Abstract
Purpose
Tamoxifen is one of many standard therapeutic option currently available for estrogen receptor-alpha (ER)-positive breast cancer patients. Emerging data have suggested that levels of ER coregulatory proteins play a significant role in acquiring resistance to anti-estrogen action. It has been suggested that high levels of ER coactivators and ER localization may enhance the estrogen agonist activity of tamoxifen and contribute to tamoxifen resistance.
Experimental Design
In an effort to understand the impact of non-genomic signaling and its contribution to hormone resistance in a whole-animal setting, we generated a transgenic mouse expressing a cytoplasmic version of PELP1 mutant defective in its nuclear translocation (PELP1-cyto) and implanted these mice with tamoxifen pellets to assess its responsiveness.
Results
We show that mammary glands from these mice developed widespread hyperplasia with increased cell proliferation and enhanced activation of mitogen-activated protein kinase (MAPK) and AKT at as early as 12 weeks of age. Treatment with tamoxifen did not inhibit this hyperplasia; instead, such treatment exaggerated hyperplasia with an enhanced degree of alteration indicative of hypersensitivity to tamoxifen. Analysis of molecular markers in the transgenic mammary glands from the tamoxifen-treated transgenic mice showed higher levels of proliferation markers PCNA and activated MAPK, than in untreated PELP1-cyto mice. We also found that nude mice with MCF-7/PELP1-cyto tumor xenografts did not respond to tamoxifen. Using immunohistochemical analysis, we found that 43% of human breast tumor samples had high levels of cytoplamic PELP1, which shows a positive correlation between tumor grade and proliferation. Patients whose tumors had high levels of cytoplasmic PELP1 exhibited a tendency to respond poorly to tamoxifen, compared with patients whose tumors had low levels of cytoplamic PELP1.
Conclusions
These findings suggest that PELP1 localization could be used as a determinant of hormone sensitivity or vulnerability. The establishment of the PELP1-cyto transgenic mouse model is expected to facilitate the development of preclinical approaches for effective intervention of breast tumors using cytoplasmic coregulators and active non-genomic signaling.
Keywords: Tamoxifen, transgenic mice, non-genomic signaling, hyperplasia, PELP1-cyto
Introduction
The steroid hormone 17-β estradiol (E2) is known to exert its cellular effects in tissues by binding to its major target, the estrogen receptor-alpha (ER). ER, a ligand-dependent transcription factor, has been implicated in the progression of breast cancer, as evidenced by the fact that almost 70% of breast tumors are ER-positive at the time of early diagnosis (1, 2). Currently, tamoxifen (TAM) represent one of many available standard treatment for ER-positive breast cancer patients (3), even though most patients who respond to TAM eventually acquire TAM resistance, allowing such tumor cells to metastasize to distant sites (4). Consequently, sustained TAM resistance continues to be one of the major limiting factors in the management of advanced breast cancer. One of the major challenges for endocrine therapy of advanced breast cancer is to better understand the cellular basis of TAM resistance and to develop novel approaches to overcoming endocrine resistance (5). Emerging data suggest that the status and functionality of ER coregulatory proteins in the nucleus as well as the cytoplasm and cross-talking between ER coregulators and the components of growth factor receptors play important roles in acquiring resistance to anti-estrogen action (6, 7).
Over the years, it has become apparent that the transcriptional effects of ER are not limited to ligand-activated ER in the nucleus but are also influenced by the participation of ER in cytoplasmic and membrane-mediated signaling (also known as non-genomic or extanuclear signaling) through complex interactions with coregulatory molecules (8, 9). However, the significance of cytoplasmic coactivators in nongenomic signaling is just beginning to be realized, and their role in resistance to selective ER modulators remains poorly understood. One such molecule with a dual role of action in the nucleus and cytoplasm is proline-, glutamic acid-, and leucine-rich protein-1 (PELP1) (10-12), a unique coactivator that plays an important role in both the genomic (13) and nongenomic actions of ER (14). PELP1 is widely expressed in breast and ovarian cancer cells, and its expression is deregulated in breast (15) and ovarian (16) tumors. PELP1 is an ER-responsive gene, and its expression is differentially regulated by selective ER modulators depending on the cell line (17). Although PELP1 is predominantly localized in the nucleus in hormonally responsive tissues (18), a large body of evidence has shown that PELP1 is localized predominantly in the cytoplasm in a significant proportion of human breast (15), endometrial (19), and salivary gland (20) carcinomas. Studies have also shown that differential compartmentalization of PELP1 may play a crucial role in modulating the status of non-genomic signaling using molecular mechanisms that remain poorly understood (15, 21).
