Abstract
Following surgery, elderly patients often suffer from postoperative cognitive dysfunction (POCD) which can persist long after physical recovery. It is known that surgery-induced tissue damage activates the peripheral innate immune system resulting in the release of inflammatory mediators. Compared to adults, aged animals demonstrate increased neuroinflammation and microglial priming that leads to an exaggerated proinflammatory cytokine response following activation of the peripheral immune system. Therefore, we sought to determine if the immune response to surgical trauma results in increased neuroinflammation and cognitive impairment in aged mice. Adult and aged mice underwent minor abdominal surgery and 24 h later hippocampal cytokines were measured and working memory was assessed in a reversal learning version of the Morris water maze. While adult mice showed no signs of neuroinflammation following surgery, aged mice had significantly increased levels of IL-1β mRNA in the hippocampus. Minor surgery did not result in severe cognitive impairment although aged mice that underwent surgery did tend to perseverate in the old target during reversal testing suggesting reduced cognitive flexibility. Overall these results suggest that minor surgery leads to an exaggerated neuroinflammatory response in aged mice but does not result in significantly impaired performance in the Morris water maze.
Keywords: aging, cytokines, brain, POCD, working memory, mice
1. Introduction
Medical advances in techniques for anesthesia and surgery allow elderly patients to undergo surgical procedures later in life with a reduced risk in mortality. However, aged individuals are at a substantially increased risk for central nervous system (CNS) dysfunction and in particular, cognitive decline following surgery. Elderly patients often suffer from postoperative delirium following a surgical procedure and these cognitive deficits can linger for months and even years after physical recovery from the operation. Postoperative cognitive dysfunction (POCD) is characterized by a persistent decline of cognitive performance after surgery as assessed by preoperative and postoperative cognitive testing, and is defined as a “deterioration of intellectual function presenting as impaired memory or concentration” (Moller et al., 1998; Rasmussen, 2006). Estimated prevalence of postoperative cognitive dysfunction in patients over the age of 60 is 15-25% with approximately 10% exhibiting symptoms three months after surgery (Dodds and Allison, 1998; Olin et al., 2005; Rasmussen and Siersma, 2004). Patients who exhibit postoperative cognitive disorders after surgery often continue to deteriorate and are three times more likely to suffer further cognitive decline one to two years after surgery (Abildstrom et al., 2000; Lewis et al., 2007).
The mechanisms underlying the pathology of this cognitive disorder are not well understood and the possible contributions of anesthetics and/or analgesics versus the contribution of the surgical trauma itself are unclear. Human studies have identified advancing age, duration of anesthesia and multiple surgeries as risk factors for POCD but are unable to distinguish between the contribution of major surgery and anesthesia to the observance of POCD (Moller et al., 1998; Newman et al., 2001; Rohan et al., 2005). Other factors such as hypoxia-ischemia, hypotension and microembolism do not seem to influence the incidence of POCD suggesting it may be the surgical trauma itself that leads to changes in cognition (Cook et al., 2007; Koch et al., 2007; Moller et al., 1998). Several large-scale patient studies have consistently found increasing age to be the most relevant risk factor in the development of POCD (Moller et al., 1998; Monk et al., 2008; Rasmussen, 2006; Williams-Russo et al., 1995). Thus, it may be that the aging brain is more vulnerable to the additional insult of a surgical procedure resulting in long-lasting cognitive impairment.
Our laboratory has shown that normal aging is associated with increased neuroinflammation and that an exaggerated inflammatory response occurs in the healthy aged brain when lipopolysaccharide (LPS) activates the peripheral innate immune system (Godbout et al., 2005). In addition to increased expression of proinflammatory mediators such as IL-1β, IL-6 and TNF-α in the brain, aged animals suffer from exaggerated sickness behavior and disrupted cognitive processing following immune system activation (Abraham et al., 2008; Chen et al., 2007; Godbout et al., 2005). Normal aging is thought to “prime” the central cytokine compartment, therefore an amplified and prolonged inflammatory response in the brain occurs when the peripheral innate immune system is activated. This exaggerated neuroinflammatory reaction may underlie the neurobehavioral impairments observed in elderly patients with an infection and explain why infection can exacerbate neurodegenerative diseases such as Multiple Sclerosis and Alzheimer’s (Perry et al., 2007).
