Abstract
Objective
Outcomes from the Women's Health Initiative have demonstrated adverse effects associated with hormone therapy (HT), and have prioritized the need to develop new alternative treatments for the management of menopause and osteoporosis. To this end, we have been investigating natural herbal medicines used by Costa Rican women to manage menopausal symptoms.
Design
Seventeen plant species were collected and extracted in Costa Rica. To establish possible mechanisms of action, and determine their potential future use for menopause or osteoporosis, the estrogenic activities of the herbal extracts were investigated in an estrogen reporter gene ERβ-CALUX® assay in U2-OS cells, and in reporter and endogenous gene assays in MCF-7 cells.
Results
Six of the plant extracts bound to the estrogen receptors. Four of the six extracts stimulated reporter gene expression in the ERβ-CALUX® assay. All six extracts modulated expression of endogenous genes in MCF-7 cells, with four extracts acting as estrogen agonists and two extracts, Pimenta dioica and Smilax domingensis, acting as partial agonist/antagonists by enhancing E2-stimulated pS2 mRNA expression, but reducing E2-stimulated PR and PTGES mRNA expression. Both P. dioica and S. domingensis induced a 2ERE-luciferase reporter gene in transient transfected MCF-7 cells, which was inhibited by the ER antagonist ICI 182780.
Conclusions
This work presents a plausible mechanism of action for many of the herbal medicines used by Costa Rican women to treat menopausal symptoms. However, it further suggests that studies of safety and efficacy are needed before these herbs should be used as alternative therapies to HT.
Keywords: Costa Rica, herbal medicine, menopause, ER-CALUX, pS2, PTGES, PR, reporter gene, safety
Introduction
Menopause is defined as the cessation of menstruation due to depletion of follicular stores, and is retrospectively determined after 12 months of amenorrhea during the midlife period.1 The menopausal transition represents a continuum of change that begins with diminished ovarian reserve, is followed by menstrual irregularity of the peri-menopause, and culminates with cessation of menses and loss of reproductive capacity in the postmenopausal female.1 Approximately 55−75% of American women experience vasomotor symptoms such as hot flashes and sweating, as well as other symptoms such as anxiety, depression, mood swings, sleep disorders, vaginal dryness, and joint pain largely due to a lack of estrogens.2 Between 10% and 25% of women suffer such severe symptoms that they will seek treatment from their health care provider.2 Prior to 2002, hormone therapy (HT; estrogen and/or progesterone) was the gold standard for the symptomatic treatment of menopause. Today, HT is still the most effective treatment for menopausal symptoms, although the adverse events associated with its use are unacceptable to most women.3,4
Estrogen is a hormone with multiple actions in reproductive tissues (such as breast, uterus, and ovary) and in many non-reproductive tissues including bone, the central nervous system, and the cardiovascular system.5,6 Estrogen exerts its effects on target tissues by interacting with two different members of the nuclear receptor super-family of hormone-regulated transcription factors, namely estrogen receptors, ERα and ERβ.6 Bound ER undergoes conformational change, interacts with chromatin, and modulates the transcription of target genes in estrogen-responsive tissues.6,7 However, despite the evidence supporting the efficacy of hormone therapy for the treatment of menopause, many women have opted to discontinue or refuse to use HT due to the fear of malignancy, adverse events such as vaginal bleeding, weight gain, and depression.8 As a consequence, many women are searching for safer alternative treatments to manage their menopausal symptoms.9,10
Results from the Study of Women's Health Across the Nation (SWAN), one of the largest multiethnic studies of menopausal women in the United States suggested that ethnicity may serve as a predictor for the prevalence of menopausal symptoms.11 The study also indicated that Hispanic-American women frequently reported having hot flashes or night sweats.11,12 However, interestingly while HT is readily available to Hispanic women living in the United States, Hispanic women are least likely to use HT to treat their menopausal symptoms, opting for more natural methods such as herbs, diet, vitamins and exercise.13 In Costa Rica, the average age at onset of menopause is 50.6 years, and Costa Rican women experience menopausal symptoms comparable to their counterparts in the United States.13,14 Similar to Hispanic women living in the United States, most Costa Rican women do not often use HT to treat menopausal symptoms, but instead opt for specific herbal medicines.13,14 While there are some limited ethnobotanical data are available for these specific herbal therapies used by Costa Rican women, many of these plant species have never been scientifically investigated for their potential use for the management of menopausal symptoms. As part of ongoing international research collaborations in Central America, we have identified and collected 17 plant species used in Costa Rica to treat women's health disorders with a focus on menopause. Since these herbal medicines are used to treat menopausal symptoms, particularly hot flashes and night sweats, we hypothesized that these plants may mediate some of their actions via the activation of the ER.
The present investigation was designed to determine if extracts of specific plants collected in Costa Rica have estrogen-like activity, and to evaluate their potential as candidates as alternative treatments for menopausal symptoms and other hormone related women's health issues. Extracts were tested in four different in vitro assays. The first, a competitive estrogen receptor-binding assay, measures the affinity of the extract for the estrogen receptors, ERα and ERβ.15 The second is a reporter gene assay, the ERβ-CALUX®, which detects the extract's ability to induce transcription of an estrogen responsive firefly luciferase reporter gene.16 The third assay utilizes the MCF-7 breast cancer cell line that expresses endogenous ERα. Increase in transcription of the endogenous estrogen responsive genes, pS2, PR, and PTGES, indicates estrogenic activity through ERα.6,7 The fourth assay utilized MCF-7 breast cancer cells transiently transfected with 2ERE-luciferase reporter construct.6,7
Methods
Chemicals
All chemicals and reagents were purchased from Fisher (Hanover Park, IL) or Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. All media for cell culture and human recombinant ER and ER were purchased from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Norcross, GA). The Dual-Luciferase Reporter Assay System from Promega (Madison, WI).