Because, under physiologic conditions, PELP1 is localized in the cytoplasm, MCF-7 breast cancer cells that mimic PELP1 cytoplasmic localization in tumors (PELP1-cyto cells) have been developed as relevant model systems for studying non-genomic signaling (15). PELP1-cyto cells are hypersensitive to estrogen but resistant to TAM (15) and form tumors in nude mice (15). In addition, relative to the parental MCF-7 cells, PELP1-cyto cells exhibit an increased association between PELP1 and Src, enhanced stimulation of mitogen-activated protein kinase (MAPK), and constitutive activation of the Akt pathway. The altered localization of PELP1 to the cytoplasm has been shown to be sufficient to trigger its interaction with the p85 subunit of phosphatidylinositol-3-kinase, leading to its activation (15). In addition, PELP1 interacts with epidermal growth factor receptor and participates in growth factor-mediated ER transactivation functions (22). Together, these findings suggest that PELP1 localization could potentially be used as a determinant of hormone sensitivity. However, our current understanding of the non-genomic action of PELP1 is derived from in vitro cell culture and xenograft models, and validation in a physiologically relevant whole-animal model awaits further investigation. In this context, we present a transgenic mouse model of PELP1-cyto non-genomic signaling and its inability to respond to the anti-estrogenic action of TAM.
Results and Discussion
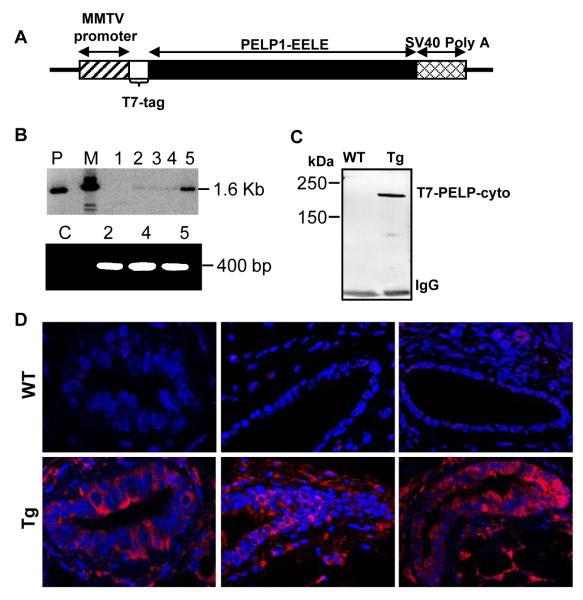
Recent studies using in vitro cell culture models have shown that cytoplasmic localization of PELP1 promotes resistance to TAM (15). To validate these findings in an in vivo animal setting and to generate a mouse model of non-genomic signaling, we generated cyto-PELP1 transgenic mice that express PELP1 in the cytoplasm by overexpression of a mutant of PELP1 (PELP1-cyto) in which the nuclear localization signal has been mutated from KKLK to EELE (Fig. 1A). As a means of targeting the expression of the PELP1-cyto transgene to the mammary gland, we placed PELP1-cyto cDNA under the control of the MMTV promoter, which directs transgene expression to mammary gland in the early stages of puberty and is hormonally regulated by progesterone during estrus and pregnancy (23). Founders with integration of the transgene constructs into the genome were screened using polymerase chain reaction (Fig 1B, lower panel), and results were confirmed by Southern blot analysis (Fig. 1B, Upper panel). Founder Line 5 of PELP1-cyto transgenic mice was used for the study. The protein product of the transgene was detected by immunoprecipitation using anti-T7 agarose beads, followed by immunoblotting with anti-T7 polyclonal antibody (Fig. 1C). Further, to assess that the levels overexpressed cytoplasmic PELP-1 in PELP1-Cyto transgenic mice are higher than the endogenous PELP1 level, we did western blot analysis with an anti-PELP1 antibody. We found that the amount of overexpressed cytoplasmic PELP-1 is comparatively much higher than the endogenous nuclear PELP-1 levels (Supplementary Fig. 1A), and thus, the phenotype reported here will be predominantly under the influence of the cytoplasmic PELP1. Also, Immunofluorescence staining of paraffin section of the mammary gland with an anti-T7 antibody revealed PELP1-cyto transgene localization exclusively in the cytoplasm (Fig. 1D).
