, of ≤16 h, and in a range of 16 to 20 h. All patients with evidence of infections at sites other than CoNS bacteremia were again excluded from all groups. We observed significant statistical differences in therapeutic approach between QBC with TP of >20 h compared to QBC with TP of ≤16 h and the positive control group. Results are reported in Table 2. Ninety-three percent (67 of 72) of patients in the two-positive-blood-culture group and 76% (63/83) of patients with a single positive QBC and TPs of ≤16 h had undergone active antibiotic treatment, whereas only 43% (17/40) of patients with a single positive QBC and TPs of >20 h had received antibiotic treatment (P < 0.001) (Table 2).
TABLE 2.
Outcome analysis among patients with a single positive blood culture and TP of >20 h, ≥16 and <20 h, or ≤16 h and the positive control group (excluding all patients with evidence of a concomitant infection from all groups)
| Patient group | Clinical significance of the blood culture | No. (%) of patients with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms (fever, chills, hypotension) | Neutropenia | Active antibiotic therapy received | Catheter
|
Relapsea within 12 wk | Complications within 12 wkb | |||||
| Removed | Retained | Exchanged | Removed or exchanged within 7 days | |||||||
| Single positive blood culture with TP of >20 h (n = 40) | Contaminant | 12 (30) | 6 (15) | 17 (43) | 7/33 (21) | 26/33 (79) | 0/33 (0) | 6/32 (19) | 2 (5) | 0 (0) |
| Single positive blood culture with TP of ≥16 and <20 h (n = 41) | Indeterminate | 20 (49) | 12 (29) | 27 (66) | 11/38 (29) | 27/38 (71) | 0/38 (0) | 10/39 (26) | 0 (0) | 2 (5) |
| Single positive blood culture with TP of ≤16 h (n = 83) | Pathogen | 50 (60) | 23 (28) | 63 (76) | 34/79 (43) | 44/79 (56) | 1/79 (1) | 34/79 (43) | 1 (1) | 2 (2) |
| Positive control group with two positive blood cultures (n = 72) | Definite diagnosis of CoNS bacteremia | 62 (86) | 32 (44) | 67 (93) | 38/68 (56) | 27/69 (39) | 4/69 (6) | 38/68 (56) | 3 (4) | 0 (0) |
All complications were septic thrombophlebitis. Relapse, a repeat positive culture within 12 weeks was available and had the same antibiogram result as the index culture.
P < 0.001 for symptoms and active antibiotic therapy received. The P value is 0.01 for neutropenia, 0.0001 for catheter-related factors, 0.47 for relapse within 12 weeks, and 0.78 for complications within 12 weeks.
Sixty-one percent (42 of 69) of patients in the two-positive-blood-culture group and 44% (35/79) of patients with a single positive QBC and TPs of ≤16 h had their catheter removed or exchanged, compared with 21% (7 of 40) of patients with a single positive QBC and TPs of >20 h (Table 2). No statistically significant differences were reported in clinical and microbiological resolution, rate of relapse, complications, or death in all three groups (P = 0.16) (Table 2).
DISCUSSION
The results of our study demonstrate that TP and QBC are useful in differentiating contaminated blood culture samples from samples representing true CoNS bacteremia. A TP of >20 h correlated with a positive QBC of <10 CFU/ml and was suggestive of CoNS bloodstream contamination. As further evidence of contaminated blood culture samples, patients in the low-colony-count group and with a TP of >20 h had a favorable outcome, without any need for active therapeutic intervention (antibiotics or CVC removal or exchange). A TP of ≤16 h was correlated with a positive QBC of >100 CFU/ml and was suggestive of true CoNS bloodstream infection. Patients in the high-colony-count group and with a TP of ≤16 h frequently required active antibiotic therapy (81% to 74%) or catheter removal or exchange (52% to 43%), like the control patients in the two-positive-blood-culture group (P = 0.001). These findings demonstrate that a simple test such as the TP method is useful at differentiating between culture samples representing CoNS blood culture contamination and those representing true CoNS bloodstream infection.