International Agreements
This study was performed under a collaborative agreement between the University of Illinois at Chicago (UIC) and the University of Costa Rica (UCR). The Memorandum of Agreement was signed by authorities from both UIC and UCR in September of 2003 and renewed in 2008.
Plant collection and extraction
An initial list of twelve plants was established by searching the databases NAPRALERT, PubMed and SciFinder for plants that had reported ethnomedical use in Costa Rica for the treatment of menopause, as well as some correlated pharmacological activity. The search terms used included but were not limited to: menopause, hot flashes, vasomotor symptoms, menopausal symptoms, estrogen, estrogenic, progesterone, progestagenic, anti-inflammatory, antioxidant and climacteric. Five additional plant species were added to this list based on their indication for the treatment of menopause in various Costa Rican medicinal herb markets.
Plant materials (1 kg dry weight) were collected at various sites throughout Costa Rica and oven dried at 37°C. The dried plant material was ground and extracted in MeOH three times for 24 hrs each, and resultant extracts were filtered and dried under reduced pressure. Herbarium specimens were identified by Jorge Gomez-Laurito at the University of Costa Rica, and were deposited in the Herbarium of the University of Costa Rica, San Jose, Costa Rica.
Competitive ER ligand binding assay
The relative binding affinity of the herbal extracts to full-length ERα and ERβ was determined in a competitive radioligand-binding assay. The methanol extracts were dissolved in DMSO and tested at 50 μg/ml as described.15 Briefly, recombinant human estrogen receptor from insect Sf9 cells (alpha or beta) was incubated with the test sample plus 0.5 nM 3H-estradiol at 4°C overnight. At the completion of incubation, 100 μl of a 50% hydroxylapatite slurry (in 40nM Tris, pH7.4, 1mM EDTA, 1mM EGTA) was added and allowed to bind the ER-ligand complex for 40 min. The hydroxylapatite was washed three times with 0.5 ml of 40 mM Tris, pH 7.4, 1mM EDTA, 1mM EGTA, and 11 mM KCL. The hydroxylapatite pellets were suspended in 1ml of ethanol and counted in 5mL of scintillation fluid, and the receptor-bound 3H-estradiol was measured. The median inhibitory concentration was determined by testing the binding affinity of the extracts to the estrogen receptors in concentrations of 20 to 100 μg/ml. All experiments were performed in triplicate, and the results were from three independent experiments.
Cell culture and RNA extraction
MCF-7 human breast cancer cells were routinely maintained in MEM (Sigma-Aldrich Corp., St. Louis, MO) supplemented with 5% calf serum (Hyclone, Logan, UT).6,7 Cell growth was quantified usin previously described protocols.6,16 Four days prior to treatment the cells were sub-cultured on to phenol red-free MEM containing 5% charcoal dextran-treated calf-serum. Media were changed on day 2 and day 4 of culture. Cells were treated with 10 nM E2 alone or in combination with 20 μg/ml of the plant extract.7 Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. RNA was further purified using RN-easy columns (Qiagen, Valencia, CA) and treatment with ribonuclease-free deoxyribonuclease 1 (Qiagen). The human osteoblastic osteosarcoma cell line U2-OS (ATCC) was cultured and maintained in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium (DF, Gibco, Grand Isand, NY) supplemented with 7.5% fetal calf serum.16
Transfection and reporter gene assays
For transient transfections, U2-OS cells were plated in 24-well tissue culture plates.16,17 After culturing for one day, cells were transfected with 1 mg reporter plasmid (33 ERE-TATA-Luc), 200 ng SV2-lacZ and 200 ng expression plasmid (pSG5-neo-hERβ) using the calcium phosphate co-precipitation method.17 Luciferase activity was corrected for transfection efficiency by measuring LacZ expression as a result of SV2-lacZ co-transfection.17 Transient transfection and luciferase assay: MCF-7 cells were co-transfected with the pERE-luciferase plasmid (2 g), which contains three copies of the Xenopus laevis vitellogenin A2 ERE upstream of fire fly luciferase and the control plasmid, Renilla luciferase to normalize transfection efficiency (Promega Corp., Madison, WI), using Lipofectamine 2000 (Invitrogen) diluted in serum-free, antibiotic-free OptiMEM (Invitrogen).7 Six hours after transfection, media were changed to phenol red-free media containing charcoal-dextran stripped serum and the cells were treated with one of the following: vehicle solvent (ethanol); vehicle solvent plus ICI 182,780 (1 μM); E2 (10 nM) dissolved in ethanol; E2 plus ICI 182,780 (1 μM; plant extract (5−20 μg/ml) plus vehicle solvent; or plant extract (20 μg/ml) plus ICI 182,780 (1 μM) for 16 h. Reporter activity was measured using dual-luciferase assay according to the manufacturer's directions (Promega Corp., Madison, WI). The vehicle solvent ethanol tested for its ability to affect gene expression in the MCF-7 cells, with little effect and was therefore used as the negative control. Luciferase data shown are the mean ± SEM from at least three independent determinations and three experiments. Group means were compared by analysis of variance with GraphPad Prism version 4.0 software (GraphPad Software, Inc, San Diego, CA). Multiple comparison tests were performed with Tukey's test for significant differences at p < 0.05. Coefficients of variation (CVs) were as follows: intra-assay CV, 9.53% at 10 nM E2, and inter-assay CV, 5.45% at 10 nM E2.