Fig. 1.
Generation of PELP1-cyto transgenic mice. (A) Schematic representation of the MMTV-T7—cyto-PELP1 transgenic construct. (B) Southern blot detection of the transgene in mouse tail genomic DNA (Upper panel). PCR(Lower panel) for genotype of transgenic mice using genomic DNA from the tail of different lines of transgenic and wild-type mice. P, plasmid as positive control; M, marker; C, control. (C) Transgene protein expression in the mammary glands as detected by T7 immunoprecipitation (IP) followed by Western blotting with T7 antibody. (D) Immunofluorescence analysis showing T7-tagged mutant PELP1 (red) in the cytoplasm of mammary epithelial cells and DAPI-stained nuclei (blue) in wild-type (top) and transgenic (bottom) mice.
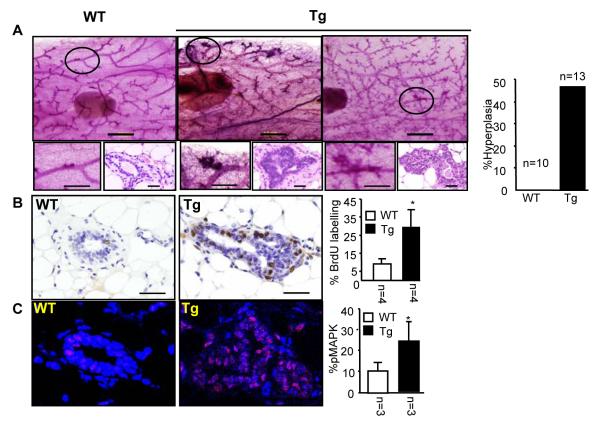
To investigate the effect of PELP1-cyto transgene on the development of mammary glands, we examined whole-mount preparations of mammary glands from littermates with matching estrous cycles at different developmental stages. We found that about 46% of transgenic females developed atypical hyperplasia at 12 weeks of age, and this became more aberrant as mice aged, whereas wild-type littermates did not exhibit such phenotypes at the same ages (Fig. 2A). To determine whether the observed phenotypic alterations were the result of increased cell proliferation, DNA synthesis was measured in an in vivo BrdU incorporation assay. In mammary glands of transgenic mice, the proliferation index, calculated as the percentage of BrdU-positive cells among total epithelial cells, was significantly higher in transgenic than in wild-type mice (Fig. 2B). Consistent with our previous in vitro data (15), phosphorylated MAPK levels were significantly higher in transgenic than wild-type mice as assayed by immunohistochemistry (Fig. 2C) and immunoblotting (Supplementary Fig. 2) with phospho-MAPK specific antibody. Also consistent with an active non-genomic signaling, we found increased levels of phospho-AKT and phospho ER-serine 118 in PELP1-cyto transgenic mice, compared with levels in wild-type mice (Supplementary Fig. 2). To our knowledge, this is the first in vivo evidence that altered localization of ER coactivator PELP1 promotes increased AKT and MAPK activation, leading to enhanced cell proliferation and thereby promoting mammary hyperplasia. However, none of the transgenic mice developed mammary gland tumors even after 2 years. This could be because we used the transgenic mice expressing MMTV-PELP1-cyto under the influence of hormones or due to the mixed genetic background of these animals which is 25% C57B6 and 75% DBA or both. It is very likely that changing the strain background might result in tumor formation, as has been observed in other mammary gland models (24).
Fig. 2.
Cytoplasmic PELP1 in mammary epithelium causes hyperplasia and promotes non-genomic signaling. (A) Whole-mount and histological analyses of MMTV—cyto-PELP1 transgenic mice (Tg) showing the mammary hyperplasia at 12 weeks of age compared with age-matched wild-type (WT) mice. (B) BrdU-positive cells for both transgenic and WT mice counted and expressed as a ratio of positive nuclei to the total number of nuclei counted (percentage). (C) Immunofluorescence analysis with anti—phospho-MAPK antibody showing increased activation of the MAPK (red) in transgenic glands (TG) compared with WT control glands. Nuclei were stained with DAPI (blue). The plot on the right panel shows the quantitative analysis of percentage of positive cells for phospho-MAPK.