During aging, increased inflammation and microglial priming set the stage for an amplified central cytokine reaction to a secondary stimulus of the innate immune system. It is known that tissue damage resulting from surgery activates the peripheral innate immune system leading to activation of the cytokine cascade and the release of many inflammatory mediators including oxygen free radicals, arachadonic acid metabolites, cytokines, nitric oxide and endothelins (Giannoudis et al., 2006; Karlidag et al., 2006; Levy and Tanaka, 2003; Lin et al., 2000). However, it is not yet known if a peripheral immune response to surgical trauma results in an exaggerated neuroinflammatory response in the aged brain. Therefore, the present study investigated whether aged mice demonstrate an increased neuroinflammatory response and prolonged deficits in cognitive function following surgery-induced activation of the peripheral innate immune system. An initial study determined that anesthetics and analgesics alone did not alter proinflammatory cytokines levels in the hippocampus of adult or aged mice. Furthermore, while adult mice showed no signs of neuroinflammation following surgery, aged mice had significantly increased levels of IL-1β mRNA in the hippocampus. Although elevation in IL-1β did not result in gross learning and memory impairment, aged mice that underwent surgery tended to perseverate in the old target quadrant during reversal testing suggesting alterations in cognitive flexibility. These results suggest that minor surgery significantly increases IL-1β production in the brain of aged animals but does not result in significant behavioral deficits in a reversal learning version of the Morris water maze.
2. Materials and Methods
2.1. Animals
Adult (4-6 months old) and aged (23-25 months old) male BALB/c mice from our in-house specific pathogen free colony were used. Mice were housed in polypropylene cages and maintained at 23°C under reverse phase 12-h light: 12-h dark cycle with ad libitum access to water and rodent chow. At the end of each study mice were examined postmortem for gross signs of disease (e.g. splenomeglia and tumors). Data from mice determined to be unhealthy were excluded from analysis. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Illinois Institutional Animal Care and Use Committee.
2.2. Surgical Procedure
A crucial asset in an animal model of surgery is the ability to control for a variety of confounding factors that make data from human studies of POCD difficult to interpret. Using an animal model, we were able to control both the length and severity of the abdominal surgery. Mice were deeply anesthetized using ketamine and xylazine (1.0 mg and 0.1 mg/10 g BW intraperitoneally (i.p.), respectively). After the surgical site was shaved and sterilized, a 1.5 cm incision was made in the upper left quadrant through the skin and muscle wall. A sterile probe was then inserted into the body cavity to gently manipulate the internal organs for 1 min. Three dissolvable sutures were used to close the muscle wall and four silk thread sutures were used to close the skin. To limit variability all surgeries were performed by one person and lasted ∼ 10 min. Animals receiving postoperative analgesics were injected subcutaneously with buprenorphine (1.0 mg/10 g BW) upon recovery from anesthesia.
2.3 Brain cytokine mRNA measurement by quantitative real-time PCR
Total RNA was isolated from homogenized brain regions using the Tri Reagent protocol (Sigma, ST. Louis, MO). A QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA) was used for cDNA synthesis with integrated removal of genomic DNA contamination according to the manufacturer protocol. In brief, RNA samples were mixed with gDNA Wipeout Buffer and RNase-free water and incubated at 42°C for 2 min. Quantiscript Reverse Transcriptase, Quantiscript RT Buffer, and RT Primer mix were added to samples and incubated at 42°C for 15 min, followed by incubation at 95°C for 3 min to inactivate Quantiscript Reverse Transcriptase. Quantitative real time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol. In short, cDNA was amplified by PCR where a target cDNA (IL-1β, Mm00434228_ml; TNF-α, m00443258_ml; IL-6, Mm00446190_ml) and a reference cDNA (glucose-3 phosphate dehydrogenase, Mn99999915_gl) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCR reactions were performed at the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold change.
2.4. Behavioral Tests
2.4.1. Locomotor Activity
To estimate locomotor activity, mice were kept in their home cage and video recorded during 3 min tests using a camera mounted directly above the cage. On video records, cages were divided into six identical rectangles and a trained observer who was blind to experimental treatments determined the frequency of line crossing. A subject was considered to have crossed a line only if its fore and hind limbs entered a new rectangle.
2.4.2. Spatial working memory - Morris water maze
A circular tank 100 cm in diameter and 30 cm deep was filled with water (24-26° C) to a depth of 25 cm. A transparent round platform 10 cm in diameter was placed ∼ 0.5 cm below the surface of the water. In this test of spatial memory the animal must learn to use distinctive distal visual cues surrounding the pool to navigate a direct path to the hidden platform. Animal training took place during a 6 day acquisition phase with three massed trials administered each day. The platform remained in a constant location during the acquisition phase. Animals were placed on the platform for 30 s preceding the start of each training session. The trials were conducted using a pseudorandom protocol in which mice were placed in the water in one of three preset entry locations. Mice were allowed to swim freely for 60 s or until the platform was reached. If the platform was not located during the 60 s, mice were guided to the platform and allowed to remain for 30 s. After completion of three consecutive trials, mice were placed in their home cage under a heat lamp for ∼ 10 min. On day 7, animals underwent surgery and 24 h later (day 8), were subjected to a reversal test in which the platform was moved to the opposite quadrant of the pool but all distal visual cues remained constant. Animals were placed on the platform for 30 s preceding the start of the reversal test and given three trials to locate the platform in the new target quadrant. Reversal learning is used as a measurement of how quickly an animal is able to extinguish their initial learning of the platform’s position and acquire a direct path to the new location (Vorhees and Williams, 2006). A video camera mounted to the ceiling directly above the center of the maze was used in conjunction with a computerized animal tracking system (Ethovision; Noldus Information Technologies, Netherlands) to monitor swim speed, latency to the platform and distance swam.