ERβ-CALUX assay
The Chemically Activated Luciferase Expression (ER-CALUX) assay was performed as previously described.16 Transfected human osteosarcoma cell line (U2-OS) ERβ CALUX cells were plated in 96-well plates (6000 cells/well) with phenol red-free DF medium supplemented with 5% dextran-coated charcoal-stripped FCS at a volume of 200 μl per well. Two days later, the medium was refreshed and the cells were incubated with compounds to be tested (dissolved in DMSO) in triplicate. After 24h, the medium was removed and the cells lysed in 30 μl Triton-lysis buffer and measured for luciferase activity using a luminometer for 0.1 min/well. Luciferase activity per well was measured as relative light units (RLUs). The results were obtained in duplicate of three individual experiments, with < 10 % variation. Fold induction was calculated by dividing the mean value of light units from exposed and non-exposed (solvent control) wells. Luciferase induction, as a percentage of maximal 17β-estradiol (E2) activity, was calculated by setting the highest fold induction of E2 at 100%. Estradiol equivalents (EEQ) were calculated by interpolating test sample data in an E2 standard curve.16
pS2, PR, PTGES expression assay
Effect on the expression of the estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells was assayed according to the previously published methods.7 Four days prior to E2 treatment, MCF-7 cells were sub-cultured onto phenol red-free MEM containing 5% charcoal-dextran-treated calf serum and the medium was changed on d 2 of culture. Cells were treated with 10 nm E2 (Sigma-Aldrich Corp., St. Louis, MO) for 4h. Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. One μg of total RNA was reverse transcribed in a total volume of 20 μl using 200 U reverse transcriptase, 50 pM random hexamer, and 1 mM deoxy-NTP (New England Biolabs, Beverly, MA). The resulting cDNA was diluted to a total volume of 100 μl with sterile H2O. Each real-time PCR reaction consisted of 1 μl diluted RT product, 1× SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA), and 50 nM forward and reverse primers: 5′-CTTCCTTTTCCTGGGCTTCG-3′ (PTGES forward), 5′-GAAGACCAGGAAGTGCATCCA-3′ (PTGES reverse), 5′-ATGGCCACCATGGAGAACAAGG-3′ (pS2 forward) and 5′-CATAAATTCACACTCCTCTTCTGG-3′ (pS2 reverse), 5′-GAGCTCATCAAGGCAATT-3′ (PR forward) and 5′-CCATCCCTGCCAATATCT-3′ (PR reverse).7 Reactions were carried out on an ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems) for 40 cycles (95 C for 15 sec, 60 C for 1 min) after an initial 10-min incubation at 95 °C. The fold change in expression of each gene was calculated using the comparative Ct method, with the ribosomal protein 36B4 mRNA as an internal control. Data are shown as the mean ± SEM of triplicates from three independent determinations.
Statistical analysis
All experiments were performed in triplicate, or as indicated. Values are expressed as means ± SEM. Data were analyzed using one-way ANOVA, followed by Dunnett test for pair-wise comparison between the vehicle control and each of the other extracts. A general linear model with two main effects and their interaction was used to analyze the real time RT-PCR data, followed by post hoc comparison (Student's Newman-Keuls test) between the control and each of the other extracts. Statistical significance was ascribed to the data when p < 0.05. Comparison of the values between runs was performed by analysis of variance, and p < 0.05 was considered to be statistically significant. The statistical software package used was GraphPad Prism version 4 software (GraphPad Software, Inc, San Diego, CA).
Results
Herbal extracts bind to the estrogen receptors, ERα and ERβ
A total of 17 plants were collected and extracted in Costa Rica based on their ethnomedical use for the treatment of menopause (Table 1). The methanol extract of each plant was then assayed for binding to ERα and ERβ. Six of the 17 plant extracts, Smilax domingensis Willd. (Smilacaceae), Pimenta dioica (L.) Merr. (Myrtaceae), Artemisia absinthium L. (Asteraceae), Plantago major L. (Plantaginaceae), Tanacetum parthenium (L.) Sch. Bip. (Asteraceae), and Hibiscus sabdariffa L. (Malvaceae) displaced [3H]-17β-estradiol from both receptor subtypes and showed significant activity (p < 0.05; 50% or greater ligand displacement at 50 μg/ml) in the binding assay. These extracts were followed up in reporter and endogenous gene transcription assays. Typical of phytoestrogens, all six active extracts bound to both ER subtypes with a slight preference to ERβ (not statistically significant). Data is shown in Table 1 as percent displacement of 3H-estradiol from the receptor subtypes and IC50s are given in μg/ml.
Table I.
ER binding data for the seventeen plant species used in Costa Rica for the treatment of menopause (tested at 50 μg/ml). Median inhibitory concentration (IC50) was obtained only for those plant extracts displacing 3H-estradiol from the receptor site at >50% at 50 μg/ml.