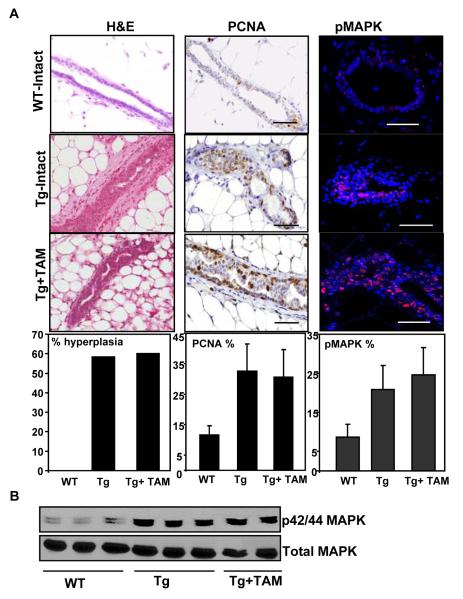
Next we used this model to confirm the hypothesis that altered localization of PELP1 could participate in the development to resistance to TAM. Transgenic and age-matched wild-type female virgin mice (n=10 each group) were implanted with TAM pellets (Innovative Research of America, Sarasota, FL) at 10 weeks of age, before the development of detectable hyperplasia. The effect of TAM was evaluated after 2.5 months of exposure (at 19 weeks of age) (Supplementary Fig. 3). We found that, compared with intact transgenic mice, which exhibited 58% typical hyperplasia, TAM treatment did not prevent or reduce the extent of hyperplasia in the treatment group (Fig. 3A). Consistent with this result, cell proliferation, as analyzed by PCNA staining, was not significantly decreased in TAM-treated mice compared with untreated transgenic mice (33 ± 9% vs 31 ± 9%; Fig. 3B). Furthermore, immunohistochemical analysis of mammary gland sections for phospho-MAPK revealed that TAM exposure did not attenuate activation of MAPK induced by transgene (21 ± 6% vs 25 ± 7%; Fig. 3A and 3B). Taken together, these results suggest that mammary epithelium harboring the PELP1-cyto transgene exhibits an increased non-genomic signaling and resists the anti-tumor activity of TAM, which in-turn could be due to the cumulative effects of the activation of the MAPK and Akt pathways, including post-translational modification of ER and its associated proteins.
Fig. 3.
Cytoplasmic PELP1 retention in mammary epithelium promotes hormonal independence. (A) Systematic implantation of TAM pellets in MMTV-cyto-PELP mice. TAM did not prevent or reduce the extent of mammary hyperplasia. Also shown are the results of immunohistochemical analysis showing a progressive increase in the levels of staining for PCNA, a marker for cell proliferation and enhanced activation of MAPK from WT to the TAM-treated group. Plots on the bottom panel show the quantitative analysis. (B) Western blot analysis showing the levels of activated MAPK in the TAM group.
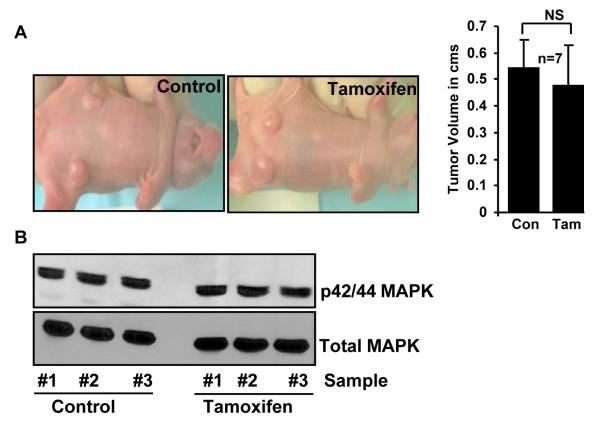
Because PELP1-cyto transgenic mice did not form tumors even though they developed mammary hyperplasia, the role of cytoplasmic PELP1 in hormone resistance in mammary gland tumors remains unknown. To address this issue, we made use of the well-characterized MCF-7/cytoplasmic PELP1-expressing clones (PELP1-cyto) (15). As expected, we found that PELP1-cyto was localized to the cytoplasm, whereas wild-type PELP1 was localized to the nucleus (Supplementary Fig. 1B). The PELP1-cyto clones are hormone independent (Supplementary Fig. 1C) and form tumors in nude mice (15). Consistent with a previous report (15), PELP1-cyto clones readily formed tumors (14/14 mice) when injected into the mammary fat pads of nude mice. However, TAM treatment did not prevent or reduce the extent of mammary tumorigenesis in these mice (Fig. 4A). Western blot analysis of lysates from these tumors consistently revealed that there was no change in the levels of activated MAPK after TAM treatment (Fig. 4B). Immunohistochemical analysis revealed that there is also no reduction in the levels of phospho Akt and PCNA after TAM treatment (Supplementary Fig. 4). Collectively, these findings suggest that cytoplamic PELP1 expression is one of the important biological variables of hormone sensitivity in breast tumors.