2.5. Experimental Protocol
The first experiment was designed to determine the contribution of the surgical procedure itself versus the possible effects that anesthesia and/or postoperative analgesics may have on cytokine mRNA expression in the brain. Both adult and aged mice were divided into 5 experimental groups (n = 8-10 for each group). One group of animals (anesthesia alone) received ketamine and xylazine (1.0 mg and 0.1 mg/10 g BW i.p., respectively) without surgery to determine the independent contribution of anesthetic drugs to changes in proinflammatory cytokine levels in the brain. Another group (analgesics alone) received only buprenorphine (1.0 mg/10 g BW s.c.) to elucidate the possible effects postoperative analgesics alone may have on cytokine expression. Animals that underwent abdominal surgery were divided into a group that received postoperative analgesics (surgery with analgesics) and a group that did not (surgery without analgesics), thus allowing us to determine how the surgical procedure alters inflammatory cytokine levels both with and without subsequent analgesia. Finally a group (saline-injected controls) was administered an i.p. injection of sterile saline to control for possible effects of handling and injection stress. Mice were killed by CO2 asphyxiation 24 h after treatment and hippocampal tissue was collected and stored in RNAlater at -80 °C until assaying for inflammatory cytokine levels.
A subsequent study was conducted to determine the effects of surgery on locomotor activity and cognitive function in adult versus aged mice. Adult (n = 8-10) and aged (n = 8-10) mice undergoing surgery without postoperative analgesics were compared to control animals (n = 8-10 for each age) both before and after treatment to determine if the surgical procedure would depress locomotor behavior. The same group of animals used for determining locomotor behavior were also tested in a reversal learning version of the Morris water maze to assess changes in hippocampal-dependent learning and memory 24 h following surgery.
2.6. Statistical analysis
Cytokine data from real-time PCR were subjected to two-way ANOVA in which age (adult or aged) and treatment (control or surgery) were dependent variables. For spatial working memory, means for each test session were calculated and behavioral data were subjected to repeated-measures ANOVA as a two-way design in which training day was a within subjects measure (i.e., repeated measure), and age (adult or aged) and treatment (control or surgery) were between subjects measures for acquisition training (days 1-6). A separate two-way ANOVA examined the effects of age (adult or aged) and treatment (control or surgery) on working memory performance during reversal testing (day 8). When the ANOVA revealed a significant main effect or interaction between main factors, post-hoc Student’s t test using Fisher’s least significant differences was employed to determine if treatment means were significantly different from one another (p < 0.05). All data are presented as means ± SEM.
3. Results
3.1. Anesthetics and analgesics do not alter cytokine levels in the hippocampus
To determine whether anesthesia or analgesics are compounding factors on cytokine production post-surgery, hippocampal IL-1β, IL-6 and TNF-α mRNA levels were measured 24 h after treatment. A complete factorial design was not possible due to the lack of a surgery without anesthesia group. Because one-way ANOVA revealed a significant effect of treatment (p < 0.01), Student’s t test using Fisher’s least significant differences was employed to determine if treatment means of each age group (saline-injected controls, anesthetics alone, analgesics alone, surgery without analgesics and surgery with analgesics) were significantly different from each other. Neither anesthetics nor analgesics significantly altered proinflammatory cytokine levels in the hippocampus of adult or aged animals when compared to their age-matched controls (data not shown). Therefore, animals that did not receive surgery (saline injected, anesthetics alone and analgesics alone) were combined by age and referred to as adult controls or aged controls. In both age groups, animals that received analgesics after surgery did not show significant differences in expression of proinflammatory cytokines compared to animals that did not receive postoperative analgesics (data not shown). Thus, all animals that received surgery (with or without analgesics) were pooled by age into adult surgery or aged surgery treatment groups.
