Summary
These are the final results of a survey of sleep-disordered breathing, which examined objective and subjective information from a large randomly selected elderly sample. We randomly selected 427 elderly people aged 65 yr and over in the city of San Diego, California. Twenty-four percent had an apnea index, AI, ≥5 and 62% had a respiratory disturbance index, RDI, ≥10. Correlates of sleep-disordered breathing included high relative weight and reports of snoring, breathing cessation at night, nocturnal wandering or confusion, daytime sleepiness and depression. Body mass index, falling asleep at inappropriate times, male gender, no alcohol within 2 hr of bedtime and napping were the best predictors of sleep-disordered breathing. Despite statistical significance, all of the associations between interview variables and apnea indices were small. No combination of demographic variables and symptoms allowed highly reliable prediction of AI or RDI.
Keywords: Sleep-disordered breathing, Sleep apnea, Aging, Prevalence, Hypopnea
In 1974, Webb (1) observed that a small sample of healthy, middle-aged men had periodic breathing with apneas. From various studies of clinic or volunteer samples, investigators have estimated sleep apnea prevalence rates in the elderly to range from 5.6% to 70% (2–9). Discrepancies among previous studies may have resulted from failure to use random population sampling methods, and, therefore, a population-based probability sample has been needed.
Some previous surveys have relied on the assumption that likely apnea cases can be identified by symptom screening, and therefore, the symptomatic correlates of sleep-disordered breathing are of interest. Snoring is often regarded as the primary symptom of sleep respiratory disturbances. The partial cessation of breathing that causes snoring and the complete cessation of breathing that causes apnea are part of the same continuum (10,11). Both excessive sleepiness and less commonly, insomnia have been described as symptoms of sleep apnea (12–14). A nocturnal increase in blood pressure secondary to sleep apnea has been well documented. Hypertension, obesity and cardiac arrhythmias have all been suggested as sleep apnea correlates (15–18). It remains to be demonstrated whether screening for these symptoms is sufficient for case finding.
Bliwise et al. (19) found that age, sex, body mass index and symptomatic status (i.e. complaints of insomnia, parasomnia or hypersomnolence) all predicted sleep-disordered breathing in an elderly sample. Nevertheless, Bliwise et al. warned that their study “… should not be considered a random sample …”.
Our study examined some of these same questions by asking in a random sample: How prevalent is sleep-disordered breathing among the elderly population? Are the symptoms characteristic of this disorder (e.g. reported snoring, obesity, daytime sleepiness) sufficiently discriminatory to predict the presence of the disorder? To address these questions, we present final results of a large survey of sleep-disordered breathing in which objective and subjective data were obtained from a randomly selected elderly sample. Results of prevalence of periodic leg movements in sleep are presented in the accompanying article.
METHODS
Subject selection
The study was done between 1981 and 1985. Target telephone exchanges were selected to provide a balanced sociodemographic sample of the city of San Diego with an oversampling of minorities to allow better detection of racial effects on apnea. Subjects were selected to represent all socioeconomic levels (i.e. from high, middle and low income areas). The Haines reverse telephone directory was used. In each targeted telephone exchange, every 20th listed number was selected. In order to reach unlisted as well as listed numbers, the number 1 was then added to each 20th telephone number to select the numbers to be called. No number was abandoned until three attempts, at different times of different days, were made. If someone 65 yr of age or older resided at a number called, a telephone interview was requested. If more than one person 65 or older lived in the household, all were asked to complete the telephone interviews.
At the end of the telephone interviews, it was explained that the purpose of the study was to learn more about sleep in older individuals. Each person was then asked to schedule a home interview within 1–2 wk of the phone interview. The home visits were done primarily by a man, but a woman was available for those subjects that preferred a female interviewer. After giving written consent, each volunteer was administered the home interview and was asked to schedule a home sleep recording. The majority of the sleep recordings were done on the same day as the home interview and almost all were within 1 wk. Of the 1,865 persons at least 65 yr of age randomly identified, 427 volunteers (23%) completed all parts of the study including home sleep recordings (Table 1). The last 76 subjects recruited were paid $10.00 for participating, but this produced no discernible improvement in cooperation.
TABLE 1.
Sampling attrition rate
| n | % Elderly identified | % of Phone interviewed | % of Home interviewed | |
|---|---|---|---|---|
| Elderly identified | 1,865 | |||
| Phone interviews | 1,526 | 82 | ||
| Home interviews | 615 | 33 | 40 | |
| Sleep recordings | 427 | 23 | 28 | 69 |
Interviews
The telephone interview consisted of 24 brief questions concerning overall sleep satisfaction, estimated sleep time, sleep complaints, demographic information and general health (presence or absence of heart disease, hypertension, stroke, etc.).
The home interview, lasting about 1 hr, included 142 questions about sleep, daytime functioning, exercise, medical history, medication use, diet, alcohol and tobacco use, family sleep history and demographic information. There were also five items on which the interviewer rated the respondent from observation. The postsleep questionnaire asked questions about quality of sleep during the recordings. Copies of the interview questionnaire can be obtained from the authors.
Sleep recording
A four-channel modified Medilog/Respitrace portable recording system was used (20,21). Two channels of uncalibrated inductance respiration (thoracic and abdominal Respitrace bands), one channel of tibialis electromyograms (EMG) summed from both legs and one channel of wrist activity were recorded. Sleep state (asleep vs. awake) was scored in 30-sec epochs using wrist activity (22), respiration and tibialis EMG data.
This portable recording system has been previously validated (23). Correlations with polysomnographic scoring were: apnea index, rs = 0.80 (p < 0.01), total sleep period, rs = 0.82 (p < 0.01), total sleep time, rs = 0.69 (p < 0.01) and wake after sleep onset, rs = 0.61 (p < 0.01).
The equipment was generally attached in the late afternoon (mean time = 1706; SD = 151 min; range = 1116–2327) and removed the following morning (meantime = 0825; SD = 81 min; range = 0331–1134). The average duration of recordings was 15 hr. The following morning, each volunteer completed a postsleep questionnaire.