Figure 4.
Cytoplasmic PELP1 induced mammary gland tumors are insensitive to tamoxifen. (A) Mammary tumor xenografts induced with MCF-7/PELP1-cyto clones in nude mice are insensitive to tamoxifen. (B) Western blot analysis showing levels of activated MAPK in lysates from the above tumors.
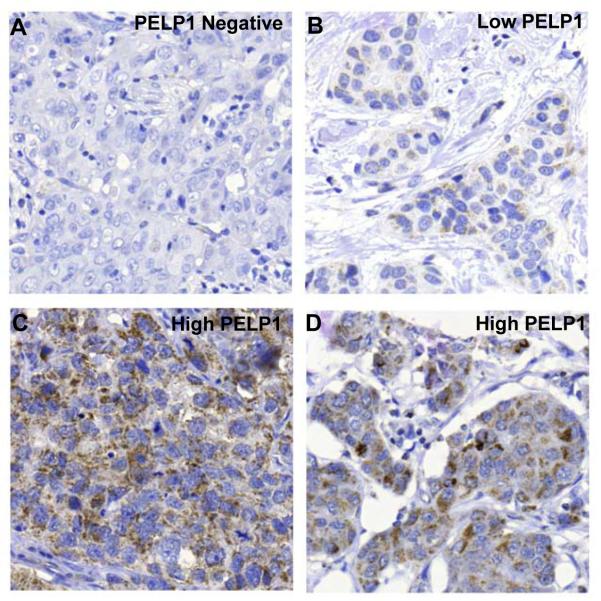
Encouraged by the above results and to valuate the clinical implication to the above data, we analyzed the cytoplasmic localization of PELP1 in a cohort of 331 breast tumor (234 ER-positive) samples (25) by immunohistochemistry. The cut-off limits between low- and high-tumors were as follows; tumors with no staining (i.e negative), weak staining or less than 50% tumor cells with a strong staining were classified as low PELP1; tumors with more than 50% tumor cells with a strong staining were classified as high PELP1. Results showed that 43% of the tumors had high levels of cytoplamic PELP1, and that high PELP1 correlated with tumor grade (Nottingham Histological Grade; NHG) and proliferation (Fig. 5A-D; Table 1). NHG is assessed based upon three parameters: tubular structure, nuclear atypia and mitotic count. The higher the score from these parameters the more malignant the tumor is. This suggests that high PELP1 expression in the cytoplasm is coupled to a more aggressive tumor.
Figure 5.
Immunohistochemical analysis of cytoplasmic PELP1 in breast cancer specimens. (A-D). Breast tumors arranged in a tissue microarray were stained with PELP1 antibody (dil 1:2500). Panel A shows a tumor negative for PELP1, panel B shows low staining and panel C and D show strong staining.
Table 1.
Distribution of cytoplasmic PELP1 staining in breast tumors according to tumor grade and proliferation.
| Variable | PELP1 cytoplamic staining |
||
|---|---|---|---|
| Negative/Low N=188 (57%) |
High N=143 (43%) |
P¶ | |
| NHG | 0.001 | ||
| I | 26 | 11 | |
| II | 79 | 42 | |
| III | 83 | 87 | |
| Unknown : 3 | |||
| Ki-67 | 0.001 | ||
| 0-10% | 83 | 44 | |
| 11-25% | 51 | 40 | |
| >25% | 37 | 50 | |
NHG — Nottingham histologic grade
Correlations were calculated using Spearman’s ρ.