| Genus species citation | Date and voucher number | Family | Part | ERα | ERβ | IC50 ERβ (μg/ml) |
|---|---|---|---|---|---|---|
| Ananas comosus (L.) Merr | AC416; 04/10/06 | Bromeliaceae | Peel | 19% ± 11.2 | 21% ± 13.4 | NT |
| Artemisia absinthium L. | BDTL102; 08/12/05 | Asteraceae | Leaves | 49% ± 4.6 | 55% ± 11.8 | 48.7 |
| Brosimum alicastrum Sw. | AC1105, 10/01/05 | Moraceae | Fruit | 48% ± 11.6 | 49% ± 14.3 | NT |
| Buddleja verticillata Kunth. | BDTL104;08/25/05 | Buddlejaceae | Leaves | 41% ± 5.5 | 43% ± 9.9 | NT |
| Citrus aurantium L. | TLBD105 8/25/05 | Rutaceae | Leaves | 2% ± 10.0 | 26% ± 21 | NT |
| Dioscorea villosa L. | AC1044;10/05/06 | Dioscoreaceae | Root | 49% ± 14.2 | 43% ± 13.1 | NT |
| Equisetum bogotense Kunth. | GM1004; 06/30/05 | Equisetaceae | Leaves | 11% ± 8.0 | 15% ± 7.5 | NT |
| Euphorbia lancifolia Schldtl | AC1106;10/01/05 | Euphorbiaceae | Leaves | 1% ± 4.4 | 7% ± 10.9 | NT |
| Hamelia patens Jacq | TLBD106;08/12/05 | Euphorbiaceae | Leaves | 23% ± 10.0 | 31% ± 14.4 | NT |
| Hibiscus sabdariffa L. | TL101;08/12/05 | Malvaceae | calices | 38% ± 4.5 | 56% ± 12.4 | 45.5 |
| Phlebodium aureum (L) J. Smith | AC417; 04/10/06 | Polypodiaceae | Rhizomes | 5% ± 4.6 | 5% ± 17.7 | NT |
| Pimenta dioica (L.) Merr. | BD101;9/10/05 | Myrtaceae | Leaves | 71% ± 19.8 | 80% ± 22.4 | 20.1 |
| Plantago major L. | TLBD106;8/30/05 | Plantaginaceae | Leaves | 49% ± 7.0 | 53% ± 11.7 | 49.3 |
| Punica granatum L. | AC420;04/10/06 | Punicaceae | Seeds | 20% ± 4.6 | 39% ± 4.6 | NT |
| Smilax domingensis Willd. | AC418;04/10/06 | Smilacaceae | Rhizomes | 67% ± 15.0 | 85% ± 21.3 | 18.8 |
| Tanacetum parthenium (L.) Sch. Bip. | GM1008;06/30/05 | Asteraceae | Leaves | 60% ± 20.2 | 71% ± 19.1 | 30.4 |
| Zingiber officinale Roscoe | AC428;10/01/05 | Zingiberaceae | Rhizomes | 47% ± 20.0 | 15% ± 12.1 | NT |
NT = Not Tested
Herbal extracts induced transcription of the estrogen responsive reporter gene in the ERβ-CALUX® assay
The six plant extracts that were active in the ER-binding assay were tested in the ERβ-CALUX® reporter gene assay which tests the extracts’ ability to induce transcription of an estrogen responsive luciferase reporter gene through ERβ. Four of the six extracts, P. dioica, A. absinthium, P. major, and T. parthenium induced transcription of the luciferase gene via ERβ in stably transfected U2-OS cells. Data are shown in Table 2 expressed in estradiol equivalents (EEQ), the concentration in ng of E2 with equivalent activity to 1 g of sample.
Table II.
Results of the ER-CALUX® reporter gene assay demonstrating the ability of the extracts to induce transcription of an estrogen responsive luciferase reporter gene through activation of ERβ in stably transfected U2-OS cells.
| Plant Extract | ERβ CALUX® EEQ (ng E2-eq./g extract) |
|---|---|
| S. domingensis | <LODa |
| P. dioica | 138 |
| A. absinthium | 18 |
| P. major | 440 |
| T. parthenium | 368 |
| H. sabdariffa | < LODa |
LOD = 1.3 ng EEQ
Data represent the mean ± SD of three separate experiments performed in triplicate.
Herbal extracts induced transcription of pS2, PTGES and PR through ERα in MCF-7 cells
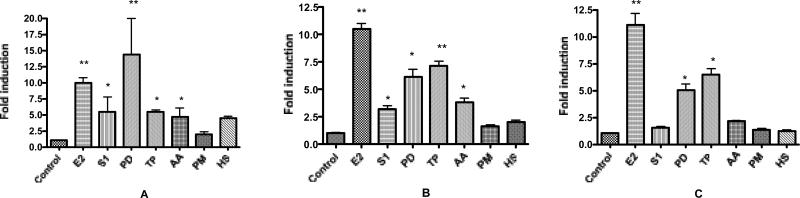
Real-time-PCR analysis was used to detect changes in levels of transcription of the estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells in the presence of each of the six extracts (Figure 1). Of the six herbal extracts tested only P. dioica (PD) and T. parthenium (TP) significantly induced transcription of the endogenous estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells via ERα. Extracts of P. dioica enhanced the expression of PR mRNA by 5.8 fold (p < 0.05), pS2 mRNA by 12.5 fold (p < 0.01) and PTGES mRNA by 4.0 fold (p < 0.05) above DMSO control (Figure 1). Extracts of TP enhanced PR mRNA expression by 6.1 fold (p < 0.05), PTGES by 5.0 fold (p < 0.05) and pS2 mRNA expression by 2.0 fold. The S. domingensis (S1) and Artemisia absinthium (AA) extracts also induced increased expression of pS2 and PR mRNA (p < 0.05). Treatment of MCF-7 cells with the extracts alone in concentrations up to 20 μg/ml did not significantly increase or decrease proliferation (data not shown).
FIG 1.
Results of RT-PCR analysis of endogenous estrogen responsive genes in MCF-7 cells after treatment with plant extracts (20 μg/ml). Data is represented as fold increase in mRNA above control for (A) pS2, (B) PR, (C) PTGES. Extracts are abbreviated: S1 = S. domingensis, PD = P. dioica, TP = T. parthenium, AA = A. absinthium, PM = P. major, HS = H. sabdariffa. Data represent the mean ± SD of three separate experiments performed in triplicate. *p<0.05 and **p<0.01 versus control.