Recordings were scored for total sleep time (TST), wake after sleep onset (WASO), number of awakenings, number of apneas (subclassified by number of obstructive, central or mixed apneas), number of hypopneas and number of leg jerks. Apnea index (AI, number of apneas per hour of sleep), obstructive apnea index (OI), central apnea index (CI), mixed apnea index (MxI), hypopnea index (HI) and respiratory disturbance index (RDI, number of apneas and hypopneas per hour of sleep) were computed. An obstructive apnea was scored when there was at least 90% reduction in respiratory movements for at least 10 sec and thoracic and abdominal channels abruptly moved 180° out of phase. Central apnea was scored when the thoracic and abdominal channels were reduced at least 90% and remained in phase. Mixed apneas were scored when the event began with a central component, followed by an obstructive component. Hypopneas were scored when there was a 50–90% decrease in respiratory signal.
Data analyses
Nonparametric tests (e.g. Kruskal-Wallis, chi-square, Spearman rank correlations) were computed. When correlating almost 200 items with five different apnea indices, we had to consider the probability that some associations might appear by chance. On the other hand, multiple testing methods (e.g. Bonferroni criteria) would have been unduly conservative, because both the apnea indices and many of the questionnaire items are highly intercorrelated. To approach this problem systematically, we generated p-value plots after the method of Schweder and Spjotvoll (24). These plots indicated that we could reasonably reject the null hypotheses of no association when p ≤ 0.05. No doubt, associations with 0.01 < p < 0.05 deserve replication before they are treated with confidence, but as will be seen, associations reached convincing statistical significance even when the strength of associations was quite weak.
As in most population-based surveys, women tended to be older than men (Kruskal-Wallis, p = 0.15). Although the confounding was not statistically significant, there was the potential for misleading results if the relationships between AI and gender and between RDI and gender were not controlled for age and, conversely, if the relationship between AI and age and between RDI and age were not controlled for gender. The controlled analyses were performed using the extended Mantel-Haenszel procedure (25).
Those variables statistically significant in univariate models were chosen for the logistic models. Separate screens for potential predictors of sleep-disordered breathing (AI ≥ 5 and RDI ≥ 10) were performed using logistic regression with backward elimination (26). The sensitivity and specificity of each model was estimated using those predictors left after the elimination.
RESULTS
The sample
There were 1,865 people 65 yr of age or older identified with randomly selected telephone numbers. Table 1 shows the sample selection. Of those identified, 1,526 (82%) agreed to a telephone interview and 615 (33%) agreed to a home interview. Of those identified, 427 (23%) agreed to undergo sleep measurement and the postsleep questionnaire.
The mean age of the 232 women was 72.4 yr (SD = 6.4; range = 65–95). The mean age of the 195 men was 72.6 yr (SD = 5.7; range = 65–91). The combined mean age was 72.5 yr (SD = 6.1). The mean BMI [body mass index, computed as weight (kg)/height (m)2] for women was 20.8 (SD = 4.1; range = 12.4–37.7). The mean BMI for men was 21.4 (SD = 3.5; range = 13.2–34.6). The combined mean BMI was 21.1 (SD = 3.9).
The 427 elderly recorded were compared on the telephone interview items with the 1,085 elderly people not recorded (i.e. the 897 elderly who completed only the telephone interview plus the 188 who completed only the telephone and home interview).
On 11 of the telephone interview items, the sample recorded did not differ significantly from those refusing to volunteer (i.e. on history of hypertension, heart disease, stroke, deviated septum and reported trouble falling asleep, total sleep time, number of nighttime awakenings, trouble going back to sleep, cessation of breathing at night, sleeping pill use and napping). However, those recorded reported both more history of leg kicks (16% vs. 10%; p < 0.0001) and more history of snoring often (70% vs. 60%; p < 0.0001) than those not recorded. There were fewer women (58%) agreeing to participate than men (72%) (p < 0.001). There were also significant differences in age (p < 0.004), education (p < 0.001), race (p < 0.02) and income (p < 0.001), with those recorded tending to be younger, better educated, white and in higher income brackets.
Demographics and distributions
Tables 2 and 3 show the distributions of age and race by gender for the sample, compared to 1980 U.S. census data for Americans aged 65 yr and older. Our sample was similar to that of the 1980 census distribution for age (Table 2). We oversampled minorities intentionally, to have a large enough minority sample for statistical comparisons (Table 3).
TABLE 2.
Age distribution of sample (n = 427) vs. U.S. 1980 census (percentage of Americans over age 65)
| Age (yr) |
||||||
|---|---|---|---|---|---|---|
| 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | 90+ | |
| Men | ||||||
| Sample Census | 19.5 (15) | 13 (11) | 8 (7) | 3 (4) | 0.7 (2) | 0.7 (0.8) |
| Women | ||||||
| Sample Census | 19.5 (19) | 17 (15) | 9 (12) | 5 (8) | 2 (4) | 1 (2) |
| Total | ||||||
| Sample Census | 39 (34) | 30 (26) | 17 (19) | 8 (12) | 3 (6) | 2 (2.8) |
TABLE 3.
Race distribution of sample (n = 427) vs. U.S. 1980 census (percentage of Americans over age 65)
| Race |
|||
|---|---|---|---|
| White | Black | Othera | |
| Men | |||
| Sample Census | 38 (36.5) | 6 (3.3) | 2 (0.5) |
| Women | |||
| Sample Census | 44 (54.2) | 7 (4.9) | 3 (0.6) |
| Total | |||
| Sample Census | 82 (90.7) | 13 (8.2) | 5 (1.1) |
Other included Hispanic, Asian, Oriental and Indian.