Because cytoplasmic PELP1 has been implicated in TAM resistance, we next analyzed the recurrence-free survival (RFS) in ER-positive samples. A significant statistical difference between RFS in untreated patients and RFS in tamoxifen treated patients was seen in patients with low PELP1 tumors (p=0.05), indicating that patients with Low PELP1 tumors respond well to tamoxifen. Conversely, in patients with High PELP1 tumors, the difference between RFS in untreated patients and RFS in tamoxifen treated patients, was not significant (p=0.3), indicating that patients with high PELP1 tumors do not respond well to tamoxifen (Supplementary Fig. 2A & B).
Recent work from other groups (26, 27) has shown that ER signaling or its coregulators are contributing factors to TAM resistance. Therefore, in addition to ER itself, ER coregulators are potential clinical intervention targets. That PELP1 localization is altered in a subset of tumors, promotes local estrogen synthesis (28), and promotes resistance to tamoxifen in animal and cancer cell models suggests that altered subcellular localization of PELP1 expression has the potential to contribute to the noted tendency of breast cancer patients with increased PELP1 expression to develop resistance to TAM therapy, presumably due to excessive stimulation of the nongenomic signaling pathways such as PI3-K (14) , Akt (15), and MAPK (this study). However, the mechanisms or modifications that promote PELP1 localization to the cytoplasm at the first place remain elusive. Given that recent evidence (29) has suggested that posttranslational modifications of coregulators could play a role in its subcellular localization, it is tempting to speculate that the reported posttranslational modification of PELP1 (30) may be involved in its exclusive cytoplasmic localization and is being persued in Dr.Vadlamudi’s laboratory.
In conclusion, these findings suggest that PELP1 localization could be potentially used as a determinant of hormone sensitivity or vulnerability. Our PELP1-cyto transgenic mouse model, which precisely mimics characteristics of hormone independence and the biochemistry of human breast cancers, might be useful in further advancing the field of hormone resistance. Preclinical investigations using this animal model might lead to the more efficient design of clinical trials by predicting hormonal effects and the mechanisms of action of developing resistance.
Materials and methods
Generation of PELP1-Cyto transgenic mice
An MMTV-human PELP-cyto transgenic construct was created by subcloning T7-tagged PELP1-cyto cDNA using sites HindIII-XbaI (blunted) into HindIII-XhoRI (blunted) of the MMTV-SV40-BssK vector (23). The transgene was excised from plasmid DNA, and the 3.9 linear fragment-containing promoter sequences, MTA1-coding and 3′ untranslated regions and SV40 polyadenylation signals was injected to the pronuclei of a B6D2F1/J mouse embryos. Finder lines were identified by Southern blotting of tail DNA digested which showed a band of 1.6 kb PEP-cyto cDNA. Genotyping was performed by using the polymerase chain reaction assay. The primers used were forward primer to the T7-epitope encoding region (5′-CAGCAAATGGGTCGGGATC) and reverse primer (5′-CTACCGAAGTTCCTCCCCAC) corresponding to PELP1-cyto cDNA. These primers only amplify T7-tagged PELP1-cyto and do not recognize endogenous mouse PELP1. As expected, these primers specifically amplified 420 bp band in PELP-cyto-transgene positive founder lines.
Mammary gland whole mounts, histology and immunodetection and immunofluorescence
For whole-mount analysis, number 4 inguinal mammary glands were stained with carmine alum, as previously described (23). Briefly, the glands were fixed with acetic acid/ethanol (1:3) for 2 hours and stained with 0.5% carmine/0.2% aluminum potassium sulfate for 16 hours. After briefly being rinsed with distilled water, the mammary glands were dehydrated using graded ethanol, and lipids were removed with two changes of acetone. Finally, the glands were preserved in methyl salicylate. For histological analysis, mammary-gland tissue was fixed in 10% neutral buffered formaldehyde and embedded in paraffin wax according to standard methods. Sections (4 μm) were stained with Hematoxylin and Eosin. For immunostaining and immunofluorescence the following primary antibodies were used. T7 tag were from Novagen (San Diego, CA), those against proliferating cell nuclear antigen (PCNA) were from Genetex (San Antonio, TX), Antibodies against AKT, phospho-AKT, and phospho-p44/42(Thr-202/Tyr-204) were purchased from Cell Signaling (Beverly, MA), Erk-1 and -2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). To detect bromodeoxyuridine (BrdU)-positive cells, a sterile solution of 5-bromo-2′-deoxyuridine (BrdU) (20 mg/ml; Sigma-Aldrich) in PBS (pH 7.4) was administered to mice by intraperitoneal injection (50 mg/kg). Mammary glands were harvested after 3 hours, embedded in paraffin wax and sectioned. BrdU incorporation was detected by immunohistochemistry using a mouse anti-BrdU monoclonal antibody.
