Abstract
Introduction
Disparities within ethnic groups are generally ignored, but in evolving populations they may have implications for public health. We examined ethnic variation in serum 25-hydroxyvitamin D [25(OH)D] and bone mineral density (BMD) among Hispanic American men.
Methods
358 Hispanic males age 30–79 y were studied. Logistic regression models assessed variation in odds of vitamin D deficiency (<20 ng/mL) and low BMD (T-score<−1) by ethnicity, with and without adjustment for risk factors (age, smoking, occupation, physical activity, body mass index, and sunlight exposure).
Results
Vitamin D deficiency was most common among Puerto Rican (26%), compared with Dominican (21%), Central American (11%), and South American (9%) men. Percentages with low BMD were: South American (44%), Puerto Rican (34%), Dominican (29%), and Central American (23%). Adjustment for age and risk factors failed to account for Hispanic subgroup differences in vitamin D deficiency and low BMD. Population estimates indicate a substantial burden of low BMD and vitamin D deficiency among Hispanic men.
Conclusions
Our findings underscore the importance of examining the skeletal health of Hispanic subgroups, and suggest that a considerable number of Hispanic men may be at elevated risk of fracture and vitamin D deficiency.
Keywords: bone densitometry, epidemiology, Hispanic, men, population study, vitamin D
INTRODUCTION
The epidemiology of osteoporosis in men and minority groups is understudied, and while advances in the understanding of gender and racial disparities in skeletal health continue to be made, there remain major gaps in knowledge. As reduced bone strength is an emerging public health concern for older men and racial/ethnic minorities [1–4], planning for osteoporosis-related health care must account for the changing demographic composition of the U.S. population. Of particular importance are annual fracture costs among the Hispanic population, which will grow from $754 million in 2005 to a projected $2 billion per year by 2025, representing the largest increase (175%) among all racial/ethnic groups [5]. The surge in health care costs will be driven by the enormous increase projected for the Hispanic population in the coming decades [6].
While between-group racial differences in variables related to skeletal status are well-documented (e.g., black/white differences in serum 25-hydroxyvitamin D [25(OH)D] or bone mineral density (BMD) [7–16]), subgroups within major racial or ethnic groups have been ignored. This is of concern for several reasons. First, there are known limitations in combining data on heterogeneous Hispanic subgroups [17], and most available data on the skeletal status of Hispanic persons come from studies of Mexican American populations [13, 18–25]. Second, the composition of the U.S. Hispanic population is changing dramatically, with migration and reproduction driving changes in the absolute and relative sizes of Hispanic age and ethnic subgroups [26]. Finally, there is evidence, although scant, of variation in fracture incidence among Hispanic subgroups [21], as well as stronger data showing substantial international, as well as within-country, variation in hip fracture incidence [22, 27–29].
Our objective was to examine variables related to skeletal status among Hispanic men enrolled in a population-based, cross-sectional, observational study of racially and ethnically diverse men aged 30–79 years. Specifically, we examined Hispanic ethnic subgroup differences in serum 25(OH)D and BMD, and determined whether differences in these outcomes are due to the confounding influence of age and other risk factors.
METHODS
Study sample
Data were obtained from men enrolled in the Boston Area Community Health/Bone (BACH/Bone) Survey, which is a cross-sectional observational study of skeletal health in 1,219 (of 1,877 eligible, 65% response rate) randomly selected Black, Hispanic, and White male Boston, MA residents aged 30 to 79 years [7]. Persons of other racial/ethnic backgrounds were ineligible. BACH/Bone subjects were a subset of 2,301 men previously enrolled in the parent Boston Area Community Health (BACH) Survey [30]. Study protocols were approved by Institutional Review Boards at New England Research Institutes (NERI) and Boston University School of Medicine (BUSM). All participants gave written informed consent separately for participation in each study.
Data Collection
BACH data collection featured a comprehensive interview which obtained detailed demographic, health, and lifestyle measures by subject self-report. Physical activity level was measured using a scale developed [31] and validated [32] by staff at NERI. Years of education, occupation, and smoking status was based on self-report. The number of smoking pack years was calculated by multiplying the number of packs per day by the number of years the subjects smoked.
Subjects’ ethnicity was determined via self-identification according to U.S. Office of Management and Budget guidelines [33]. Subjects were considered Hispanic if they answered “yes” to the question “Do you consider yourself to be Spanish, Hispanic, or Latino?”; those who did so were subsequently asked whether they considered themselves one or more of: Mexican American/Chicano, Puerto Rican, Cuban, Spanish, Central American, South American, or Dominican. Subjects were permitted to choose more than one response, or could specify a designation not listed, state that they did not know, or refuse to answer. Subjects were then asked “What race do you consider yourself to be?”, with the option of choosing multiple races from the following responses: American Indian or Alaskan Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, White or Caucasian, and Other (Specify). Additional information relevant to Hispanic ethnicity included place of birth, year of immigration, and language preference (English vs. Spanish).
Data collection for BACH/Bone occurred at the BUSM General Clinical Research Center, where staff obtained a non-fasting blood sample, administered a brief health questionnaire, and obtained bone density, anthropometric, and functional measurements. Data were collected between November 2002 and July 2005. Subjects’ height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) were obtained using a stadiometer and digital scale; body mass index (BMI, kg/m2) was obtained from these by calculation. Information on weekly sunlight exposure and multivitamin use was obtained via self-report.
Serum measures
Serum 25(OH)D [25(OH)D2 + 25(OH)D3] was measured (throughout the year) in duplicate (the average of the two are presented) at the Core Laboratory, BUSM, using a competitive binding protein (CPB) assay without prior chromatography [34]. Inter-assay coefficients of variation are 10–15%. The reference range is 20–100 ng/mL (50–250 nmol/L). The following cut-points for 25(OH)D were used: deficient: < 20 ng/mL (< 50 nmol/L), insufficient: 20–30 ng/mL (50–75 nmol/L), sufficient: > 30 ng/mL (> 75 nmol/L) [35]. Serum bio-intact parathyroid hormone (1–84 PTH) was measured in duplicate (the average of the two are presented) with the Nichols Advantage System (Nichols Institute Diagnostics, San Clemente, CA). The sensitivity of the assay is 4.0 pg/mL and the inter-assay CVs are 5.6–8.3%.