General health
In this sample of elderly, 19% reported being somewhat or very troubled with their sleep whereas 81% reported being moderately or very satisfied. Twenty-one percent felt they got too little sleep, 2% reported getting too much sleep and 77% reported enough sleep each night. In addition, 25% reported trouble falling asleep at least once per week while 75% reported no trouble falling asleep at night. When asked about experiences with excessive daytime sleepiness (EDS, i.e. feeling sleepy or struggling to stay awake during the daytime), 39% reported experiencing EDS at least one per week while 61% said they never experienced EDS.
Sixty-three percent reported snoring and 35% reported snoring very loudly. Note that only 278 people could answer this question; the rest did not know if they snored.
Fifty-one percent had been hospitalized at least once in the previous 5 yr (see Table 4 for medical history and medication use). When asked about smoking history, 40% reported having never smoked, 42% used to smoke and 18% still smoked.
TABLE 4.
Reported medical history and use of medications (in percentage)
| Never had | Had or currently have | None | At least once/week | |
|---|---|---|---|---|
| Medical history | ||||
| Arthritis | 33 | 67 | ||
| Asthma | 91 | 9 | ||
| Diabetes | 90 | 10 | ||
| Heart disease | 74 | 26 | ||
| Hypertension | 53 | 47 | ||
| Kidney disease | 83 | 17 | ||
| Stroke | 93 | 7 | ||
| Tonsils and adenoids removed | 37 | 63 | ||
| Medication use | ||||
| Analgesics | 75 | 25 | ||
| Anti-hypertensives | 73 | 27 | ||
| Cardiac drugs | 81 | 19 | ||
| Diuretics | 73 | 27 | ||
| Major tranquilizers | 99 | 1 | ||
| Minor tranquilizers | 94 | 6 | ||
| Sedative-hypnotics | 94 | 6 | ||
Sleep-disordered breathing results
Prevalence of sleep-disordered breathing
A criterion of AI ≥ 5 (i.e. ≥5 apneas per hour of sleep) was arbitrarily chosen (27). Of the total sample of 427, respiration data were lost on 7 people; of the remaining 420, 24% (n = 100) had an AI ≥5 (14% had just AI ≥ 5 alone, and 10% had AI ≥ 5 plus a myoclonus index ≥ 5). Women showed significantly less sleep-disordered breathing than men with 20% of women having an AI ≥ 5 compared to 28% of the men (p < 0.05). The mean AI for the n = 100 with AI ≥ 5 was 13.2 (SD = 11.2, range = 5.3–81.7) (see Fig. 1). Table 5 summarizes these apnea data. The percentages of men and women with apnea indices at different criterion levels and different ages are shown in Table 6.
FIG. 1.
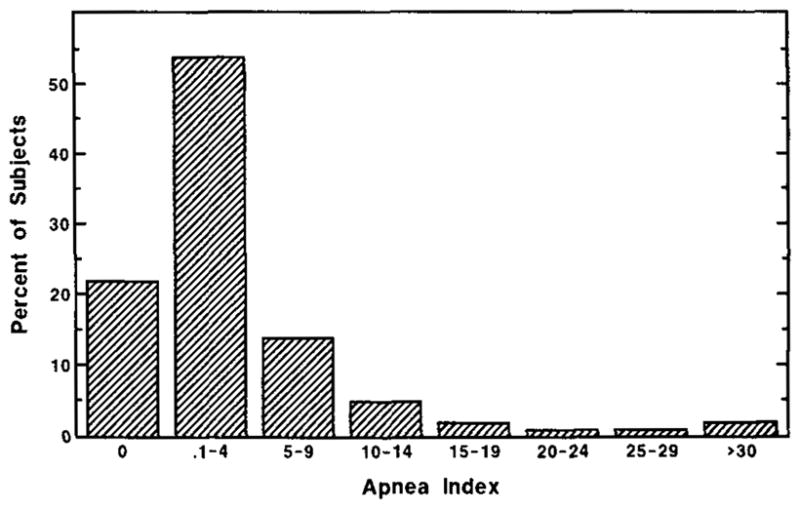
Distribution of apnea index.
TABLE 5.
Apnea variables
| Apnea index | Longest apnea (sec) | No. of apneas | No. of obstructive apneas | No. of central apneas | No. of mixed apneas | |
|---|---|---|---|---|---|---|
| Total group (n = 420) | ||||||
| Mean | 4.0 | 25.1 | 23.8 | 13.9 | 7.7 | 2.3 |
| SD | 7.6 | 22.3 | 40.2 | 27.8 | 23.5 | 7.0 |
| Median | 1.2 | 18.0 | 8.0 | 2.0 | 1.0 | 0.0 |
| Range | 0–81.7 | 0–138 | 0–233 | 0–203 | 0–216 | 0–55 |
| Group with AI > 5 (n = 100) | ||||||
| Mean | 13.2 | 47.8 | 77.4 | 45.7 | 23.5 | 8.1 |
| SD | 11.2 | 22.9 | 52.3 | 41.7 | 43.4 | 12.4 |
| Median | 9.1 | 42.0 | 59.0 | 35.0 | 5.0 | 2.0 |
| Range | 5.3–81.7 | 18–138 | 17–233 | 0–203 | 0–216 | 0–55 |
| Group with AI < 5 (n = 320) | ||||||
| Mean | 1.0 | 17.9 | 6.8 | 3.7 | 2.7 | 0.5 |
| SD | 1.3 | 16.7 | 8.4 | 6.4 | 5.3 | 1.2 |
| Median | 0.5 | 18.0 | 3.0 | 0 | 0 | 0 |
| Range | 0–4.9 | 0–90 | 0–36 | 0–31 | 0–29 | 0–9 |
TABLE 6.