Animal Husbandry and treatment
Mice were housed at the animal care facility at the University of Texas, MD Anderson Cancer Center. All procedures involving mice were conducted in accordance with NIH regulations concerning the use and care of experimental animals and were approved by the institutional review board. Mice were maintained on a mixed C57/BL6: DBA background. Line 5 of PELP1-cyto transgenic mice were used for further study. For tamoxifen pellet implantation, each group (intact and TAM) included 10 mice. Age-matched wild-type (most of them are littermates of the transgenic) were used as controls. Virgin mice were implanted with TAM pellets (NE-361, Innovative Research of America, Sarasota, FL) at 10 weeks of age. Tissue sample were obtained at 19 weeks of age for analysis.
Immunoblot and Immunoprecipitation Analysis
About 50 μg of total protein extract from cultured cells or mammary glands was used in the immunoblot assays. The lysates to immunoprecipitate T7-tagged proteins were immunoprecipitated for 2 h at 4 °C using 50 μl of T7-agarose beads per milligram of protein.
Mouse xenograft studies
For the tumorigenesis studies, 4-week-old ovariectomized female athymic nude mice (Charles River, Wilmington, MA) were bilaterally injected with 5 × 106 MCF-7 PELP1 cyto cells into the mammary fat pads, as previously described (15). Mice were divided in to two groups. One group received tamoxifen pellets and the other group is the control Tumors were allowed to grow for 5 weeks, and tumor size was measured every 15 days. All animal procedures were done in compliance with the Institute Animal Care and Use Committee and the NIH Policy on Humane Care and Use of Laboratory Animals.
Supplementary Material
Acknowledgements
We thank Dr.Robert D.Cardiff, University of California, Davis, CA 95616 for analyzing the slides.
Grant Support: NIH grant CA 90970 to R.K.
Footnotes
STATEMENT OF TRANSLATIONAL RELEVANCE
PELP1 is widely expressed in breast cancer cells and its expression is deregulated in breast tumors. In this study, we show that mammary glands from a transgenic mouse expressing a cytoplasmic version of PELP1 mutant defective in its nuclear translocation (PELP1-cyto) developed widespread hyperplasia and enhanced activation of extra nuclear signaling events. Treatment with tamoxifen did not inhibit this hyperplasia; rather exaggerated hyperplasia with an enhanced degree of alteration indicative of hypersensitivity to tamoxifen. In the future, preclinical investigations using this animal model system that accurately mimic characteristics of estrogen independence would greatly assist in more efficient design of clinical trials by predicting hormonal effects and their mechanisms of action of developing resistance.
References
- 1.Cordera F, Jordan VC. Steroid receptors and their role in the biology and control of breast cancer growth. Semin Oncol. 2006;33:631–41. doi: 10.1053/j.seminoncol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:195–216. [PubMed] [Google Scholar]
- 3.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 4.Jordan VC, O’Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25:5815–24. doi: 10.1200/JCO.2007.11.3886. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK. Mechanisms for tamoxifen resistance in breast cancer: possible role of tamoxifen metabolism. J Steroid Biochem Mol Biol. 1993;47:83–9. doi: 10.1016/0960-0760(93)90060-a. [DOI] [PubMed] [Google Scholar]
- 6.Graham JD, Bain DL, Richer JK, Jackson TA, Tung L, Horwitz KB. Thoughts on tamoxifen resistant breast cancer. Are coregulators the answer or just a red herring? J Steroid Biochem Mol Biol. 2000;74:255–9. doi: 10.1016/s0960-0760(00)00101-1. [DOI] [PubMed] [Google Scholar]
- 7.Gururaj AE, Rayala SK, Vadlamudi RK, Kumar R. Novel mechanisms of resistance to endocrine therapy: genomic and nongenomic considerations. Clin Cancer Res. 2006;12:1001s–7s. doi: 10.1158/1078-0432.CCR-05-2110. [DOI] [PubMed] [Google Scholar]
- 8.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 9.Iwase H, Omoto Y, Toyama T, et al. Clinical significance of AIB1 expression in human breast cancer. Breast Cancer Res Treat. 2003;80:339–45. doi: 10.1023/A:1024916126532. [DOI] [PubMed] [Google Scholar]