The significance of CoNS grown from blood cultures of febrile patients can be difficult to determine because it can reflect bloodstream infection (catheter related or not), skin contamination, or even intravascular catheter colonization. In 2003, Wisplinghoff et al. (15) found an increased incidence of gram-positive organisms, especially CoNS, accounting for the predominant pathogens of all bloodstream infections in cancer patients. Furthermore, in patients with CVCs, catheter-related bloodstream infections are a prominent cause of morbidity and excess hospital costs (3). It is important to accurately identify true catheter-related CoNS bloodstream infections and differentiate them from skin, intraluminal catheter, and hub contamination to avoid unnecessary catheter removal or exchange. This distinction is also necessary to establish adequate active antibiotic administration and to reduce the selective pressure caused by inappropriate or unnecessary antibiotic treatment. In several studies, high rates of vancomycin or broad-spectrum cephalosporin usage were associated with an increased prevalence of vancomycin-resistant enterococci, especially in the intensive-care-unit setting, ultimately leading to the emergence of vancomycin-intermediate and -resistant Staphylococcus aureus (5). The urgent need for accurate diagnosis and treatment led to our proposal of conservative diagnostic techniques, such as TP and QBC.
The use of quantitative methods such as isolator tubes and resin-containing Bactec 26 Plus bottles can aid in the interpretation of QBC findings (13) and was suggested by the Infectious Disease Society of America as an alternative method of diagnosing catheter-related bloodstream infections. This technique relies on the ratio of QBCs of paired blood samples that have been obtained through the CVC and a peripheral vein (10). Diagnosis of true CoNS bloodstream infection becomes particularly difficult when only a single blood culture sample is obtained (4-11). We previously reported that a cutoff point of >15 CFU/ml and positive clinical findings were useful for differentiating culture samples representing true bacteremia from contaminated blood culture samples (2). Furthermore, QBCs are not widely performed in all hospitals and may not be routinely available to clinicians.
The TP method has been proposed as a surrogate test for initial bacterial density, as defined by CFU/ml (11). In our study, a high colony count (>100 CFU/ml) was suggestive of a high-grade bacterial density, whereas a low colony count (<10 CFU/ml) was consistent with contamination. Furthermore, given the fact that, worldwide, most hospital laboratories use an automated blood culture system that automatically provides TP information, this test is simple and readily available in many hospitals and cost free.
We found that a TP of >20 h was associated with a low initial bacterial density (<10 CFU/ml) and represented contamination. A TP of ≤16 h was associated with a high initial bacterial density (>100 CFU/ml), represented high-grade bacteremia, and required an active therapeutic approach. Our data were consistent with those of prior studies. In an experimental study, pediatric Bactec bottles inoculated with Staphylococcus epidermidis at <10 CFU/ml had TPs of >20 h. Bottles with initial bacterial densities of ≥50 CFU/ml had TPs of ≤15 h (8). In a more recent study of pediatric patients, a TP of <15 h had a positive predictive value of 84% for diagnosing true CoNS bloodstream infections, and a TP of >22 h had a positive predictive value of 87% for diagnosing CoNS blood culture contamination (7).
The TP method may be subjected to many variations and limitations, such as those for the volumes of blood drawn and the incubation conditions (12). Such limitations were not observed in our study. Blood culture samples with appropriate 10-ml blood draw volumes were included. All blood draws of <8 ml were excluded. Incubation conditions were always stable at our institution because of the automation involved in our microbiologic procedures. Furthermore, specific teams were assigned to different services, and blood culture bottles were placed in the Bactec machine within 2 h of the blood draw.
The strength of this study resides in the fact that we verified our data by comparing the clinical outcomes of different colony counts and TP groups in order to verify the clinical validity of two microbiological parameters, TP and QBC.
Similar to positive control patients in the two-positive-blood-culture group, patients with QBC in the high-colony-count group or TPs of ≤16 h required active therapeutic approach with either antibiotic administration or catheter removal or exchange, whereas most of the patients with QBC in the low-colony-count group and TPs of >20 h experienced favorable outcome and complete microbiologic resolution without any need for antibiotic treatment or catheter removal.
Our study is subject to several limitations. First, our analysis was based on a retrospective evaluation of laboratory records. Second, our patient population included only those with hematologic or nonhematologic cancers. These findings may not be applicable to noncancer patients. Third, different CoNS species may exhibit different growth kinetics in this blood culture system, which would affect the reliability of TP.
In conclusion, we demonstrated that a CoNS-positive single blood culture at a TP of ≤16 h reflects high-grade bacteremia (>100 CFU/ml) and patients require adequate therapeutic management to achieve complete symptomatic and microbiologic resolution. On the other hand, a TP of >20 h is associated with contamination, and low bacterial density is associated with <10 CFU/ml. These patients demonstrate favorable outcomes and did not necessarily require therapy.