Distribution of men and women, by decade, with AI <5, ≥5, ≥10 and ≥20
| AI <5 |
AI ≥ 5 |
AI ≥ 10 |
AI ≥ 20 |
|||||
|---|---|---|---|---|---|---|---|---|
| n | %a | n | % | n | % | n | % | |
| Age | ||||||||
| 65–69 | ||||||||
| Men (n = 83) | 58 | 70 | 25 | 30 | 12 | 15 | 5 | 6 |
| Women (n = 83) | 65 | 78 | 18 | 22 | 8 | 10 | 1 | 1 |
| Total (n= 166) | 123 | 74 | 43 | 26 | 20 | 12 | 6 | 4 |
| 70–79 | ||||||||
| Men (n = 90) | 64 | 71 | 26 | 29 | 6 | 7 | 4 | 4 |
| Women (n = 111) | 94 | 85 | 17 | 15 | 7 | 6 | 2 | 2 |
| Total (n = 201) | 158 | 79 | 43 | 21 | 13 | 6 | 6 | 3 |
| 80–89 | ||||||||
| Men(n= 18) | 14 | 78 | 4 | 22 | 2 | 11 | 2 | 11 |
| Women (n = 35) | 25 | 71 | 10 | 29 | 8 | 23 | 2 | 6 |
| Total (n = 53) | 39 | 74 | 14 | 26 | 10 | 19 | 4 | 8 |
| 65–99 | ||||||||
| Men(n = 191) | 136 | 72 | 55 | 28 | 20 | 11 | 11 | 6 |
| Women (n = 229) | 184 | 80 | 45 | 20 | 23 | 10 | 5 | 2 |
| Total (n = 420) | 320 | 76 | 100 | 24 | 43 | 11 | 16 | 4 |
Percentages = within gender and within age group.
Hypopnea data were available for 385 people (171 men and 214 women). Subjects had far more hypopneas than apneas. When RDI was computed, 81% had an RDI ≥ 5, 62% had an RDI ≥ 10 and 44% had an RDI ≥ 20. The mean RDI for those with RDI ≥ 10 was 48.0 (SD = 50.3, range = 10–349.8). The mean RDI for the entire sample was 32.2 (SD = 45). The percentages of men and women with RDI at different criterion levels and different ages are shown in Table 7 (see also Fig. 2).
TABLE 7.
Distribution of men and women, by decade, with RDI <10, ≥10, ≥20 and ≤ 40
| RDI < 10 |
RDI ≥ 10 |
RDI ≥ 20 |
RDI ≥ 40 |
|||||
|---|---|---|---|---|---|---|---|---|
| n | %a | n | % | n | % | n | % | |
| Age | ||||||||
| 65–69 | ||||||||
| Men (n = 75) | 19 | 25 | 56 | 75 | 38 | 51 | 21 | 28 |
| Women (n = 77) | 40 | 52 | 37 | 48 | 28 | 36 | 16 | 21 |
| Total (n= 152) | 59 | 39 | 93 | 61 | 66 | 43 | 37 | 24 |
| 70–79 | ||||||||
| Men (n = 79) | 26 | 33 | 53 | 67 | 38 | 48 | 22 | 28 |
| Women (n = 102) | 42 | 41 | 60 | 59 | 39 | 38 | 16 | 16 |
| Total (n= 181) | 68 | 38 | 113 | 62 | 77 | 43 | 38 | 21 |
| 80–89 | ||||||||
| Men(n= 17) | 6 | 35 | 11 | 65 | 11 | 65 | 4 | 24 |
| Women (n = 35) | 12 | 34 | 23 | 66 | 1 | 46 | 13 | 37 |
| Total (n = 52) | 18 | 35 | 34 | 65 | 27 | 52 | 17 | 33 |
| 65–99 | ||||||||
| Men(n= 171) | 51 | 30 | 120 | 70 | 87 | 51 | 47 | 28 |
| Women (n = 214) | 94 | 44 | 120 | 56 | 83 | 39 | 45 | 21 |
| Total (n = 385) | 145 | 38 | 240 | 62 | 170 | 44 | 92 | 24 |
Percentages = within gender and within age group.
FIG. 2.
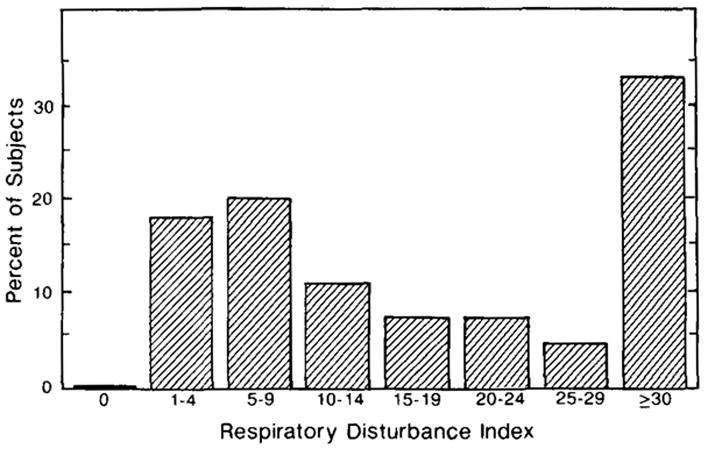
Distribution of respiratory index.
Association of age and gender with degree of sleep-disordered breathing
We wished to investigate the trends evident in Tables 6 and 7, treating AI and RDI as continuous variables and taking into account the confounding between age and gender. On average, the women were slightly older than the men but tended to have slightly lower AI. Nevertheless, none of these trends were statistically significant. The association of gender with AI controlled for age yielded a Mantel–Haenszel (M–H) p = 0.14 and the association of age with AI controlled for gender yielded an M–H p = 0.66. The association of gender with RDI controlled for age yielded an M–H p = 0.14 and the association of age with RDI controlled for gender yielded an M–H p = 0.13.
Items associated with sleep-disordered breathing
Home interview items significantly related to respiratory disturbances are shown in Tables 8 and 9. Higher apnea indices were found in low income areas and among subjects reporting snoring, kicking and wandering at night. Higher apnea indices were also found among subjects who had never had tonsillectomies or adenoidectomies and among former smokers. Higher respiratory disturbance indices were found among snorers.
TABLE 8.