Bone densitometry
Proximal femur (femoral neck and total hip) BMD was measured by DXA using a QDR 4500W densitometer (Hologic, Inc., Waltham, MA). The DXA system was monitored weekly for drift. Participants were categorized as osteoporotic or osteopenic [36] according to femoral neck and total hip BMD T-scores (T-score < −1 at either site), computed from the Third National Health and Nutrition Examination Survey (NHANES III) young non-Hispanic white male reference database [13]. We refer to men who met this criterion as having “low BMD”. Too few men met the criteria for osteoporosis (T ≤ −2.5) to be analyzed separately.
Analysis sample
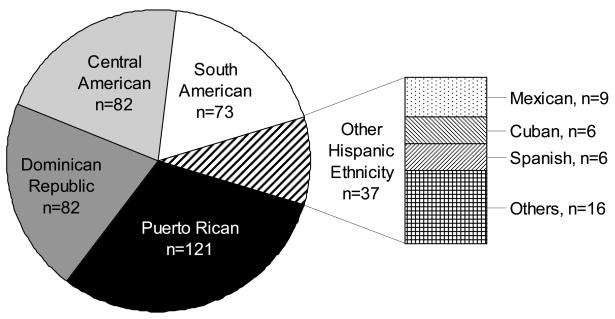
A total of 401 BACH/Bone subjects identified themselves as Hispanic. Four Hispanic subjects did not have DXA scans and 2 were missing hip BMD measures. See Figure 1 for a detailed breakdown of Hispanic ethnicity among these 395 participants. We excluded from our analyses 37 “Other Hispanic” men who either self-identified as having two or more Hispanic ethnicities (n = 16) or stated that they considered themselves to be of Mexican American, Cuban, or Spanish descent (n = 21), because the subgroups themselves are too small to analyze, and there is no justification for combining them into one “Other” group. This left a total of 358 subjects available for the BMD analysis. An additional 27 men were missing data on serum 25(OH)D, leaving 331 men for analyses considering 25(OH)D.
FIGURE 1.
Distribution of self-identified ethnic origin among 395 Hispanic respondents with non-missing DXA data. The group “Others, n = 16” includes men who self-identified as having two or more Hispanic ethnicities.
Statistical analysis
Mean (standard deviation) and N (percent) were used to describe the population with respect to the outcomes and covariates of interest according to Hispanic ethnic group. Associations between ethnic group and continuous (femoral neck and total hip BMD) and binary (vitamin D deficiency and low BMD) outcomes were quantified by linear and logistic regression models, respectively, specifying the appropriate distribution (binomial for logistic models and normal for linear models) and link function (logit for logistic models and identity for linear models). In logistic regression models, we used Puerto Rican men as the reference category for computing odds ratios (OR) and 95% confidence intervals (CI). Multivariate logistic regression was used to examine Hispanic ethnic differences in vitamin D deficiency and low BMD in two regression models: (i) adjusted for age only; and (ii) adjusted for age plus risk factors. The potential covariates considered in this analysis were: age (continuous), smoking pack years (ordinal), occupation (categorical), BMI (continuous), sunlight exposure (ordinal), physical activity (ordinal), years of education (continuous), multivitamin supplement use (binary), and height (continuous). Details on the categorizations used for non-continuous variables are shown in Table 1. The statistical significance of regression effects was determined using Wald hypothesis tests. Pairwise comparisons between each of the Hispanic ethnic groups were obtained using CONTRAST statements in the SAS (SAS Institute, Cary, NC, USA) procedure PROC GENMOD. Visual data displays were constructed using Splus version 7.0 (Insightful Corp., Seattle, WA, USA) and SAS/GRAPH software (SAS Institute, Cary, NC, USA).
TABLE 1.
Study sample characteristics by Hispanic ethnic group (N = 358).
| Mean ± Standard Deviation or N (Percent) |
||||
|---|---|---|---|---|
| Variable | Puerto Rican (N = 121) | Dominican Republic (N = 82) | Central American (N = 82) | South American (N = 73) |
| Racial and ethnic group | ||||
| Hispanic Only | 99 (80.5%) | 66 (81.5%) | 67 (81.7%) | 55 (75.3%) |
| Hispanic and any race | 24 (19.5%) | 15 (18.3%) | 15 (18.3%) | 18 (24.7%) |
| Other and Hispanic | 4 (3.3%) | 4 (4.9%) | 5 (6.1%) | 4 (5.5%) |
| White and Hispanic | 18 (14.6%) | 7 (8.6%) | 6 (7.3%) | 13 (17.8%) |
| Black and Hispanic | 2 (1.6%) | 4 (4.9%) | 4 (4.9%) | 1 (1.4%) |
| Age, y | 50.4 ± 11.6 | 52.6 ± 12.3 | 46.3 ± 10.8 | 46.6 ± 11.4 |
| Education, y | 9.8 ± 4.6 | 10.0 ± 4.8 | 9.0 ± 5.4 | 12.1 ± 5.3 |
| Occupation | ||||
| No work | 75 (61.0%) | 24 (29.6%) | 14 (17.1%) | 9 (12.3%) |
| Non-physical work | 18 (14.6%) | 12 (14.8%) | 21 (25.6%) | 20 (27.4%) |
| Moderately physical work | 17 (13.8%) | 27 (33.3%) | 25 (30.5%) | 30 (41.1%) |
| Heavy physical work | 13 (10.6%) | 18 (22.2%) | 22 (26.8%) | 14 (19.2%) |
| Multivitamin supplement use | 26 (21.1%) | 17 (20.7%) | 17 (21.0%) | 9 (12.3%) |
| Sun Exposure (hrs/wk) | ||||
| < 1 hrs/wk | 10 (8.1%) | 15 (18.3%) | 4 (4.9%) | 3 (4.1%) |