3.2. Surgery increases IL-1β mRNA levels in the hippocampus of aged mice
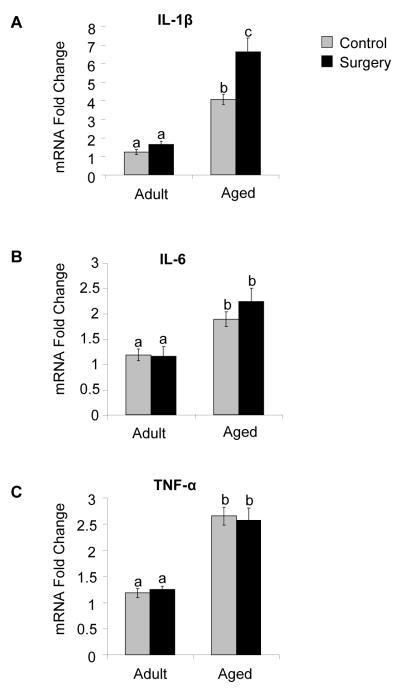
Studies from our lab suggest that the hippocampus of aged animals is vulnerable to cytokine-induced disruption of cognitive processing after peripheral immune system activation by LPS (Chen et al., 2007; Sparkman et al., 2006). In addition, the hippocampus is known to be especially sensitive to aging and is involved in the types of cognitive impairment seen in elderly patients following surgical trauma (Price et al., 2008). Therefore, hippocampal cytokine mRNA levels of IL-1β, IL-6 and TNF-α were measured 24 h after the surgical procedure in both adult and aged mice. Two-way ANOVA examined the effects of age (adult or aged) and treatment (control or surgery) on proinflammatory cytokine mRNA expression. Analysis of hippocampal IL-1β mRNA levels revealed a main effect of age (p< 0.001) and treatment (p < 0.001) as well as a significant age × treatment interaction (p < 0.01) indicating that surgery induced an increase in IL-1β in the hippocampus that was enhanced in the aged mice (Fig. 1A). While there was a main effect of age for both IL-6 and TNF-α (p < 0.001), there was not a significant effect of treatment or an age × treatment interaction indicating that although these cytokines are elevated in the aged hippocampus, the surgical procedure employed here did not result in further amplification (Fig. 1B-C).
Fig. 1.
Surgery increases IL-1β mRNA levels in the hippocampus of aged mice. IL-1β (A), IL-6 (B), and TNF-α (C) mRNA levels were measured in the hippocampus of adult and aged mice 24 h following surgery. Bars represent means ±S.E.M. Means with different letters (a, b, or c) are significantly different (p <0.05) from each other.
3.3. Locomotor activity is not depressed in adult or aged mice following surgery
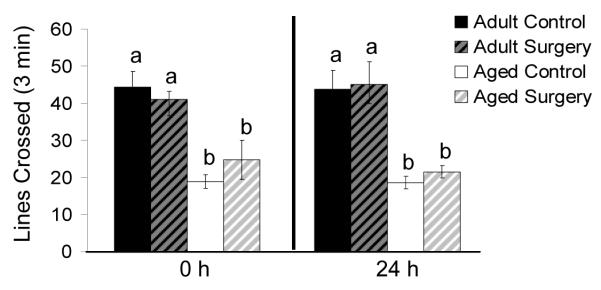
To determine if surgery depressed spontaneous motor activity (i.e. no motivational stimulus was provided), locomotor behavior was assessed in both adult and aged animals immediately before and 24 h following surgery (Fig. 2). Results from two-way ANOVA (age × treatment) show that while there was a significant main effect of age (p < 0.001) both before and after surgery, there was no main effect of treatment or an age × treatment interaction suggesting that general motor activity decreases with age but surgery alone does not depress locomotor behavior in mice when compared to their age-matched controls.
Fig. 2.
Locomotor activity is not depressed in adult or aged mice following surgery. Locomotor behavior (lines crossed) was measured both immediately before (0 h) and 24 h after surgery in adult and aged mice. Bars represent means ±S.E.M. Means with different letters (a or b) are significantly different (p <0.05) from each other.