Herbal extracts modulate the effects of estradiol on pS2, PR and PTGES expression in MCF-7 cells
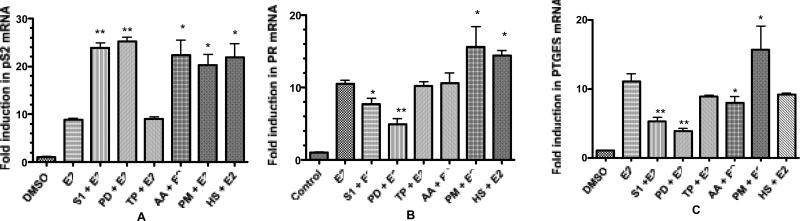
The agonist or antagonistic effects of the herbal extracts in the presence of E2 in the MCF cells was also investigated (Figure 2). In the pS2 assay, S. domingensis (S1), P. dioica (PD), A. absinthium (AA), P. major (PM), and H. sabdariffa (HS) in combination with E2 significantly (*p < 0.01; **p < 0.05) enhanced pS2 expression by 10 to 15 fold above E2 controls, suggesting additive, or possibly synergistic effects. The T. parthenium (TP) extract exhibited no additive or synergistic activity with E2 (Figure 2). Both the HS and PM extracts significantly increased PR mRNA expression in combination with E2 (p < 0.05), and only the PM extract significantly increased PTGES mRNA expression in combination with E2 in MCF-7 cells.
FIG 2.
MCF-7 cells were treated with extracts (20 μg/ml) + E2 (10 nM) to assess synergistic or antagonistic effects. Data is represented as fold increase in mRNA above control for (A) pS2, (B) PR, (C) PTGES. Extracts are abbreviated: S1 = S. domingensis, PD = P. dioica, TP = T. parthenium, AA = A. absinthium, PM = P. major, HS = H. sabdariffa. Data represent the mean ± SD of three separate experiments performed in triplicate. *p<0.05 and **p<0.01 versus E2.
Conversely, co-treatment of the MCF-7 cells with the PD or S1 extracts plus E2 resulted in significant (p < 0.05) decreases in both PTGES and PR mRNA expression, thereby demonstrating estrogen antagonistic effects (Figure 2). Interestingly, while PD and S1 extracts showed an enhancement of pS2 expression, they did not stimulate proliferation of MCF-7 cells in vitro at concentrations up to 20 μg/ml. Thus, of the seventeen herbal extracts used to treat menopause in Costa Rica, six bind to the ERs, with extracts of AA, TP, PM and HS acting as estrogen agonists, and extracts of PD and S1 exhibiting partial estrogen agonist/antagonist effects.
ER antagonist, ICI 182,780 inhibits the effects of herbal extracts in transient transfected MCF-7 cells
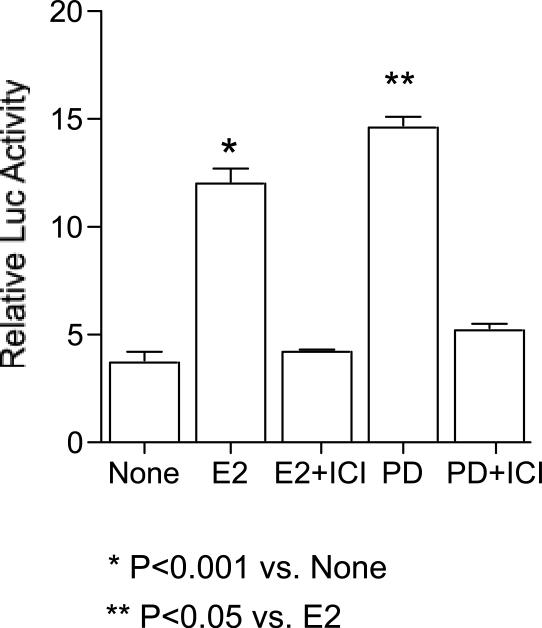
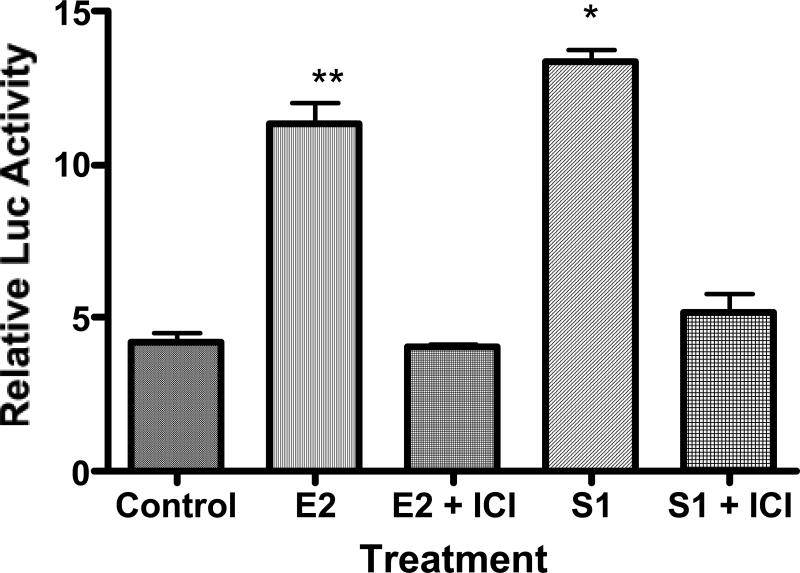
Due to the interesting agonist/antagonist activities of the P. dioica and S. domingensis, we investigated the impact of the estrogen antagonist ICI 182,780 on the agonist activities of these two extracts. Transient transfection assays were performed to determine if ICI 182,780 would inhibit the effects of both P. dioica and S. domingensis extracts in an ERE-regulated reporter gene assay. MCF-7 cells were transfected with a 2ERE-luciferase reporter construct and the control plasmid, Renilla luciferase. Both extracts and the E2 control increased ERE-transcriptional activation, as measured by the fold increase in luciferase activity in MCF-7 cells (Fig. 3 and 4). E2 significantly increased luciferase activity by approximately 12. 5 fold (p < 0.001), PD by ∼ 14.8 fold (p < 0.05) and S1 by ∼ 13 fold (p < 0.05). Both the extracts and E2 induction of ERE-dependent luciferase activity was inhibited by the co-treatment of the cells with ICI 182,780 (Fig. 3 and 4), indicating that the activities of these two plants may be mediated via the activation of the ER. The estimated EC50 of P. dioica and S. domingensis in activation of ERE-dependent luciferase activity was 10 μg/ml, and the concentration of extract required for maximal stimulation of luciferase activity was 20 μg/ml.