- 10.Vadlamudi RK, Wang R, Mazumdar A, et al. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem. 2001;276:38272–23. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- 11.Rajhans R, Vadlamudi RK. Comprehensive analysis of recent biochemical and biologic findings regarding a newly discovered protein-PELP1/MNAR. Clin Exp Metastasis. 2006;23:1–7. doi: 10.1007/s10585-006-9019-9. [DOI] [PubMed] [Google Scholar]
- 12.Brann DW, Zhang QG, Wang RM, Mahesh VB, Vadlamudi RK. PELP1-A novel estrogen receptor-interacting protein. Mol Cell Endocrinol. 2008;290:2–7. doi: 10.1016/j.mce.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004;64:6416–23. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- 14.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity—crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci U S A. 2002;99:14783–8. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Vadlamudi RK, Manavathi B, Balasenthil S, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–32. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimple C, Nair SS, Rajhans R, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–9. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- 17.Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair S, Vadlamudi RK. Emerging significance of ER-coregulator PELP1/MNAR in cancer. Histol Histopathol. 2007;22:91–6. doi: 10.14670/hh-22.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadlamudi RK, Balasenthil S, Broaddus RR, Gustafsson JA, Kumar R. Deregulation of estrogen receptor coactivator proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor in human endometrial tumors. J Clin Endocrinol Metab. 2004;89:6130–8. doi: 10.1210/jc.2004-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vadlamudi RK, Balasenthil S, Sahin AA, et al. Novel estrogen receptor coactivator PELP1/MNAR gene and ERbeta expression in salivary duct adenocarcinoma: potential therapeutic targets. Hum Pathol. 2005;36:670–5. doi: 10.1016/j.humpath.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Rayala SK, Hollander P, Balasenthil S, et al. Hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) interacts with PELP1 and activates MAPK. J Biol Chem. 2006;281:4395–403. doi: 10.1074/jbc.M510368200. [DOI] [PubMed] [Google Scholar]
- 22.Manavathi B, Nair SS, Wang RA, Kumar R, Vadlamudi RK. Proline-, glutamic acid-, and leucine-rich protein-1 is essential in growth factor regulation of signal transducers and activators of transcription 3 activation. Cancer Res. 2005;65:5571–7. doi: 10.1158/0008-5472.CAN-04-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagheri-Yarmand R, Talukder AH, Wang R-A, Vadlamudi RK, Kumar R. Metastasis associated protein 1 deregulation causes inappropriate mammary gland development and tumorigenesis. Development. 2004;131:3469–79. doi: 10.1242/dev.01213. [DOI] [PubMed] [Google Scholar]
- 24.Rose-Hellekant TA, Gilchrist K, Sandgren EP. Strain background alters mammary gland lesion phenotype in transforming growth factor-alpha transgenic mice. Am J Pathol. 2002;161:1439–47. doi: 10.1016/s0002-9440(10)64419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryden L, Jirstrom K, Bendahl P-O, et al. Tumor-specific expression of VEGFR2 is associated with impaired tamoxifen response in premenopausal breast cancer. J Clin Oncol. 2005;23:4695–704. doi: 10.1200/JCO.2005.08.126. [DOI] [PubMed] [Google Scholar]
- 26.Schiff R, Massarweh SA, Shou J, et al. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother Pharmacol. 2005;56(Suppl 1):10–20. doi: 10.1007/s00280-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 27.Kirkegaard T, McGlynn LM, Campbell FM, et al. Amplified in breast cancer 1 in human epidermal growth factor receptor - positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res. 2007;13:1405–11. doi: 10.1158/1078-0432.CCR-06-1933. [DOI] [PubMed] [Google Scholar]
- 28.Rajhans R, Nair HB, Nair SS, et al. Modulation of in situ estrogen synthesis by proline-, glutamic acid-, and leucine-rich protein-1: potential estrogen receptor autocrine signaling loop in breast cancer cells. Mol Endocrinol. 2008;22:649–64. doi: 10.1210/me.2007-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lonard DM, O’Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Nagpal JK, Nair S, Chakravarty D, et al. Growth factor regulation of estrogen receptor coregulator PELP1 functions via protein Kinase A pathway. Mol Cancer Res. 2008;6:851–61. doi: 10.1158/1541-7786.MCR-07-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.