3.4. Aged mice demonstrate impaired performance on basic parameters in the Morris water maze but these deficits are not exacerbated by the minor surgical procedure
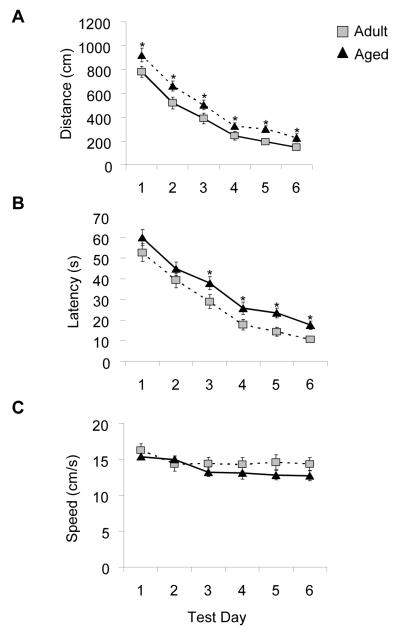
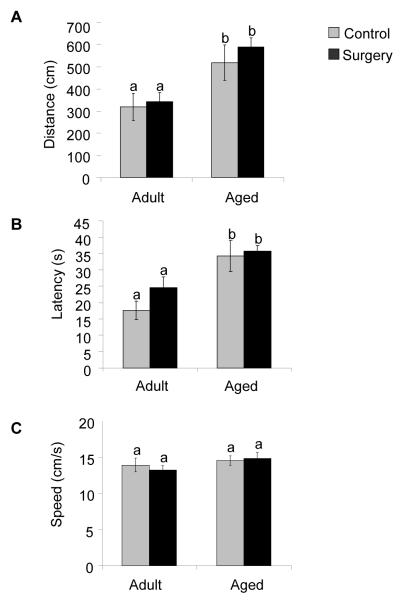
Because elevated levels of IL-1β have been shown to impair cognitive function, we next tested mice in the Morris water maze to determine if the surgery-induced increase in IL-1β in the brain of aged animals would disrupt hippocampal-dependent learning and memory. Adult and aged mice received 6 days of acquisition training with the platform in a fixed location. On day 7 animals underwent surgery and 24 h later (day 8) were subjected to a reversal test in which the platform was moved to the opposite quadrant of the pool while all distal visual cues remained constant. Repeated-measures ANOVA revealed that during the acquisition phase there was a main effect of day (p < 0.001) and age (p < 0.001) for both distance and latency. These data demonstrate that while the performance of both adult and aged mice improved over time, the aged mice swam further and longer in order to locate the platform during the acquisition phase (Fig. 3A-B). The reduced performance of aged mice did not appear to be due to lack of general motor ability or motivation because swim speed did not significantly differ by age across test sessions of the acquisition phase (Fig. 3C). A separate two-way ANOVA examined the effects of age (adult or aged) and treatment (control or surgery) on working memory performance during reversal testing. Analysis of swim data revealed a significant main effect of age for distance swam (p <0.001) and latency to platform (p < 0.001) but no main effect of treatment or an age × treatment interaction (Fig. 4A-B). There was no main effect of age, treatment or an age × treatment interaction for swim speed (Fig. 4C). The results indicate that while aging alone seems to impair learning in the acquisition phase as well as during reversal testing as assessed in the Morris water maze, these deficits in performance are not exacerbated by the minor surgical procedure.
Fig. 3.
Performance of adult and aged mice during a 6 day acquisition phase in the Morris water maze. (A) Distance swam to platform across testing days. (B) Latency to find the platform across testing days. (C) Swim speed across testing days. Data are represented as means ±S.E.M. Means with * are significantly different (p <0.05).
Fig. 4.
Surgery does not impair basic performance parameters in the Morris water maze. After 6-days of acquisition training, mice were evaluated in a reversal test 24 h after surgery. (A) Distance swam to platform during reversal testing. (B) Latency to find the platform during reversal testing. (C) Swim speed during reversal testing. Bars represent means ±S.E.M. Means with different letters (a or b) are significantly different (p <0.05) from each other.