Interview responses associated with total, obstructive, central and mixed apneas (Kruskall–Wallis p values)
| AI | OI | CI | MxI | RDI | |
|---|---|---|---|---|---|
| Residence area | |||||
| High income area (n = 150) | 4.2a | — | — | 0.5 | — |
| Middle income area (n = 167) | 3.4 | — | — | 0.3 | — |
| Low income area (n = 104) | 4.6 | — | — | 0.4 | — |
| p = 0.03 | p < 0.003 | ||||
| Snoring | |||||
| Snores 0–20 days per month (n = 175) | 3.0 | 1.8 | — | — | 28.7 |
| Snores every night (n = 97) | 5.8 | 3.0 | — | — | 42.8 |
| p = 0.05 | p = 0.2 | p = 0.002 | |||
| Stopping breathing at night | |||||
| Never (n = 259) | — | — | 1.0 | — | 28.8 |
| Once or more per month (n = 19) | — | — | 3.5 | — | 48.8 |
| p = 0.01 | p = 0.02 | ||||
| Leg kicks | |||||
| Never kicks (n = 279) | 3.8 | — | — | 0.3 | — |
| Kicks one night or more per month (n = 61) | 5.1 | — | — | 0.9 | — |
| p = 0.02 | p < 0.001 | ||||
| Waking up confused or wandering at night | |||||
| Never (n = 406) | 3.9 | 2.3 | — | — | — |
| Once or more per month (n = 7) | 6.7 | 4.5 | — | — | — |
| p = 0.05 | p = 0.009 | ||||
| Shares a bed | |||||
| Bedmate(n= 131) | — | — | — | 0.3 | — |
| No bedmate (n = 287) | — | — | — | 0.4 | — |
| p = 0.04 | |||||
| Emphysema | |||||
| Never had (n = 388) | — | — | 1.3 | — | — |
| Has now (n = 21) | — | — | 0.6 | — | — |
| p = 0.01 | |||||
| Tonsils and adenoids | |||||
| Never removed (n = 156) | — | 3.0 | — | — | — |
| Removed (n = 262) | — | 2.0 p = 0.009 | — | — | — |
| Smoking | |||||
| Never smoked (n = 167) | — | — | 1.1 | — | — |
| Used to smoke (n = 177) | — | — | 1.5 | — | — |
| Smokes now (n = 76) | — | — | 0.9 | — | — |
| p = 0.009 | |||||
| Dentures | |||||
| Wears dentures (n = 219) | — | 2.5 | — | — | — |
| No dentures (n = 197) | — | 2.2 | — | — | — |
| p = 0.03 | |||||
| Family history of leg jerks | |||||
| Present (n = 37) | — | 2.9 | |||
| Absent (n = 200) | — | 2.0 | — | — | — |
| p = 0.01 | — | — | — | ||
Mean apnea indices (AI = apnea index; OI = obstructive index; CI = central index; MxI = mixed index). Only those with p ≤ 0.05 are presented.
TABLE 9.
Home interview rank correlations (p values) with apnea indices
| AI | OI | CI | MxI | RDI | |
|---|---|---|---|---|---|
| Total sleep time | −0.12 (0.007) | −0.12 (0.006) | |||
| Stop breathing during sleep | 0.15 (0.006) | 0.13 (0.03) | |||
| Confusion or wandering at night | 0.13 (0.004) | ||||
| Fall asleep reading not in bed | 0.12 (0.008) | 0.15 (0.001) | 0.14 (0.007) | ||
| Fall asleep while in conversation | 0.13 (0.005) | 0.08 (0.06) | |||
| Weight | 0.17 (0.001) | 0.13 (0.004) | 0.19 (0.001) | ||
| Height | 0.20 (0.001) | 0.12 (0.008) | 0.12 (0.009) | ||
| Alcohol within 2 hr of bedtime | −0.14 (0.002) | −0.11 (0.01) | |||
| Exercise | 0.15 (0.001) | ||||
| Observer rating of depression | 0.13 (0.004) | 0.13 (0.006) | 0.15 (0.001) | 0.09 (0.03) |
Correlates of sleep-disordered breathing were: nocturnal wandering or confusion, reports of breathing cessation at night, daytime sleepiness in several settings, greater weight and more depression. Apnea was negatively correlated with total sleep time and with use of alcohol within 2 hr of bedtime, but unexpectedly, central apnea and reported exercise were positively correlated (see Table 9).
In a study of this scope, negative results are also of interest. There were no significant differences between those with and without sleep-disordered breathing in age, total sleep time reported, number of car accidents or near car accidents or in reported history of heart disease, stroke, asthma, nasal polyps, sinus problems, deviated septum, broken nose or thyroid disease. In addition, there were no differences in reported family histories of dying in sleep, stopping breathing during sleep, loud snoring, excessive daytime sleepiness or sudden infant death syndrome.
Logistic regression results
Backward elimination logistic regression provided only two independently significant predictors of AI ≥ 5: BMI and falling asleep while sitting with friends talking. BMI was clearly the more powerful of the two factors (Table 10).
TABLE 10.
Logistic regression coefficients by dependent variables and factors
| Dependent variable | Factor | Coefficient | p value | p value Hosmer–Leme-show goodness-of-fita |
|---|---|---|---|---|
| AI ≥ 5 | Constant | −3.1645 | < 0.001 | 0.15 |
| Body mass index | 0.3810 | 0.002 | ||
| Falling asleep talking with friends | 0.2982 | 0.037 | ||
| RDI ≥ 10 | Constant | −1.7260 | 0.010 | 0.074 |
| Body mass index | 0.0869 | 0.0067 | ||
| Alcohol within 2 hr of bedtime | −0.2115 | 0.011 | ||
| Gender | 0.5208 | 0.025 | ||
| Napping | 0.4104 | 0.033 | ||
| Falling asleep reading | 0.1024 | 0.046 |
The estimated probability of AI ≥ 5 is then equal to 100/[1 − exp(−Y)] = 22%.
BMI was also the strongest predictor of RDI ≥ 10, with no alcohol within 2 hr of bedtime, gender, amount of napping and falling asleep reading also being significant factors. The distribution and effect magnitude of risk factors for AI and RDI are shown in Tables 11 and 12.