Our findings support the use of TP (≤16 h and >20 h) as a predictor of colony count bacterial density and thus as a method to differentiate culture samples representing CoNS bacteremia from those representing contamination. This approach is useful to every health care provider and physician. It helps differentiate CoNS-positive blood culture contamination from true bacteremia and may minimize excessive antibiotic use and unnecessary catheter removal.
Footnotes
Published ahead of print on 19 August 2009.
REFERENCES
- 1.Blot, F., E. Schmidt, G. Nitenberg, C. Tancrède, B. Leclercq, A. Laplanche, and A. Andremont. 1998. Earlier positivity of central venous versus peripheral blood cultures is highly predictive of catheter-related sepsis. J. Clin. Microbiol. 36:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatzinikolaou, I., H. Hanna, R. Darouiche, G. Samonis, J. Tarrand, and I. Raad. 2006. Prospective study of the value of quantitative culture of organisms from blood collected through central venous catheters in differentiating between contamination and bloodstream infection. J. Clin. Microbiol. 44:1834-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 4.Favre, B., S. Hugonnet, L. Correa, H. Sax, P. Rohner, and D. Pittet. 2005. Nosocomial bacteremia: clinical significance of a single blood culture positive for coagulase-negative staphylococci. Infect. Control Hosp. Epidemiol. 26:697-702. [DOI] [PubMed] [Google Scholar]
- 5.Fridkin, S. K., J. R. Edwards, J. M. Courval, H. Hill, F. C. Tenover, R. Lawton, R. P. Gaynes, and J. E. McGowan, Jr., for the Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project and the National Nosocomial Infections Surveillance (NNIS) System Hospitals. 2001. The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 U.S. adult intensive care units. Ann. Intern. Med. 135:175-183. [DOI] [PubMed] [Google Scholar]
- 6.Fridkin, S. K., J. R. Edwards, S. C. Pichette, E. R. Pryor, J. E. McGowan, Jr., F. C. Tenover, D. H. Culver, and R. P. Gaynes. 1999. Determinants of vancomycin use in adult intensive care units in 41 United States hospitals. Clin. Infect. Dis. 28:1119-1125. [DOI] [PubMed] [Google Scholar]
- 7.Haimi-Cohen, Y., S. Shafinoori, V. Tucci, and L. G. Rubin. 2003. Use of incubation time to detection in BACTEC 9240 to distinguish coagulase-negative staphylococcal contamination from infection in pediatric blood cultures. Pediatr. Infect. Dis. J. 22:968-974. [DOI] [PubMed] [Google Scholar]
- 8.Haimi-Cohen, Y., E. M. Vellozzi, and L. G. Rubin. 2002. Initial concentration of Staphylococcus epidermidis in simulated pediatric blood cultures correlates with time to positive results with the automated, continuously monitored BACTEC blood culture system. J. Clin. Microbiol. 40:898-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huebner, J., and D. A. Goldmann. 1999. Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50:223-236. [DOI] [PubMed] [Google Scholar]
- 10.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. Raad, N. O'Grady, J. S. Harris, D. E. Craven, et al. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 11.Mirrett, S., M. P. Weinstein, L. G. Reimer, M. L. Wilson, and L. B. Reller. 2001. Relevance of the number of positive bottles in determining clinical significance of coagulase-negative staphylococci in blood cultures. J. Clin. Microbiol. 39:3279-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plorde, J. J., F. C. Tenover, and L. G. Carlson. 1985. Specimen volume versus yield in the BACTEC blood culture system. J. Clin. Microbiol. 22:292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers, M. S., and B. A. Oppenheim. 1998. The use of continuous monitoring blood culture systems in the diagnosis of catheter-related sepsis. J. Clin. Pathol. 51:635-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarrand, J. J., C. Guillot, M. Wenglar, J. Jackson, J. D. Lajeunesse, and K. V. Rolston. 1991. Clinical comparison of the resin-containing BACTEC 26 Plus and the Isolator 10 blood culturing systems. J. Clin. Microbiol. 29:2245-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisplinghoff, H., H. Seifert, R. P. Wenzel, and M. B. Edmond. 2003. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 36:1103-1110. [DOI] [PubMed] [Google Scholar]