| 1 to < 2 hrs/wk | 20 (16.3%) | 17 (20.7%) | 21 (25.6%) | 17 (23.3%) |
| 2 to < 4 hrs/wk | 24 (19.5%) | 16 (19.5%) | 15 (18.3%) | 14 (19.2%) |
| 4 to < 7 hrs/wk | 19 (15.5%) | 10 (12.2%) | 9 (11.0%) | 9 (12.3%) |
| ≥ 7 hrs/wk | 50 (40.7%) | 24 (29.3%) | 33 (40.2%) | 30 (41.1%) |
| Smoking status | ||||
| Never smoker | 45 (37.2%) | 49 (59.8%) | 40 (48.8%) | 36 (49.3%) |
| > 0 to <10 pack years | 26 (21.5%) | 18 (22.0%) | 30 (36.6%) | 19 (26.0%) |
| ≥ 10 pack years | 50 (41.3%) | 15 (18.3%) | 12 (14.6%) | 18 (24.7%) |
| Height, cm | 168.7 ± 7.0 | 169.6 ± 6.5 | 167.3 ± 6.3 | 168.7 ± 5.7 |
| Body Mass Index, kg/m2 | 30.1 ± 5.6 | 27.7 ± 4.5 | 29.0 ± 4.0 | 27.1 ± 3.2 |
| Physical activity scale for the elderly (PASE) | ||||
| Inactive (PASE < 100) | 59 (48.8%) | 27 (33.3%) | 8 (9.9%) | 14 (19.2%) |
| Active (PASE 100 to < 250) | 42 (34.7%) | 40 (49.4%) | 46 (56.8%) | 37 (50.7%) |
| Very active (PASE ≥ 250) | 20 (16.5%) | 14 (17.3%) | 27 (33.3%) | 22 (30.1%) |
| Age at Immigration | 17.8 (14.5) | 33.4 (14.6) | 31.5 (12.7) | 35.0 (11.9) |
| Immigration | ||||
| Born in U.S. | 22 (18.0%) | 2 (2.5%) | 1 (1.2%) | 0 (0.0%) |
| Immigrated as child | 37 (30.3%) | 8 (9.9%) | 6 (7.3%) | 3 (4.11%) |
| Immigrated as adult (≥ 18 y) | 63 (51.6%) | 71 (87.7%) | 75 (91.5%) | 70 (95.9%) |
| Preferred language English | 47 (38.2%) | 9 (11.0%) | 17 (23.3%) | 11 (13.4%) |
We estimated the number of prevalent cases of vitamin D deficiency and low BMD among men between 50 and 79 years of age based on 2000 Census data [37]. To obtain these estimates, we multiplied age- and ethnic group-specific prevalence estimates times the number of men in each age and ethnic group; the sum of these products represents an estimate of the number of men with vitamin D deficiency and low BMD in the specified population. In order to broaden the applicability of these estimates, we used published estimates of the prevalence of vitamin D deficiency [15] and low BMD [38] in Mexican American men from NHANES III.
RESULTS
Of the 358 Hispanic men who comprised the base analysis sample, 121 (34%) self-identified as Puerto Rican, 82 (23%) as Dominican, 82 (23%) as Central American, and 73 (20%) as South American. There was no difference between the groups with regard to season of measurement. Selected descriptive characteristics are displayed in Table 1. Most subjects (80%) did not identify a racial group, choosing to report Hispanic ethnicity only. Among Hispanic subjects reporting a racial group, Puerto Rican and South American men were more likely to report being white as well as Hispanic, and Dominican and Central American men were more likely to report being Black and Hispanic. Puerto Rican men appeared to exhibit considerably poorer health behaviors (e.g., a higher percentage were heavy smokers and sedentary, with higher BMI). Multivitamin use was lower, though not significantly (p = 0.10), among South American men (12%) compared with the other groups (21%). The distribution of self-reported sunlight exposure was similar in all ethnic groups except Dominican men, who reported less exposure. Ninety-six percent of South Americans, 92% of Central Americans, 88% of Dominicans, and 52% of Puerto Ricans immigrated to the U.S. as adults. Additionally, while English was the language of preference in 38% of Puerto Rican and 23% of Central American men, a lower percentage of Dominican (11%) and South American (13%) men preferred English.
Differences in mean 25(OH)D across ethnic group were not statistically significant (Table 2). Median (25th, 75th percentile) PTH levels in the ethnic subgroups were: Puerto Rican: 24.8 (18.5, 38.1) pg/mL; South American: 25.9 (21.8, 37.2) pg/mL; Central American: 28.0 (20.1, 40.2) pg/mL; Dominican: 30.3 (21.9, 44.0) pg/mL. Puerto Rican subjects exhibited the highest percentage of men who were vitamin D deficient (26%). Vitamin D deficiency was relatively uncommon among Central American (11%) or South American (9%) men. The relatively substantial differences in the percentage of men with vitamin D deficiency despite similar mean 25(OH)D across groups is illustrated in Figure 2. Mean femoral neck BMD in South American men (0.84 g/cm2) was approximately 6% lower than femoral neck BMD in Central American men (p = 0.02). Mean total hip BMD was significantly lower in South American men as compared to Puerto Rican (p = 0.03), Dominican (p = 0.03), and Central American (p = 0.01) men. The percentage of men with low BMD differed substantially across the groups, with 44% of South American, 34% of Puerto Rican, 29% of Dominican, and 23% of Central American men having low BMD at either the femoral neck or total hip.
TABLE 2.