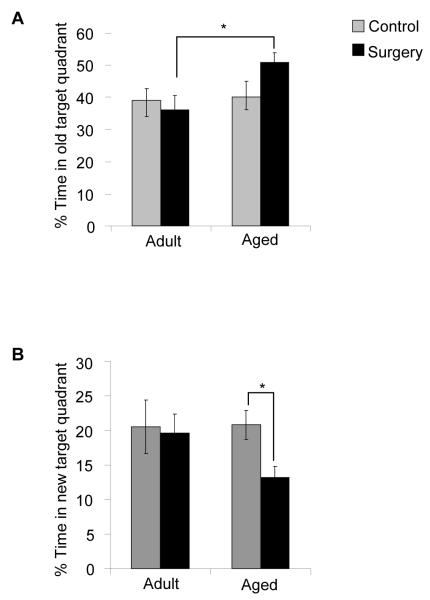
3.5. Aged mice tend to perseverate in the old target quadrant and spend less time in the new target quadrant during reversal testing in the Morris water maze
In addition to looking at basic performance parameters such as latency to the platform and distance swam, we also wanted to determine if surgery affected the amount of time an animal spent searching the old quadrant location after the platform had been moved to the new target quadrant for reversal testing. Perseveration in the old target quadrant may be due to impaired spatial extinction or impaired acquisition of the new spatial representation, both active learning processes occurring during reversal learning (Colon-Cesario et al., 2006). Perseveration was calculated as the percentage of total time in the maze in which the animal was searching the old target quadrant, an indication of reduced ability to change search strategy and impaired cognitive flexibility. Two-way ANOVA examined the effects of age (adult or aged) and treatment (control or surgery) on perseveration. Results revealed a trend for aged mice to spend more time in the old target quadrant (p = 0.07), but no significant effect of treatment. In addition, two-way ANOVA revealed a trend between age × treatment (p = 0.11), suggesting the possibility that surgery affected adult and aged animals differently. Post-hoc analyses revealed that aged mice that underwent surgery spent significantly more time perseverating in the old target quadrant than adult mice that underwent the same procedure (p = 0.02). In addition, there was a trend for aged surgical animals to spend more time in swimming in the old target quadrant than both adult controls (p = 0.06) and aged controls (p = 0.07) (Fig. 5A). Post-hoc analyses also revealed that aged surgical mice spent significantly less time searching for the platform in the new target location when compared to aged controls (p = 0.04). There was also a trend for aged surgical mice to spend less time in the new target quadrant than both adult surgical mice (p = 0.09) and adult control mice (p = 0.06) (Fig. 5B). Overall, these results suggest that in aged mice, minor surgery contributes to increased perseverative behavior suggesting reduced cognitive flexibility during reversal learning.
Fig. 5.
Effects of surgery on performance of adult and aged mice during reversal testing in the Morris water maze. (A) Aged mice that underwent surgery spent significantly more time perseverating in the old target quadrant than adult mice that underwent the same procedure. (B) In addition, aged surgical mice spent significantly less time in the new target quadrant compared to their age-matched controls. Data are represented as means ±S.E.M. Means with * are significantly different (p <0.05).
4. Discussion
It is expected that persons age 65 years and older will represent the most rapidly increasing age group for nearly all surgical subspecialties (Cohendy et al., 2005; Etzioni et al., 2003). Despite the fact that POCD is precisely defined and a variety of diagnostic and evaluation tools are available, its neurobiological basis remains poorly understood. Therefore, we sought to establish if surgery results in increased neuroinflammation and cognitive dysfunction in aged mice. The current study determined anesthetics and analgesics to have no significant effect on brain cytokine levels suggesting the surgical procedure itself was the main factor contributing to increased inflammation in the aged brain. The most important finding was that while adult mice showed no signs of neuroinflammation following surgery, aged mice had significantly increased levels of hippocampal IL-1β 24 h after the surgical procedure. Our results indicate that aging alone leads to increased levels of neuroinflammation and coincident impairment in learning ability during both the acquisition phase and reversal testing in the Morris water maze. These deficits were not significantly exacerbated by the minor surgical procedure. In addition, aged mice that underwent surgery tended to perseverate in the original target quadrant during reversal learning suggesting the possibility of surgery-induced alterations in cognitive strategy.
The brain is a target organ for both anesthetic and analgesic drugs and there is increasing evidence that the effects of these agents in the CNS outlast their pharmacological action (Lewis et al., 2007). Changes in pharmacokinetics and pharmacodynamics increase the sensitivity of the elderly patients to anesthetics and analgesics making the aged brain particularly vulnerable to long-term neurological changes (Cohendy et al., 2005; Lewis et al., 2007). Therefore, it is of relevance to determine what effect drugs used in the surgical setting have on both inflammatory and cognitive parameters. Both the anesthetic agent ketamine, and analgesic buprenorphine, have been shown to exert transient suppression of immune mediators in the periphery 2-4 h after administration (Carrigan et al., 2004; Martucci et al., 2004; Taniguchi et al., 2003). In contrast, we found no effect of ketamine or buprenorphine on proinflammatory cytokine expression. This difference may be due in part because we measured central instead of peripheral cytokine levels or because expression was evaluated at a later time point (24 h) after administration. Our data are in accordance with a recent study finding no association of anesthesia with neuroinflammatory changes or cognitive dysfunction in young rats that had undergone splenectomy (Wan et al., 2007). The finding that central cytokine levels were not altered by anesthetics or analgesics suggest that the surgical procedure itself increased hippocampal IL-1β in the aged brain and lends support to the idea that neuroinflammation may be an underlying cause of POCD.