FIG. 3.
The ER antagonist ICI 182780 specifically blocks the induction of luciferase activity by PD through ERα in MCF-7 cells transiently transfected with a pERE-luciferase plasmid. Cells were treated with 1 nM E2 or 1 nM E2 + 10 nM ICI 182780 or 20 μg/ml PD or 20 μg/ml PD + 10 nM ICI 182780 to assess induction. Results are expressed as fold induction above control (vehicle solvent). Abbreviations: E2 = 17β-estradiol; PD = Pimenta dioica; ICI = ICI182780. Data represent the mean ± SD of three separate experiments performed in triplicate. * P < 0.001 versus control solvent (none) or **p < 0.05 versus E2.
FIG. 4.
The ER antagonist ICI 182780 specifically blocks the induction of luciferase activity by S1 through ERα in MCF-7 cells transiently transfected with a pERE-luciferase plasmid. Cells were treated with 20 μg/ml of the S1 extract or 1 nM 17β-estradiol (E2) with or without ICI 182780 (10 nM) for 16 h before they were lysed and reacted with substrate luciferin. Relative luciferase units were measured by luminometer. The S1 extract and E2 treatment significantly increased estrogen-dependent activation of luciferase gene transcription in MCF-7 cells. ICI 182780 specifically blocked the actions of the S1 extract and E2 in MCF-7 cells. Abbreviation: S1 = S. domingensis. Data represent the mean ± SD of three separate experiments performed in triplicate. Data represent the mean ± SD of three separate experiments performed in triplicate. * P < 0.001 versus control solvent (none) or **p < 0.05 versus E2.
Discussion
It is estimated that by the year 2030, there will be over 60 million postmenopausal women in the United States, and 1.2 billion postmenopausal women worldwide.9,13 Understanding the onset of this transition, as well as the development of new therapeutic alternatives for the treatment of menopause has become increasingly important due to concerns regarding the safety of HT, particularly in light of 21st century age demographics.9,10 In the United States, herbal extracts are commonly used Hispanic women to manage their menopausal symptoms, and their use of HT is low, although readily available.13,19 These data mirror the situation in Costa Rica, where there is lower use of HT, and women use specific herbs to manage symptoms during the menopausal transition.13,20-21 Thus, investigations of commonly used therapies from other cultures may lead to the development of alternative therapies for menopause.
Based on our ethnomedical investigation of herbal medicines commonly used for the management of menopausal symptoms by Costa Rican women, we collected and extracted 17 plant species. In view of the fact that HT is the most effective treatment for menopausal symptoms, and that many plants contain phytoestrogens, the most logical first step was to assess the potential estrogenic activities of the extracts. Using a combination of a competitive estrogen receptor binding assays and cell-based reporter and endogenous gene transcription assays we identified six extracts that not only bound to the estrogen receptors but also induced transcription of estrogen responsive genes. Extracts of Smilax domingensis Willd. (Smilacaceae), Pimenta dioica (L.) Merr. (Myrtaceae), Artemisia absinthium L. (Asteraceae), Plantago major L. (Plantaginaceae), Tanacetum parthenium (L.) Sch. Bip. (Asteraceae), and Hibiscus sabdariffa L. (Malvaceae) bound to both ER-α and ER-β subtypes with a slight preference to ER-β. The difference in tissue distribution of the two estrogen receptors has been extensively investigated, and it has been suggested that the proliferative effects of HT in ER-responsive MCF-7 cells may be mediated through ERα, rather than ERβ.22 Since many plant extracts bind to both ERs, it is possible that any potential proliferative effects of the extracts mediated through ERα maybe negated by the effects of the plant extracts on ERβ. For this reason, plant extracts that may bind to ERβ are especially interesting as alternatives to HT. However, since the herbal extracts tested in this study bind both to ERs, the issue of whether such extracts are safe for use, especially in women with familial or past history of breast cancer, is an important question to answer prior to initiating any human studies.
From the seventeen plant extracts commonly used by Costa Rican women to treat menopausal symptoms, six plant extracts were active in the ERα and ERβ competitive binding assays. These six plant extracts also activated ER, as they were able to induce or inhibit the transcription of estrogen responsive reporter or endogenous genes in MCF-7 and U2-OS cells. In addition, the ER antagonist ICI 182,780 inhibited reporter gene induction by two of the extracts indicating that they exert their effect via ER. Interestingly, the activity of the extracts in the U2-OS cell based assay (ERβ-CALUX®) did not correspond well to the extracts’ binding affinity for the ERβ, in that herbal extracts that induced gene transcription in the ERβ-CALUX® assay, actually had higher IC50s in the ERβ binding assay. These results point out the necessity for ER ligand binding studies to be combined with other functional assays, due to the possibility that the compounds contained in herbal extracts may bind to the ER subtypes, but only block the receptor site and not induce the conformational change in the receptor necessary to induce gene transcription. The ER competitive binding assay is however, a relatively high throughput, inexpensive screen useful for prioritizing extracts for study in more specific assays, as well as for bioassay-guided fractionation of the active chemical constituents.