Serum 25(OH)D and BMD by Hispanic ethnic group.
| Mean ± Standard Deviation or N (Percent) |
|||||
|---|---|---|---|---|---|
| Variable | Puerto Rican (N = 121) | Dominican Republic (N = 82) | Central American (N = 82) | South American (N = 73) | p-value† |
| Mean 25(OH)D, ng/mL* | 33.0 ± 16.3 | 36.7 ± 23.6 | 35.0 ± 13.7 | 36.4 ± 19.1 | 0.48 |
| 25(OH)D category | < 0.01 | ||||
| Deficient, <20 ng/mL | 30 (26.1%)c,d | 15 (21.1%)d | 8 (10.8%)a | 6 (8.5%)a,b | |
| Insufficient, 20–30 ng/mL | 21 (18.3%) | 19 (26.8%) | 18 (24.3%) | 21 (29.6%) | |
| Sufficient, >30 ng/mL | 64 (55.6%) | 37 (52.1%) | 48 (64.86%) | 44 (62.0%) | |
| Mean femoral neck BMD, g/cm2 | 0.88 ± 0.16 | 0.87 ± 0.14 | 0.89 ± 0.12d | 0.84 ± 0.12c | 0.14 |
| Femoral neck BMD category | 0.05 | ||||
| Low BMD, T-score < −1 SD | 40 (33.1%) | 23 (28.1%) | 17 (20.7%)d | 30 (41.1%)c | |
| Normal BMD, T-score ≥ −1 SD | 81 (66.9%) | 59 (72.0%) | 65 (79.3%) | 43 (58.9%) | |
| Mean total hip BMD, g/cm2 | 1.02 ± 0.17d | 1.03 ± 0.15d | 1.03 ± 0.13d | 0.97 ± 0.11a,b,c | 0.05 |
| Total hip BMD category | 0.19 | ||||
| Low BMD, T-score < −1 SD | 24 (19.8%) | 15 (18.3%) | 10 (12.2%)d | 19 (26.0%)c | |
| Normal BMD, T-score ≥ −1 SD | 97 (80.2%) | 67 (81.7%) | 72 (87.8%) | 54 (74.0%) | |
| Femoral neck or total hip BMD category‡ | 0.05 | ||||
| Low BMD, T-score < −1 SD | 41 (33.9%) | 24 (29.3%) | 19 (23.2%)d | 32 (43.8%)c | |
| Normal BMD, T-score ≥ −1 SD | 80 (66.1%) | 58 (70.7%) | 63 (76.8%) | 41 (56.2%) | |
To convert 25(OH)D to nmol/L, multiply values by 2.496. There are n = 331 subjects with non-missing 25(OH)D data.
p-value from Wald test for the null hypothesis of no difference among Hispanic subgroups. For the 25(OH)D category row, the outcome was deficient vs. others. Superscripted letters refer to Wald tests for the null hypothesis of no pairwise (mean) difference between specific Hispanic subgroups (obtained with CONTRAST statements in PROC GENMOD).
Met criterion for low BMD on either femoral neck or total hip BMD.
p < 0.05 vs. Puerto Rican
p < 0.05 vs. Dominican Republic
p < 0.05 vs. Central American
p < 0.05 vs. South American
FIGURE 2.
Density estimates of (A) serum 25(OH)D and (B) femoral neck BMD by Hispanic ethnic origin, with reference lines at 20 ng/mL for 25(OH)D and T-score of −1 (0.797 g/cm2) for BMD. Despite similar mean levels of 25(OH)D across ethnic groups, the prevalence of vitamin D deficiency [25(OH)D < 20 ng/mL] exhibited considerable variation by ethnic group. Notably, a tighter 25(OH)D distribution among South American men, combined with the location of the cutpoint for 25(OH)D, results in a lower prevalence of vitamin D deficiency in spite of means similar to those observed in other Hispanic ethnic groups.
We examined whether the differences observed in Table 2 depended on age group by stratifying the sample into two groups [<50 years (n = 206) and ≥ 50 years (n = 152)]. There was a slight suggestion of an interaction between age and Hispanic ethnicity (e.g., the OR for low BMD in South American vs. Puerto Rican men < 50 years was 2.49 (95% CI: 1.03, 6.02) whereas in men ≥ 50 years it was 1.50 (95% CI: 0.58, 3.87). However, cell sizes among the older group were small (e.g., there were 27 and 25 Central and South American men, respectively, aged 50 years or older). Furthermore, models that included the statistical interaction term for age (dichotomous, < 50 and ≥ 50 years) and Hispanic ethnicity did not meet the level of statistical significance in predicting vitamin D deficiency (p = 0.90) or low BMD (p = 0.21).
Table 3 presents covariate-adjusted logistic regression models for vitamin D deficiency and low BMD. Models for vitamin D deficiency consistently showed that South American men were at much lower risk as compared with Puerto Rican or Dominican men. Models for low BMD indicated that South American men were at much higher risk of low BMD as compared with Dominican or Central American men. Adjustment for risk factors (smoking, occupation, physical activity, BMI, and sunlight exposure) appeared to affect OR estimates for vitamin D deficiency and low BMD similarly; for instance, the multivariate-adjusted ORs for vitamin D deficiency and low BMD comparing South American and Puerto Rican men changed by ~25% from age-adjusted ORs. Observed differences generally persisted with multivariate adjustment. Consistent with previously reported analyses [39], we found no correlation between 25(OH)D and BMD in this subsample of Hispanic men. Additional analyses (not shown) conducted on the showed no evidence of variation in the effect of 25(OH)D on BMD by Hispanic ethnic subgroup.
TABLE 3.
Multivariate-adjusted logistic regression models of vitamin D deficiency and low BMD on Hispanic ethnic group.
| Vitamin D Deficient, 25(OH)D < 20 ng/mL |
Low BMD*, T-score < −1 SD |
|||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-value† | OR | 95% CI | p-value† |
| i. Age-adjusted | ||||||
| Hispanic ethnic group | < 0.01 | < 0.01 | ||||
| Puerto Rican | 1.00 | - | c,d | 1.00 | - | d |
| Dominican Republic | 0.81 | 0.40, 1.66 | d | 0.68 | 0.35, 1.30 | d |
| Central American | 0.34 | 0.14, 0.79 | a | 0.77 | 0.39, 1.51 | d |
| South American | 0.25 | 0.10, 0.65 | a,b | 2.12 | 1.11, 4.06 | a,b,c |
| ii. Multivariate-adjusted‡ | ||||||
| Hispanic ethnic group | 0.07 | < 0.01 | ||||
| Puerto Rican | 1.00 | - | d | 1.00 | - | |
| Dominican Republic | 0.90 | 0.29, 2.07 | d | 0.40 | 0.17, 0.90 | d |
| Central American | 0.42 | 0.16, 1.10 | 0.66 | 0.29, 1.53 | d | |
| South American | 0.31 | 0.11, 0.87 | a,b | 1.59 | 0.71, 3.53 | b,c |
Met criterion for low BMD on either femoral neck or total hip BMD.
p-value from Wald test for the null hypothesis of no difference among Hispanic subgroups. Superscripted letters refer to Wald tests for the null hypothesis of no pairwise difference between specific Hispanic subgroups (obtained with CONTRAST statements in PROC GENMOD).