Most importantly, the current study demonstrates that aged, but not adult, mice have increased levels of the proinflammatory cytokine IL-1β in the hippocampus following minor surgery. Elevated levels of IL-1β in hippocampus have been shown to impair synaptic plasticity leading to deficits in long-term potentiation (LTP) that can impair learning and memory function (Maher et al., 2006; Pickering and O’Connor, 2007). Previous studies have shown that high levels of IL-1β can impede performance during hippocampal-dependent learning and memory tests including the Morris water maze and contextual fear-conditioning (Barrientos et al., 2006; Gibertini et al., 1995; Oitzl et al., 1993; Pugh et al., 1998). Recently, our lab has shown that when the peripheral innate immune system is activated by LPS, only aged mice show impairment of working memory in a matching to place radial arm water maze (Chen et al., 2007). Aged animals demonstrated deficits in learning and memory 4 h after LPS injection, a time point shown to correspond with IL-1β mRNA levels in the hippocampus that were 100-fold greater in aged animals that received LPS compared to saline-injected controls (Chen et al., 2007). In the current study, aged animals were not severely impaired in a reversal learning version of the Morris water maze when tested 24 h after surgery, a time point corresponding to a 7-fold increase in hippocampal IL-1β mRNA levels. Although the peripheral immune system is activated by both LPS and surgical trauma, the resulting neuroinflammatory response in the brains of aged animals is amplified to a much greater extent after LPS challenge. A 7-fold increase in hippocampal IL-1β, although significant, may not be high enough to produce pronounced deficits in spatial working memory 24 h after the surgical procedure. The fact that this change in IL-1β was not sufficient to significantly depress locomotor activity, a behavior sensitive to cytokine effects, further demonstrates that neuroinflammation 24 h after surgery does not reach the level of elevation necessary to elicit exaggerated sickness behavior or severely impair cognition.
The severity of the surgery influences the magnitude of the immune response and the size of the incision has been shown to correlate with the degree of postoperative inflammation (Huang et al., 2005; Nelson and Lysle, 1998). The surgical procedure we used was modeled after laparoscopy, a relatively minor, often outpatient procedure in humans. It is likely that a more “major” surgery would induce a state of neuroinflammation aged animals that could result in considerable cognitive deficits. Previous work has shown that the incidence of POCD in elderly patients after minor surgery (primarily laparoscopy) was significantly lower than after cardiac and noncardiac major surgery suggesting that both age and extent of surgery contribute to postoperative brain dysfunction (Canet et al., 2003). The minor surgical procedure did not result in profound learning and memory deficits in a reversal learning version of the Morris water maze but aged surgical mice did tend to perseverate in the old target quadrant as well as spend less time swimming in the new target location. Perseveration indicates impaired ability to modulate behavior based upon new or changing information and may reflect a disruption in the integration of stored memory with current contextual information (Freudenberg et al., 2007). It has been previously shown that hippocampal-lesioned animals can acquire a place response in a water maze task but are impaired during reversal learning due to perseverative returns to the previous correct location (Whishaw and Tomie, 1997). Aged animals that underwent surgery demonstrate a similar pattern of reduced cognitive flexibility during reversal testing suggesting that surgery-induced neuroinflammation may contribute to alterations in hippocampal function.
In summary, the present study demonstrates that surgery significantly increases IL-1β in the hippocampus of aged animals, but does not depress locomotor behavior or severely impair spatial working memory in the Morris water maze. It is possible that a more severe “major” surgery could lead to a greater amplification of proinflammatory cytokines in the brain and hence impair cognitive function to a greater extent. We also observed that aged mice tended to spend more time perseverating in the old target quadrant during reversal testing suggesting that the surgery-induced increase in IL-1β may contribute to changes in cognitive flexibility. The exaggerated proinflammatory response that occurs in the aged brain after surgery may contribute to the cognitive deficits often seen in the elderly following surgical recovery and therapeutics aimed at decreasing surgery-induced neuroinflammation may be useful in preventing postoperative cognitive decline in aged patients.