Up-regulation of the expression of endogenous genes pS2, PR and PTGES in MCF-7 cells is indicative of estrogenic effects via ERα.5,7,22 It is well known that pS2 expression is regulated in MCF-7 cells by estrogens, and that pS2 is predominantly, but not exclusively, expressed in ER-dependent cancers.23 Interestingly, results of clinical investigations of pS2 indicate that there is a clear association between the pS2 presence in primary tumors and a more positive response to chemotherapy.24 In addition to pS2, mRNA expression of the PR and PTGES genes in MCF-7 cells are also regulated in a characteristic manner by estrogenic compounds.5,7,22 How commonly used herbal extracts may impact the expression of these genes, and the potential implications for women taking these herbal remedies has not been well investigated.
In this work, RT-PCR was performed to determine the change of pS2, PR and PTGES mRNA production in MCF-7 cells treated with the herbal extracts. Four of the extracts induced mRNA production of all three genes, suggesting that in MCF-7 cells these extracts may act as estrogen agonists. Plantago major (PM) was the only herbal extract that enhanced the expression of all pS2, PR and PTGES mRNAs in the presence of E2, also indicating an estrogen agonist effect. Conversely, in the presence of E2, both P. dioica and S. domingensis extracts suppressed the expression of the PR and PTGES mRNAs, indicating potential estrogen antagonist effects. This novel finding is interesting as PTGES encodes for prostaglandin E2 synthase 1 (mPGES-1), a microsomal enzyme that is up-regulated in pre-malignant and malignant breast disease.7, 25 PTGES mRNA levels in breast tumors is correlated with ER expression, and PTGES may be up-regulated by proinflammatory cytokines, as well as E2.7 Thus, treatments that may down-regulate PTGES expression, especially in the presence of E2 may reduce mPGES-1 levels and have implications for breast cancer chemoprevention.
While differences in gene expression induced by the extracts in U2-OS and MCF-7 cells may be attributed to the fact that the U2-OS cells express only ERβ, and the MCF-7 cells express primarily ERα, the differences in the relative activity of the extracts for different endogenous estrogen responsive genes in MCF-7 cells are intriguing. Up-regulation of specific estrogen responsive genes and down-regulation of others within MCF-7 cells may be due to the variety of chemical constituents present within the extracts, and the ability of these constituents to modulate gene expression through alternative mechanisms. Another possible explanation is that slight differences in the sequences of estrogen response elements (EREs) in the promoter regions of these genes might affect the response to the ER depending on the ligand that is bound. It has been shown that many natural EREs deviate substantially from the consensus sequences.26
In the United States, herbal medicine are sold as botanical dietary supplements, and only very limited data on safety and efficacy are required for the sale of these products.27 The regulatory situation is similar in Costa Rica. As more menopausal women turn away from HT to use herbal products for the treatment of menopausal symptoms, the lack of safety and efficacy data for these herbal medicines becomes problematic due to the unknown impact of these products on women's health in general, and their potential to influence estrogen-responsive cancers. This problem is not limited to Costa Rica, as many of the herbal medicines used to treat menopause in Costa Rica, are used by women in the United States for other conditions other than menopause. For example, while T. parthenium (feverfew) is also widely used in the United States for the treatment of fever, women's ailments, inflammatory conditions, psoriasis, toothache, insect bites, rheumatism, asthma and stomachache.28 In addition, over the past 20 years, feverfew has also been used for migraine prophylaxis, particularly for migraine headaches associated with the menstrual cycle.28 Other herbal remedies included in this study, such as Smilax, Artemisia, Plantago and Hibiscus species are also widely used in the United States.
Our investigation of specific herbal extracts from Costa Rica, used ethnomedically to manage menopausal symptoms indicates that at least six extracts act as estrogen agonists or partial agonist/antagonists in vitro. The in vitro estrogenic data suggest a plausible mechanism of action for these herbal remedies in the management of menopausal symptoms. However, it also presents some serious concerns in regards to the safety of these products in view of their possible estrogenicity and its potential impact on estrogen-dependent cancers. Considering the large number of women in the United States that are opting to use alternative therapies for the management of their menopausal symptoms, and that specific ethnic groups and women in developing countries use herbal medicines in general, an understanding of how these products impact women's health, particularly estrogen-responsive cancers, is extremely important. Thus, continued investigation of botanicals extracts currently used by women to manage menopausal symptoms is critical for two specific reasons. First and foremost, determination of the safety of widely used botanical products for women's health is an important public health issue. Investigations of the effect of herbal extracts and their active compounds on estrogen-responsive cancers in appropriate in vitro tumor and animal models are essential. Secondly, research of herbal medicines used by women in other countries and cultures may ultimately lead to the development of much needed alternative treatments for the management of menopausal symptoms. Currently, we are in the process of isolating the active chemical constituents from these herbal extracts and testing them in animal models to more fully understand their multiple effects on estrogen-dependent genes.
Acknowledgements
This study was made possible by Grant Number R21-AT02381 from the National Center for Complementary and Alternative Medicine, NIH. We would like to gratefully acknowledge Mr. Juan-Carlos Brenes, University of Costa Rica for his assistance in the plant collections and technical help, as well as Dr. Harry Besselink, BioDetection Systems B.V., Amsterdam, The Netherlands for his assistance with the ERβ CALUX assay.
Footnotes
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or NIH.