Models adjusted for age (continuous and centered at 50 y), smoking (ordinal), occupation (categorical), physical activity (ordinal), BMI (continuous), and sunlight exposure (ordinal) (see Table 1).
p < 0.05 vs. Puerto Rican
p < 0.05 vs. Dominican Republic
p < 0.05 vs. Central American
p < 0.05 vs. South American
We estimated the number of U.S. Hispanic men (of Puerto Rican, Dominican, Central American, or South American origin from our study and Mexican Americans from NHANES III) aged 50–79 years with vitamin D deficiency and low BMD. In 2000, there were 2,024,936 U.S. Hispanic males between the ages of 50–79 y. Of these, 459,843 (23%) were of Puerto Rican, Dominican, Central American, or South American descent and 1,045,649 (52%) were Mexican American. It is estimated that there are approximately 394,000 Hispanic men aged 50–79 y with vitamin D deficiency and 574,500 with low BMD (Table 4). Major differences between groups are related to differences in both the prevalence of these conditions as well as population size.
TABLE 4.
Estimated number of Hispanic men between 50–79 years of age with vitamin D deficiency and low BMD in the year 2000.
| Vitamin D Deficient, 25(OH)D < 20 ng/mL |
Low BMD†, T-score < −1 SD* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hispanic ethnic group | Age group | Population in 2000† | N‡ | Proportion | (95% CI)§ | Estimated number of cases | N‡ | Proportion | (95% CI)§ | Estimated number of cases |
| Puerto Rican | 50–59 y | 130,397 | 34 | 0.21 | (0.09, 0.38) | 26,849 | 35 | 0.40 | (0.24, 0.58) | 52,159 |
| 60–79 y | 104,218 |
23 | 0.22 | (0.07, 0.44) | 22,657 |
25 | 0.60 | (0.43, 0.82) | 62,531 |
|
| Sub-total | 234,615 | 49,506 | 114,690 | |||||||
| Dominican | 50–59 y | 28,086 | 14 | 0.21 | (0.05, 0.51) | 6,019 | 16 | 0.44 | (0.20, 0.71) | 12,288 |
| 60–79 y | 19,023 |
23 | 0.17 | (0.05, 0.39) | 3,308 |
24 | 0.29 | (0.13, 0.51) | 5,549 |
|
| Sub-total | 47,109 | 9,327 | 17,837 | |||||||
| Central American | 50–59 y | 48,460 | 16 | 0.06 | (0.00, 0.30) | 3,029 | 16 | 0.38 | (0.15, 0.65) | 18,173 |
| 60–79 y | 24,830 |
9 | 0.22 | (0.03, 0.60) | 5,517 |
11 | 0.64 | (0.31, 0.89) | 15,802 |
|
| Sub-total | 73,290 | 8,546 | 33,974 | |||||||
| South American | 50–59 y | 62,651 | 15 | 0.00 | – | 0 | 15 | 0.53 | (0.27, 0.79) | 33,412 |
| 60–79 y | 42,178 |
10 | 0.20 | (0.78, 1.00) | 8,436 |
10 | 0.70 | (0.35, 0.93) | 29,525 |
|
| Sub-total | 104,829 | 8,436 | 62,936 | |||||||
| Mexican American | 50–59 y | 583,282 | 0.30¶ | 174,985 | 0.33# | 192,483 | ||||
| 60–79 y | 462,367 |
0.31¶ | 143,334 |
0.33# | 152,581 |
|||||
| Sub-total | 1,045,649 | 318,318 | 345,064 | |||||||
| All groups | Total | 1,505,492 | 394,133 | 574,501 | ||||||
Met criterion for low BMD on either femoral neck or total hip BMD.
Source: Census 2000, U.S. Census Bureau [37].
N = age-specific sample size.
Exact 95% CI.
Source: NHANES III, Table 2 in Looker et al. [15] and low BMD. Estimate for 50–59 yr-old Mexican American men based on prevalence of vitamin D deficiency (< 20 ng/mL) among Mexican American men aged 30–59 y. Estimate for 60–79 yr-old Mexican American men based on prevalence of low 25(OH)D among Mexican American men aged 60+ y.
Source: NHANES III, from Figure 1 in Looker et al. [38]. Estimates are the sum of the proportion of Mexican American men aged ≥ 50 y with osteopenia and osteoporosis.
DISCUSSION
To our knowledge, this is the first to study to examine differences in serum 25(OH)D and BMD among these ethnic subgroups of Hispanic men. Even with adjustment for known risk factors for vitamin D deficiency and low BMD, we observe relatively substantial differences in risk among the Hispanic ethnic groups studied. Puerto Rican and Dominican men are at higher risk of vitamin D deficiency. South American men are at much higher risk for low BMD yet had the lowest prevalence of vitamin D deficiency. We estimate that a substantial number of Hispanic men between 50–79 years of age have vitamin D deficiency and low BMD, even though our estimates for the year 2000 are made only with respect to approximately 75% of U.S. male Hispanic population in this age range.