TABLE 11.
Distribution of AI risk factors
| Risk factors | Distribution (n) | Prevalence (%)a |
|||
|---|---|---|---|---|---|
| AI < 5 | AI ≥ 5 | AI ≥ 10 | AI ≥ 20 | ||
| BMIb | |||||
| 10–19 | 175 | 79 | 21 | 9 | 3 |
| 20–29 | 227 | 74 | 26 | 10 | 4 |
| 30–40 | 17 | 59 | 41 | 29 | 18 |
| Falling asleep talking with friends | |||||
| Never | 401 | 77 | 23 | 10 | 4 |
| ≥1 time/week | 20 | 50 | 50 | 15 | 5 |
Percent apnea = percent within each group.
BMI = body mass index (weight/height2, i.e. kg/m2).
TABLE 12.
Distribution of RDI risk factors
| Prevalence(%)a |
|||||
|---|---|---|---|---|---|
| Risk factors | Distribution (n) | RDI < 10 | RDI ≥ 10 | RDI ≥ 20 | RDI ≥ 40 |
| BMIb | |||||
| 10–19 | 164 | 42 | 58 | 40 | 22 |
| 20–29 | 200 | 37 | 63 | 46 | 24 |
| 30–40 | 16 | 13 | 87 | 63 | 38 |
| Falling asleep reading | |||||
| Never | 218 | 44 | 56 | 39 | 21 |
| ≥ 1 time/week | 157 | 31 | 69 | 49 | 25 |
| Napping | |||||
| Never | 256 | 41 | 59 | 43 | 22 |
| ≥ 1 time/day | 128 | 30 | 70 | 46 | 27 |
| Alcohol within 2 hr of bedtime | |||||
| Never | 352 | 36 | 64 | 45 | 26 |
| ≥ 1 time per week | 32 | 59 | 41 | 31 | 6 |
Percent apnea = percent within each group.
BMI = body mass index (weight/height2, i.e. kg/m2).
Neither of the models described above may be recommended for use as predictors. The model for AI ≥ 5 is highly specific (>99%), but very insensitive. Only 6% of subjects with AI ≥ 5 are given a probability exceeding 50% for having the condition. The reverse situation was obtained for RDI ≥ 10. High sensitivity was achieved (84%) at the cost of low specificity (27%). Further, the goodness-of-fit of the RDI model was poor (Table 10).
DISCUSSION
In a randomly selected probability sample of community-dwelling persons 65 yr and older, the prevalence rate of sleep-disordered breathing was 24% for AI ≥ 5 and 62% for RDI ≥ 10.
Overall, the severity of sleep-disordered breathing in this sample was somewhat mild. Of the 427 volunteers studied, 10% had AI ≥ 10, 4% had AI ≥ 20 [which some authorities regard as an indication for prompt intervention (28)] and 1% had AI ≥ 40. However, the group had a very high number of hypopneas with 44% having RDI ≥ 20. In a previous study in an elderly nursing home population, it was shown that RDI ≥ 50 was predictive of increased mortality. Most of the nursing home patients were also severely ill with multiple illnesses (29). Nevertheless, in the current sample, 18% had RDI ≥ 50.
It seems quite certain that the prevalence of sleep-disordered breathing is greater among the elderly than among younger adults (30,31). There were no significant correlations between age and apnea indices in this sample, which started at age 65 and covered a narrow age range. It appears that most of the increase in apnea indices associated with aging occurs before age 65 is reached. One cannot ignore the possibility that people with the more severe sleep-disordered breathing died before reaching age 65, as there are now several reports linking severe sleep apnea with increased mortality (28,29,32). We are continuing to study these questions with further research.
In sleep clinic samples, sleep apnea has appeared predominantly to be a disease of men (30). The rate of sleep-disordered breathing was significantly higher among men in this study also, and male gender was a predictor of RDI ≥ 10. The rate of AI ≥ 5 in these postmenopausal women, however, was substantially higher than that reported in premenopausal women (21,33).
Given the high prevalence of sleep-disordered breathing, it is important to inquire whether clinicians can reliably recognize sleep-disordered breathing by history alone. In our experience they cannot. Our logistic regression models indicate that there are only a few significant independent predictors of AI ≥ 5 and RDI ≥ 10. Despite statistical significance, all of the associations between interview variables and respiratory disturbance indices were small. No combination of demographic variables and symptoms allowed reliable prediction of the apnea index. Neither model was both sensitive and specific. New factors that are clear-cut indicators of the conditions, or of their absence, are needed before a useful predictive model can be developed.
Our results are consistent with the hypothesis that sleep-disordered breathing causes symptoms of excessive daytime sleepiness and disturbed sleep at night in the elderly (34). Histories of waking up confused or wandering at night, lack of a bed partner, use of dentures, reported stoppage of breathing at night and reported leg kicks were associated with higher apnea in univariate analyses (Tables 8 and 9), but these associations were not independently significant in the multivariable analyses. In general, BMI was the strongest predictor. Only 65% of subjects could report whether or not they snored. Among those who could report, snoring was as strong a correlate of OI as BMI; however, snoring did not contribute to the discriminative prediction of RDI. Reported total sleep time had little correlation with apnea indices. Elsewhere, we examined this association and showed that higher apnea indices are found both among subjects with under 7 hr reported sleep and among those reporting over 8 hr sleep (i.e. a U-shaped association) (35).
At the time this study was begun, it did not seem practical to calibrate the Respitrace. More recently, we have examined the issue of calibration by blindly scoring calibrated and uncalibrated Respitrace records. Correlations for total apneas (rs = 0.84), total hypopneas (rs = 0.95), total events (rs = 0.88) and type of apnea (obstructive, rs = 0.87; central, rs = 0.71; mixed, rs = 0.68) were all medium to high and were all significant (21). In the uncalibrated records, apneas tended to be underscored while hypopneas were over-scored. Therefore, the uncalibrated recordings used in this study may actually give too conservative an estimate of apneas.