References
- 1.Houmard BS, Seifer DB. Predicting the Onset of Menopause. In: Seifer DB, Kennard EA, editors. Menopause: Endocrinology and Management. Humana Press; Totwa, New Jersey: 1999. pp. 109–118. [Google Scholar]
- 2.Wijma K, Melin A, Nedstrand E, Hammar M. Treatment of menopausal symptoms with applied relaxation: A pilot study. J Behav Ther Exp Psychiatry. 1997;28:251–261. doi: 10.1016/s0005-7916(97)00030-x. [DOI] [PubMed] [Google Scholar]
- 3.Cass I, Runowicz CD. Non-hormonal alternatives to treating menopausal symptoms. Am J Manag Care. 1998;4:732–735. [PubMed] [Google Scholar]
- 4.Taylor M. Alternatives to conventional hormone replacement therapy. Compr Ther. 1997;23:514–532. [PubMed] [Google Scholar]
- 5.Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) alpha or ERbeta in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145:3473–3486. doi: 10.1210/en.2003-1682. [DOI] [PubMed] [Google Scholar]
- 6.Frasor J, Danes JM, Komm B, Chang KC, Lyttle R, Katzenellenbogen B. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 7.Frasor J, Weaver AE, Pradhan M, Metha K. Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17β-estradiol and pro-inflammatory cytokines. Endocrinology. 2008;149:6272–6279. doi: 10.1210/en.2008-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guay MP, Dragomir A, Pilon D, Moride Y, Perreault S. Changes in pattern of use, clinical characteristics and persistence rate of hormone replacement therapy among postmenopausal women after the WHI publication. Pharmacoepidemiol Drug Saf. 2007;16:17–27. doi: 10.1002/pds.1273. [DOI] [PubMed] [Google Scholar]
- 9.Doyle BJ, Mahady GB. Phytotherapies for Menopause. Drugs of the Future. 2007;32:897–905. [Google Scholar]
- 10.Mahady GB, Huang Y, Doyle BJ, Locklear TD. Black cohosh (Actaea racemosa) for the mitigation of menopausal symptoms: recent developments in clinical safety and efficacy. J. Women's Health. 2006;2:773–784. doi: 10.2217/17455057.2.5.773. [DOI] [PubMed] [Google Scholar]
- 11.Avis NE, Ory M, Matthews KA, Schocken M, Bromberger J, Colvin A. Health-related quality of life in a multiethnic sample of middle-aged women: Study of Women's Health Across the Nation (SWAN). Med Care. 2003;41:1262–1276. doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 12.Gold EB, Block G, Crawford S. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;159:1189–1199. doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- 13.Locklear TD, Doyle BJ, Caceres A, Perez A, Mahady GB. Menopause, a Universal Female Experience, Lessons from Central America. Current Reviews in Women's Health. 2007;4:1–10. [Google Scholar]
- 14.Tan D, Darmasetiawan S, Haines CJ, Huang KE, Jaisamram U, Limpaphayom KK, Lin SQ, et al. Guidelines for hormone replacement therapy of Asian women during the. [DOI] [PubMed]
- 15.Obourn JD, Koszewski NJ, Notides AC. Hormone- and DNA binding mechanisms of the recombinant human estrogen receptor. Biochemistry. 1993;32:6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- 16.Sonneveld E, Jansen HJ, Riteco JA, Brouwer A, van der Burg B. Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol Sci. 2005;83:136–148. doi: 10.1093/toxsci/kfi005. [DOI] [PubMed] [Google Scholar]
- 17.Legler J, van den Brink CE, Brouwer A, Murk AJ, van der Saag PT, Vethaak AD, van der Burg B. Development of a stably transfected estrogen receptor-mediated luciferase reporter gene assay in the human T47D breast cancer cell line. Toxicol Sci. 1999;48:55–66. doi: 10.1093/toxsci/48.1.55. [DOI] [PubMed] [Google Scholar]
- 18.Kalkhoven E, Kwakkenbos-Isbrucker L, de Laat SW, van der Saag PT, van der Burg B. Synthetic progestins induce proliferation of breast tumor cell lines via the progesterone or estrogen receptor. Mol Cell Endocrinol. 1994;102:45–52. doi: 10.1016/0303-7207(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 19.Longworth JC. Hispanic women's experience with “el cambio de vida”. J Am Acad Nurse Pract. 2003;15:266–275. doi: 10.1111/j.1745-7599.2003.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 20.Michel J, Duarte RE, Caceres A, Yao P, Huang Y, Bolton J, Soejarto DD, Mahady GB. Q'eqchi Maya medicine for women's health: In vitro evaluation of Guatemalan plants in estrogen and serotonin bioassay. J. Ethnopharmacol. 2007;114:92–101. doi: 10.1016/j.jep.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel J, Mahady GB, Caceres A, Soejarto DD. Attitudes and traditional medicine treatments for menopause in Guatemala. Soc. Sci. Med. 2006;63:732–736. doi: 10.1016/j.socscimed.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overk CR, Yao P, Chen S, Deng S, Imai A, Main M, Schinkovitz A, Farnsworth NR, Pauli GF, Bolton JL. High-content screening and mechanism-based evaluation of estrogenic botanical extracts. Comb Chem High Throughput Screen. 2008;11:283–293. doi: 10.2174/138620708784246022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 24.Pichon MF, Milgrom E. Clinical significance of the estrogen regulated pS2 protein in mammary tumors. Crit Rev Oncol Hematol. 1993;15:13–21. doi: 10.1016/1040-8428(93)90017-x. [DOI] [PubMed] [Google Scholar]
- 25.Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S. Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J. Pathol. 2006;208:356–363. doi: 10.1002/path.1907. [DOI] [PubMed] [Google Scholar]
- 26.O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 27.Amato P, Christophe S, Mellon PL. Estrogenic activity of herbs commonly used as remedies for menopausal symptoms. Menopause. 2002;9:145–150. doi: 10.1097/00042192-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2004;(1):CD002286. doi: 10.1002/14651858.CD002286.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S. Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J Pathol. 2006;208:356–363. doi: 10.1002/path.1907. [DOI] [PubMed] [Google Scholar]
- 30.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]