Comparison with other studies on vitamin D deficiency and low BMD among Hispanic men
Harris and colleagues studied 19 Hispanic men aged ≥ 64 years from Boston, and found that more than 50% had 25(OH)D < 20 ng/mL (measured by CPB in winter) [40]. This is higher than our estimate and could be due to season of measurement as well as the ages of the participants. NHANES III reported that approximately 9%–13% of Mexican American men aged ≥ 30 years had 25(OH)D levels < 15 ng/mL, and 30% had 25(OH)D levels < 20 ng/mL [15]. Estimates from our study were somewhat lower than those obtained from NHANES III data, with 9% having 25(OH)D < 15 ng/mL and only 19% having 25(OH)D < 20 ng/mL. Small differences could be explained by the fact that BACH/Bone data were obtained throughout the year, whereas these data from NHANES III were from samples obtained in winter. Perhaps more important is the possibility that different assay techniques yield different results [41]. NHANES III used the Diasorin radioimmunoassay (RIA) whereas BACH/Bone used the CPB assay. The CPB assay used in this study and RIA generally overestimate serum 25(OH)D levels by 15–20%. This is due to the fact that the CPB and RIA assays recognize not only 25(OH)D, but also 24, 25-dihydroxyvitamin D [24, 25(OH)D] as well as other metabolites in the serum sample. Typically these metabolites circulate at a level of about 10–20% of the 25(OH)D levels which could cause a small overestimation that is within the CV for the assay. Our CPB assay was compared to liquid chromatography-tandem mass spectrometry with a correlation coefficient of 0.70 [35]. Furthermore, Lips et al. have observed that routine assays for serum 25(OH)D can discriminate well between low, average, and high values [42]. As a result, previous work indicates that prevalence estimates of vitamin D insufficiency or deficiency with our assay are nearly identical to those obtained with liquid chromatography-mass spectrometry [35]. In summary, our estimates of the percentage of men with vitamin D deficiency, while not fundamentally different from other studies, may exhibit a small low bias, which would mean that the population prevalence may therefore be greater than the estimates we are reporting here.
NHANES III is the only other study to report low BMD data among men of Hispanic descent [38]. According to NHANES III estimates, approximately 40% of Mexican American men aged ≥ 50 years had low femoral neck BMD. This is somewhat lower than that observed in our data, with 47% of all Hispanic men aged ≥ 50 years having low BMD at the femoral neck. This is likely attributable to differences in Hologic machines used for DXA scans (NHANES III: Hologic QDR 1000; BACH/Bone: Hologic QDR 4500). In fact, the application of NHANES III cutpoints to the current data set could be problematic given differences in instrument technologies [pencil-beam (NHANES) vs. fan-beam (BACH/Bone)]. Tothill and colleagues have shown that the pencil-beam yields 3–9% higher BMD than fan-beam [43]. We examined how assuming the worst-case scenario (i.e., decreasing NHANES III BMD cutpoint values by 9%) would affect prevalence estimates, and observed that they were reduced from 32% to 22% in the sample overall, with a range of reductions among Hispanic subgroups from 29% to 21% in Dominican men to 34% to 21% among Puerto Rican men. In Hispanic men 50 years or older, prevalence was decreased from 47% to 33%. It should be noted that NHANES III reference data are generally incorporated into densitometer data bases without such scaling, so the application of these reference values to our population is not without precedent.
Associations of vitamin D status with BMD and sunlight exposure
The finding that South American men were at much lower risk for vitamin D deficiency yet at much higher risk for low BMD merits attention. This mirrors the apparent paradox in the observation that white men and women, despite being at much lower risk of vitamin D deficiency [15], are at much higher risk of osteoporosis [13, 38] and fracture [8] as compared to black men and women. A previous investigation from the larger BACH/Bone sample shows that among Hispanic and black men in this cohort, there are essentially no associations between 25(OH)D levels and BMD, but modest associations are observed among white men [39]. This is consistent with other data [15]. This apparent protection against the negative skeletal consequences of suboptimal vitamin D might be explained by differences in the vitamin D receptor [44–47]. One could speculate that a similar phenomenon, i.e., subgroup variation in the association between 25(OH)D and BMD, might occur among Hispanic ethnic subgroups as well. Unfortunately, the small size of the ethnic subgroups limits our ability to examine this in detail.
The relationship between sunlight exposure and vitamin D status in this study deserves comment, as the ethnic patterns in self-reported sun exposure observed do not map neatly onto ethnic differences in vitamin D deficiency. For example, despite similar levels of sun exposure in Puerto Rican and South American men, Puerto Rican men had a much higher rate of vitamin D deficiency. Despite the important role played by latitude and season in vitamin D synthesis [48], differences in circulating vitamin D levels associated with these factors cannot explain this finding since there was no variation between ethnic groups in latitude or in time of year of sampling. It is possible that heterogeneity in skin pigmentation contributes to observed differences [49–51], but this was not addressed in this study. Alternatively, the self-reported measure of sun exposure may not capture actual differences in exposure.
Limitations and strengths
Limitations to this study beyond those previously noted should be acknowledged. Our study population had too few Cuban or Mexican American subjects to include in the analysis. However, our study sample is consistent with the distribution of Hispanic subgroups in Boston, the geographic area from which the study sample was drawn. Nonetheless, we attempted to minimize this limitation in making population estimates of burden by using published estimates of the prevalence of vitamin D deficiency and low BMD among Mexican American men from NHANES III. Another potential limitation is that it is unclear which reference group should be used to compute T-scores in studies of men and minority populations. Interim recommendations from the International Society for Clinical Densitometry suggest the use of young Caucasian males as the reference group for non-Caucasians [52], as was done in this report. Finally, although we provide descriptive information on very crude but widely used [53] proxy measures for acculturation (i.e., language and nativity/immigration status), their lack of formal validation prohibits us from addressing hypotheses about the role of acculturation in the differences observed between Hispanic subgroups. Further work with a validated measure of acculturation could provide useful information about differences between subgroups.