The current study was only successful in obtaining full data on 23% of the random sample identified. It is unlikely that any study that requires objective sleep recording can obtain high compliance from a random population sample. Paying the volunteers a minimal amount had no effect on cooperation. Larger monetary rewards or other inducements powerful enough to produce high compliance could bias the sample in other ways. To reduce this problem, the study was prospectively designed to identify those randomly selected volunteers that refused to be recorded and to assess the extent to which compliance would bias our conclusions by selecting for particular types of subjects. Thus, demographic features of persons identified by telephone interview and home interview (without recording) were obtained for comparison with volunteers completing the study. Although we did demonstrate that there were some sampling biases in this volunteer sample, we also tried to demonstrate that no sampling bias distorted the results to a serious extent. None of the variables in which significant biases were demonstrated were retained by the final logistic models. In addition, the low correlations of these factors with measured apnea indicated that these sampling biases could not have seriously affected prevalence estimates.
To determine point prevalence, the single-night recordings that we utilized seemed adequate, but the question arises whether a single overnight recording is a reliable predictor of sleep apnea over an extended period of time. In three studies, using our technology, we have found that the night-to-night correlations for apnea indices range from rs = 0.76 to rs = 0.94 (p < 0.01). Others have reported similar findings (36). We have also been able to rerecord 30 of the initial volunteers for this study after an average lapse of 4.6 yr. The correlation of apnea indices over these 4.6 yr was rs = 0.50 (p < 0.01). The correlation for RDI was rs = 0.69 (p < 0.001) (37). These results demonstrate substantial night-to-night variability in apnea indices with even greater variability over several years. A substantial proportion of the variance between questionnaire-predicted and observed apnea indices may be related to such night-to-night variability in sleep apnea. This variability must likewise affect the reliability of sleep clinic polysomnograms. In other words, we would probably have obtained higher correlations of apnea indices and symptoms if we could have recorded more nights for each subject.
At the time this study was planned, very little was known about the prevalence of sleep-disordered breathing in any age group. This study showed that mild sleep-disordered breathing is exceptionally prevalent among elderly Americans. These disturbances are significant, but weakly associated with various symptoms. The clinician could not reliably screen for these sleep disturbances on the basis of demographic factors, signs and symptoms. In this final report, we now see that in the elderly mild sleep-disordered breathing is usually occult. It is now necessary to go beyond the question of prevalence with longitudinal designs. Repeated recordings are needed to better assess the impact of sleep-disordered breathing on morbidity and mortality in the elderly.
Acknowledgments
This work was supported by NIA AG02711, RSDA MH0017 (to D.F.K.), NIA AG08415, NHLBI HL40930 and the Department of Veterans Affairs. Parts of this manuscript were presented at the meetings of the Association of Professional Sleep Societies.
References
- 1.Webb P. Periodic breathing during sleep. J Appl Physiol. 1974;37:899–903. doi: 10.1152/jappl.1974.37.6.899. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RM, Miles LE, Guilleminault CC, Zarcone VP, van den Hoed J, Dement WC. Sleep–wake disorders in the elderly: a polysomnographic analysis. J Am Geriatr Soc. 1981;29:289–96. doi: 10.1111/j.1532-5415.1981.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoch CC, Reynolds CF, III, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry. 1986;47:499–503. [PubMed] [Google Scholar]
- 4.Krieger J, Mangin P, Kurtz D. Respiration changes during sleep in healthy elderly subjects (French) Rev Electroencephalogr Neurophysiol. 1980;10:177–85. doi: 10.1016/s0370-4475(80)80051-7. [DOI] [PubMed] [Google Scholar]
- 5.McGinty DJ, Littner M, Beahm E, Ruiz-Primo E, Young E, Sowers J. Sleep-related breathing disorders in older men: a search for underlying mechanisms. Neurobiol Aging. 1982;3:337–50. doi: 10.1016/0197-4580(82)90022-7. [DOI] [PubMed] [Google Scholar]
- 6.Roehrs T, Zorick F, Sicklesteel J, Wittig R, Roth T. Age-related sleep–wake disorders at a sleep disorder center. J Am Geriatr Soc. 1983;31:364–70. doi: 10.1111/j.1532-5415.1983.tb05748.x. [DOI] [PubMed] [Google Scholar]
- 7.Smallwood RG, Vitiello MV, Giblin EC, Prinz P. Sleep apnea: relationship to age, sex, and Alzheimer’s dementia. Sleep. 1983;6:16–22. doi: 10.1093/sleep/6.1.16. [DOI] [PubMed] [Google Scholar]
- 8.Kreis P, Kripke DF, Ancoli-Israel S. Sleep apnea: a prospective study. West J Med. 1983;139:171–3. [PMC free article] [PubMed] [Google Scholar]
- 9.Ancoli-Israel S. Epidemiology of sleep disorders. In: Roth T, editor. Clinics in geriatric medicine. Philadelphia, PA: W. B. Saunders Company; 1989. pp. 347–62. [PubMed] [Google Scholar]
- 10.Fairbanks DNF. Snoring: an overview with historical perspectives. In: Fairbanks DNF, Fujita S, Ikematsu T, Simmons FB, editors. Snoring and obstructive sleep apnea. New York: Raven Press; 1987. pp. 1–18. [Google Scholar]