Acknowledgements
This research was supported by NIH grants AG016710 and AG023580.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, Moller JT. Cognitive dysfunction 1-2 years after non-cardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction. Acta Anaesthesiol Scand. 2000;44:1246–1251. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Canet J, Raeder J, Rasmussen LS, Enlund M, Kuipers HM, Hanning CD, Jolles J, Korttila K, Siersma VD, Dodds C, Abildstrom H, Sneyd JR, Vila P, Johnson T, Corsini L. Munoz, Silverstein JH, Nielsen IK, Moller JT. Cognitive dysfunction after minor surgery in the elderly. Acta Anaesthesiol Scand. 2003;47:1204–1210. doi: 10.1046/j.1399-6576.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- Carrigan KA, Saurer TB, Ijames SG, Lysle DT. Buprenorphine produces naltrexone reversible alterations of immune status. Int Immunopharmacol. 2004;4:419–428. doi: 10.1016/j.intimp.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohendy R, Brougere A, Cuvillon P. Anaesthesia in the older patient. Curr Opin Clin Nutr Metab Care. 2005;8:17–21. doi: 10.1097/00075197-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Colon-Cesario WI, Martinez-Montemayor MM, Morales S, Felix J, Cruz J, Adorno M, Pereira L, Colon N, Maldonado-Vlaar CS, de Ortiz S. Pena. Knockdown of Nurr1 in the rat hippocampus: implications to spatial discrimination learning and memory. Learn Mem. 2006;13:734–744. doi: 10.1101/lm.407706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DJ, Huston J, 3rd, Trenerry MR, Brown RD, Jr., Zehr KJ, Sundt TM., 3rd Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83:1389–1395. doi: 10.1016/j.athoracsur.2006.11.089. [DOI] [PubMed] [Google Scholar]
- Dodds C, Allison J. Postoperative cognitive deficit in the elderly surgical patient. Br J Anaesth. 1998;81:449–462. doi: 10.1093/bja/81.3.449. [DOI] [PubMed] [Google Scholar]
- Etzioni DA, Liu JH, O’Connell JB, Maggard MA, Ko CY. Elderly patients in surgical workloads: a population-based analysis. Am Surg. 2003;69:961–965. [PubMed] [Google Scholar]
- Freudenberg F, Dieckmann M, Winter S, Koch M, Schwabe K. Selective breeding for deficient sensorimotor gating is accompanied by increased perseveration in rats. Neuroscience. 2007;148:612–622. doi: 10.1016/j.neuroscience.2007.06.034. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, Chalidis B, Hall GM. Surgical stress response. Injury. 2006;37(Suppl 5):S3–9. doi: 10.1016/S0020-1383(07)70005-0. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Huang TJ, Hsu RW, Li YY, Cheng CC. Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res. 2005;23:406–411. doi: 10.1016/j.orthres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Karlidag R, Unal S, Sezer OH, Karabulut A. Bay, Battaloglu B, But A, Ozcan C. The role of oxidative stress in postoperative delirium. Gen Hosp Psychiatry. 2006;28:418–423. doi: 10.1016/j.genhosppsych.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Koch S, Forteza A, Lavernia C, Romano JG, Campo-Bustillo I, Campo N, Gold S. Cerebral fat microembolism and cognitive decline after hip and knee replacement. Stroke. 2007;38:1079–1081. doi: 10.1161/01.STR.0000258104.01627.50. [DOI] [PubMed] [Google Scholar]
- Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Nevo I, Paniagua MA, Ben-Ari A, Pretto E, Eisdorfer S, Davidson E, Matot I, Eisdorfer C. Uncomplicated general anesthesia in the elderly results in cognitive decline: does cognitive decline predict morbidity and mortality? Med Hypotheses. 2007;68:484–492. doi: 10.1016/j.mehy.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117–126. doi: 10.1067/msy.2000.101584. [DOI] [PubMed] [Google Scholar]
- Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–1571. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain. 2004;110:385–392. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Lysle DT. Severity, time, and beta-adrenergic receptor involvement in surgery-induced immune alterations. J Surg Res. 1998;80:115–122. doi: 10.1006/jsre.1998.5429. [DOI] [PubMed] [Google Scholar]
- Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schobitz B, de Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Olin K, Eriksdotter-Jonhagen M, Jansson A, Herrington MK, Kristiansson M, Permert J. Postoperative delirium in elderly patients after major abdominal surgery. Br J Surg. 2005;92:1559–1564. doi: 10.1002/bjs.5053. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Pickering M, O’Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Rasmussen LS. Postoperative cognitive dysfunction: incidence and prevention. Best Pract Res Clin Anaesthesiol. 2006;20:315–330. doi: 10.1016/j.bpa.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Rasmussen LS, Siersma VD. Postoperative cognitive dysfunction: true deterioration versus random variation. Acta Anaesthesiol Scand. 2004;48:1137–1143. doi: 10.1111/j.1399-6576.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- Rohan D, Buggy DJ, Crowley S, Ling FK, Gallagher H, Regan C, Moriarty DC. Increased incidence of postoperative cognitive dysfunction 24 hr after minor surgery in the elderly. Can J Anaesth. 2005;52:137–142. doi: 10.1007/BF03027718. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Takemoto Y, Kanakura H, Kidani Y, Yamamoto K. The dose-related effects of ketamine on mortality and cytokine responses to endotoxin-induced shock in rats. Anesth Analg. 2003;97:1769–1772. doi: 10.1213/01.ANE.0000085634.72426.ED. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME. Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. Jama. 1995;274:44–50. [PubMed] [Google Scholar]