These limitations must be balanced against the strengths of this study, which include a random, population-based study of Hispanic men in Boston and measurement of factors that might confound differences in vitamin D and BMD status between these groups. In addition, we presented data on the major Hispanic subgroups in our study. Our data are consistent with the one study that has shown considerable variation in fracture incidence among Hispanic subgroups [21]. These data support the notion of Melton and colleagues that persons of particular races should not be viewed as members of a uniform class, and differences among component ethnic groups should be explored [54]. Additional study of these subgroups, perhaps with individual-level estimates of genetic ancestry [55], might be revealing.
These findings could have implications for public health. BMD is strongly predictive of fracture risk [56, 57], and it has been recently argued that low BMD (or osteopenia) is analogous to prehypertension, impaired fasting glucose, and borderline high cholesterol in defining an intermediate-risk group [58]. Based on our prevalence estimates, we estimate that a large number of Hispanic men comprise this intermediate-risk group who may be at risk of fracture (as indicated by low BMD) and vitamin D deficiency. When considered in light of U.S. demographic trends, recent data of a rising incidence of hip fracture in California Hispanics [25], and the wide range of chronic diseases associated with suboptimal vitamin D status (e.g., cancer, multiple sclerosis, diabetes, and cardiovascular disease [48]), these data highlight the need for additional studies on Hispanic subgroups, and could have important implications for service planning, as well as education and prevention efforts for fractures, osteoporosis, and the sequelae of vitamin D deficiency.
Acknowledgments
Grant Support: The BACH/Bone Survey was supported by grant AG 20727 from the National Institute on Aging (NIA). The parent study (BACH) was supported by grant DK 56842 from the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support from MO RR00533.
Footnotes
None of the authors have a conflict of interest
Contributor Information
Thomas G. Travison, Email: ttravison@neriscience.com.
Gretchen R. Esche, Email: gesche@neriscience.com.
Michael F. Holick, Email: mfholick@bu.edu.
Tai C. Chen, Email: taichen@bu.edu.
John B. McKinlay, Email: jmckinlay@neriscience.com.
References
- 1.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. U.S. Department of Health and Human Services, Office of the Surgeon General; Rockville, MD: 2004. [Google Scholar]
- 2.National Institutes of Health. Osteoporosis prevention, diagnosis, and therapy. National Institutes of Health Consensus Development Conference Statement. Jama. 2001;285:785–795. [Google Scholar]
- 3.Heaney RP. Bone mass, the mechanostat, and ethnic differences. J Clin Endocrinol Metab. 1995;80:2289–2290. doi: 10.1210/jcem.80.8.7629221. [DOI] [PubMed] [Google Scholar]
- 4.Orwoll ES, Klein RF. Osteoporosis in men. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Census Bureau. [accessed 9 February 2007];We the People: Hispanics in the United States. 2004 http://wwwcensusgov/ipc/www/usinterimproj/
- 7.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 8.Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, Tosteson T. Racial differences in fracture risk. Epidemiology. 1994;5:42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Cummings SR, Cauley JA, Palermo L, Ross PD, Wasnich RD, Black D, Faulkner KG. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4:226–229. doi: 10.1007/BF01623243. [DOI] [PubMed] [Google Scholar]
- 10.Dawson-Hughes B. Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr. 2004;80:1763S–1766S. doi: 10.1093/ajcn/80.6.1763S. [DOI] [PubMed] [Google Scholar]
- 11.Farmer ME, White LR, Brody JA, Bailey KR. Race and sex differences in hip fracture incidence. Am J Public Health. 1984;74:1374–1380. doi: 10.2105/ajph.74.12.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George A, Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in bone mineral density in older men. J Bone Miner Res. 2003;18:2238–2244. doi: 10.1359/jbmr.2003.18.12.2238. [DOI] [PubMed] [Google Scholar]
- 13.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 14.Looker AC. The skeleton, race, and ethnicity. J Clin Endocrinol Metab. 2002;87:3047–3050. doi: 10.1210/jcem.87.7.8779. [DOI] [PubMed] [Google Scholar]
- 15.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 16.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20:1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 17.Villa ML. Cultural determinants of skeletal health: the need to consider both race and ethnicity in bone research. J Bone Miner Res. 1994;9:1329–1332. doi: 10.1002/jbmr.5650090902. [DOI] [PubMed] [Google Scholar]
- 18.Bauer RL. Ethnic differences in hip fracture: a reduced incidence in Mexican Americans. Am J Epidemiol. 1988;127:145–149. doi: 10.1093/oxfordjournals.aje.a114774. [DOI] [PubMed] [Google Scholar]
- 19.Bauer RL, Deyo RA. Low risk of vertebral fracture in Mexican American women. Arch Intern Med. 1987;147:1437–1439. [PubMed] [Google Scholar]
- 20.Espino DV, Palmer RF, Miles TP, Mouton CP, Wood RC, Bayne NS, Markides KP. Prevalence, incidence, and risk factors associated with hip fractures in community-dwelling older Mexican Americans: results of the Hispanic EPESE study. Establish Population for the Epidemiologic Study for the Elderly. J Am Geriatr Soc. 2000;48:1252–1260. doi: 10.1111/j.1532-5415.2000.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 21.Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. Hip fracture incidence among elderly Hispanics. Am J Public Health. 1998;88:1245–1247. doi: 10.2105/ajph.88.8.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggi S, Kelsey JL, Litvak J, Heyse SP. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232–241. doi: 10.1007/BF03187467. [DOI] [PubMed] [Google Scholar]