- 11.Lugaresi E, Coccagna G, Cirignotta F. Snoring and its clinical implications. In: Guilleminault C, Dement WC, editors. Sleep apnea syndromes. New York: Alan R. Liss; 1978. pp. 13–22. [Google Scholar]
- 12.Roehrs T, Conway W, Wittig R, Zorick F, Sicklesteel J, Roth T. Sleep–wake complaints in patients with sleep-related respiratory disturbances. Am Rev Respir Dis. 1985;132:520–3. doi: 10.1164/arrd.1985.132.3.520. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Eldridge FL, Dement WC. Insomnia with sleep apnea: a new syndrome. Science. 1973;181:856–8. doi: 10.1126/science.181.4102.856. [DOI] [PubMed] [Google Scholar]
- 14.Kales A, Bixler EO, Soldatos CR, Vela-Bueno A, Caldwell AB, Cadieux RJ. Biopsychobehavioral correlates of insomnia, part 1: role of sleep apnea and nocturnal myoclonus. Psychosomatics. 1982;23:589–600. doi: 10.1016/S0033-3182(82)73359-6. [DOI] [PubMed] [Google Scholar]
- 15.Lavie P, Ben-Yosef R, Rubin AE. Prevalence of sleep apnea syndrome among patients with essential hypertension. Am Heart J. 1984;108:373. doi: 10.1016/0002-8703(84)90628-8. [DOI] [PubMed] [Google Scholar]
- 16.Guilleminault C. Natural history, cardiac impact and long term follow-up of sleep apnea syndrome. In: Guilleminault C, Lugaresi E, editors. Sleep/wake disorders: natural history, epidemiology, and long-term evolution. New York: Raven Press; 1983. pp. 107–24. [Google Scholar]
- 17.Wittels EH. Obesity and hormonal factors in sleep and sleep apnea. Med Clin North Am. 1985;69:1265–80. doi: 10.1016/s0025-7125(16)30986-5. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher EC, DeBehnke RD, Lovoi MS, Gorin AB. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med. 1985;103:190–5. doi: 10.7326/0003-4819-103-2-190. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL, Feldman DE, Bliwise NG, Carskadon MA, Kraemer HC, North CS, Petta DE, Seidel WF, Dement WC. Risk factors for sleep disordered breathing in heterogeneous geriatric populations. J Am Geriatr Soc. 1987;35:132–41. doi: 10.1111/j.1532-5415.1987.tb01342.x. [DOI] [PubMed] [Google Scholar]
- 20.Mason WJ, Kripke DF, Messin S, Ancoli-Israel S. The application and utilization of an ambulatory recording system for the screening of sleep disorders. Am J EEG Technol. 1986;26:145–56. [Google Scholar]
- 21.Ancoli-Israel S. Ambulatory cassette recording of sleep apnea. In: Ebersole JS, editor. Ambulatory EEG monitoring. New York: Raven Press; 1989. pp. 299–315. [Google Scholar]
- 22.Mullaney DJ, Kripke DF, Messin S. Wrist-actigraphic estimation of sleep time. Sleep. 1980;3:83–92. doi: 10.1093/sleep/3.1.83. [DOI] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S, Kripke DF, Mason W, Messin S. Comparisons of home sleep recordings and polysomnograms in older adults with sleep disorders. Sleep. 1981;4(3):283–91. doi: 10.1093/sleep/4.3.283. [DOI] [PubMed] [Google Scholar]
- 24.Schweder T, Spjotvoll E. Plots of P-values to evaluate many tests simultaneously. Biometrika. 1982;69:493–502. [Google Scholar]
- 25.Mantel N. Chi-square tests with one degree of freedom: extensions of the Mantel–Haenszel procedure. J Am Stat Assoc. 1963;58:690–700. [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 27.Guilleminault C, Dement WC. Sleep apnea syndromes. New York: Alan R. Liss; 1978. pp. 1–372. [Google Scholar]
- 28.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 29.Ancoli-Israel S, Klauber MR, Kripke DF, Parker L, Cobarrubias M. Sleep apnea in female patients in a nursing home: increased risk of mortality. Chest. 1989;96(5):1054–8. doi: 10.1378/chest.96.5.1054. [DOI] [PubMed] [Google Scholar]
- 30.Block AJ, Boysen PG, Wynne JW, Hunt LA. Sleep apnea, hypopnea and oxygen desaturation in normal subjects. A strong male predominance. N Engl J Med. 1979;300:513–7. doi: 10.1056/NEJM197903083001001. [DOI] [PubMed] [Google Scholar]
- 31.Bixler EO, Kales A, Soldatos CR, Vela-Bueno A, Jacoby JA, Scarone S. Sleep apneic activity in a normal population. Res Commun Chem Pathol Pharmacol. 1982;36:141–52. [PubMed] [Google Scholar]
- 32.Partinen M, Jamieson A, Guilleminault C. Long-term outcome for obstructive sleep apnea syndrome patients—mortality. Chest. 1988;94:1200–4. doi: 10.1378/chest.94.6.1200. [DOI] [PubMed] [Google Scholar]
- 33.Block AJ, Wynne JW, Boysen PG. Sleep-disordered breathing and nocturnal oxygen desaturation in postmenopausal women. Am J Med. 1980;69:75–9. doi: 10.1016/0002-9343(80)90502-1. [DOI] [PubMed] [Google Scholar]
- 34.Berry DT, Phillips BA, Cook YR, Schmitt FA, Honeycutt NA, Arita AA, Allen RS. Geriatric sleep apnea syndrome: a preliminary description. J Gerontol. 1990;45(5):M169–74. doi: 10.1093/geronj/45.5.m169. [DOI] [PubMed] [Google Scholar]
- 35.Kripke DF, Ancoli-Israel S, Mason WJ, Kaplan O. Sleep apnea: association with deviant sleep durations and increased mortality. In: Guilleminault C, Partinen M, editors. Obstructive sleep apnea syndrome: clinical research and treatment. New York: Raven Press; 1990. pp. 9–14. [Google Scholar]
- 36.Mosko SS, Dickel MJ, Ashurst J. Night-to-night variability in sleep apnea and sleep in sleep-related periodic leg movements in the elderly. Sleep. 1988;11:340–8. [PubMed] [Google Scholar]
- 37.Mason WJ, Ancoli-Israel S, Kripke DF. Apnea revisited: a longitudinal follow-up. Sleep. 1989;12(5):423–9. doi: 10.1093/sleep/12.5.423. [DOI] [PubMed] [Google Scholar]