- 23.Silverman SL, Madison RE. Decreased incidence of hip fracture in Hispanics, Asians, and blacks: California Hospital Discharge Data. Am J Public Health. 1988;78:1482–1483. doi: 10.2105/ajph.78.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villa ML, Marcus R, Ramirez Delay R, Kelsey JL. Factors contributing to skeletal health of postmenopausal Mexican-American women. J Bone Miner Res. 1995;10:1233–1242. doi: 10.1002/jbmr.5650100814. [DOI] [PubMed] [Google Scholar]
- 25.Zingmond DS, Melton LJ, 3rd, Silverman SL. Increasing hip fracture incidence in California Hispanics, 1983 to 2000. Osteoporos Int. 2004;15:603–610. doi: 10.1007/s00198-004-1592-7. [DOI] [PubMed] [Google Scholar]
- 26.Rumbaut RG. The making of a people. In: Tienda M, Mitchell F, editors. Hispanics and the Future of American Panel on Hispanics in the United States. The National Academies Press; Washington, DC: 2006. pp. 16–65. [Google Scholar]
- 27.Clark P, de la Pena F, Gomez Garcia F, Orozco JA, Tugwell P. Risk factors for osteoporotic hip fractures in Mexicans. Arch Med Res. 1998;29:253–257. [PubMed] [Google Scholar]
- 28.Elffors I, Allander E, Kanis JA, Gullberg B, Johnell O, Dequeker J, Dilsen G, Gennari C, Lopes Vaz AA, Lyritis G, et al. The variable incidence of hip fracture in southern Europe: the MEDOS Study. Osteoporos Int. 1994;4:253–263. doi: 10.1007/BF01623349. [DOI] [PubMed] [Google Scholar]
- 29.Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK. International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res. 2002;17:1237–1244. doi: 10.1359/jbmr.2002.17.7.1237. [DOI] [PubMed] [Google Scholar]
- 30.McKinlay JB, Link CL. Measuring the Urologic Iceberg: Design and Implementation of The Boston Area Community Health (BACH) Survey. Eur Urol. 2007 doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 32.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 33.Wallman K. Data on race and ethnicity: Revising the federal standard. Am Stat. 1997:31–35. [Google Scholar]
- 34.Chen TC, Turner AK, Holick MF. Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem. 1990;1:315–319. doi: 10.1016/0955-2863(90)90067-u. [DOI] [PubMed] [Google Scholar]
- 35.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. WHO Technical Report Series 843: Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group. World Health Organization; Geneva: 1994. [PubMed] [Google Scholar]
- 37.U.S. Census Bureau. [Accessed: 30 April 2007];2000 Summary File 4--United States/prepared by the U.S. Census Bureau. 2003 http://www.census.gov/Press-Release/www/2003/SF4.html.
- 38.Looker AC, Orwoll ES, Johnston CC, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 39.Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93:40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85:4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 41.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 42.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 43.Tothill P, Hannan WJ. Comparisons between Hologic QDR 1000W, QDR 4500A, and Lunar Expert dual-energy X-ray absorptiometry scanners used for measuring total body bone and soft tissue. Ann N Y Acad Sci. 2000;904:63–71. doi: 10.1111/j.1749-6632.2000.tb06422.x. [DOI] [PubMed] [Google Scholar]
- 44.Cooper GS, Umbach DM. Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis. J Bone Miner Res. 1996;11:1841–1849. doi: 10.1002/jbmr.5650111203. [DOI] [PubMed] [Google Scholar]
- 45.Fang Y, van Meurs JB, d’Alesio A, Jhamai M, Zhao H, Rivadeneira F, Hofman A, van Leeuwen JP, Jehan F, Pols HA, Uitterlinden AG. Promoter and 3′-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the rotterdam study. Am J Hum Genet. 2005;77:807–823. doi: 10.1086/497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris SS, Eccleshall TR, Gross C, Dawson-Hughes B, Feldman D. The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J Bone Miner Res. 1997;12:1043–1048. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- 47.Nelson DA, Vande Vord PJ, Wooley PH. Polymorphism in the vitamin D receptor gene and bone mass in African-American and white mothers and children: a preliminary report. Ann Rheum Dis. 2000;59:626–630. doi: 10.1136/ard.59.8.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 49.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Kohn N, Martinello S, Berkowitz R, Holick MF. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choudhry S, Coyle NE, Tang H, Salari K, Lind D, Clark SL, Tsai HJ, Naqvi M, Phong A, Ung N, Matallana H, Avila PC, Casal J, Torres A, Nazario S, Castro R, Battle NC, Perez-Stable EJ, Kwok PY, Sheppard D, Shriver MD, Rodriguez-Cintron W, Risch N, Ziv E, Burchard EG. Population stratification confounds genetic association studies among Latinos. Hum Genet. 2006;118:652–664. doi: 10.1007/s00439-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 51.Hanis CL, Hewett-Emmett D, Bertin TK, Schull WJ. Origins of U.S. Hispanics. Implications for diabetes. Diabetes Care. 1991;14:618–627. doi: 10.2337/diacare.14.7.618. [DOI] [PubMed] [Google Scholar]
- 52.Binkley NC, Schmeer P, Wasnich RD, Lenchik L. What are the criteria by which a densitometric diagnosis of osteoporosis can be made in males and non-Caucasians? J Clin Densitom. 2002;5 Suppl:S19–27. doi: 10.1385/jcd:5:3s:s19. [DOI] [PubMed] [Google Scholar]
- 53.Lara M, Gamboa C, Kahramanian MI, Morales LS, Bautista DE. Acculturation and Latino health in the United States: a review of the literature and its sociopolitical context. Annu Rev Public Health. 2005;26:367–397. doi: 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melton LJ, 3rd, Marquez MA, Achenbach SJ, Tefferi A, O’Connor MK, O’Fallon WM, Riggs BL. Variations in bone density among persons of African heritage. Osteoporos Int. 2002;13:551–559. doi: 10.1007/s001980200072. [DOI] [PubMed] [Google Scholar]
- 55.Bonilla C, Shriver MD, Parra EJ, Jones A, Fernandez JR. Ancestral proportions and their association with skin pigmentation and bone mineral density in Puerto Rican women from New York city. Hum Genet. 2004;115:57–68. doi: 10.1007/s00439-004-1125-7. [DOI] [PubMed] [Google Scholar]
- 56.Leslie WD, Tsang JF, Caetano PA, Lix LM. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab. 2007;92:77–81. doi: 10.1210/jc.2006-1415. [DOI] [PubMed] [Google Scholar]
- 57.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khosla S, Melton LJ. Osteopenia. N Engl J Med. 2007;356:2293–2300. doi: 10.1056/NEJMcp070341. [DOI] [PubMed] [Google Scholar]